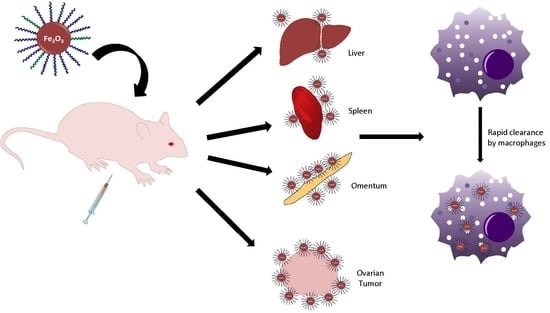
Biodistribution and Clearance of Stable Superparamagnetic Maghemite Iron Oxide Nanoparticles in Mice Following Intraperitoneal Administration
Abstract
:1. Introduction
2. Results
2.1. Generation and In Vitro Stabilization Studies of the SPIONS
2.1.1. Macro-RAFT and Short Chain Diblock Stabilizers with Functionalized End Groups
2.1.2. Steric Stabilization Achieved through the Combination of SPIONs with RAFT Derived Diblocks with a Desired Ratio of Various Functional End Groups
2.2. Biodistribution of s-SPIONs in Nude Mice
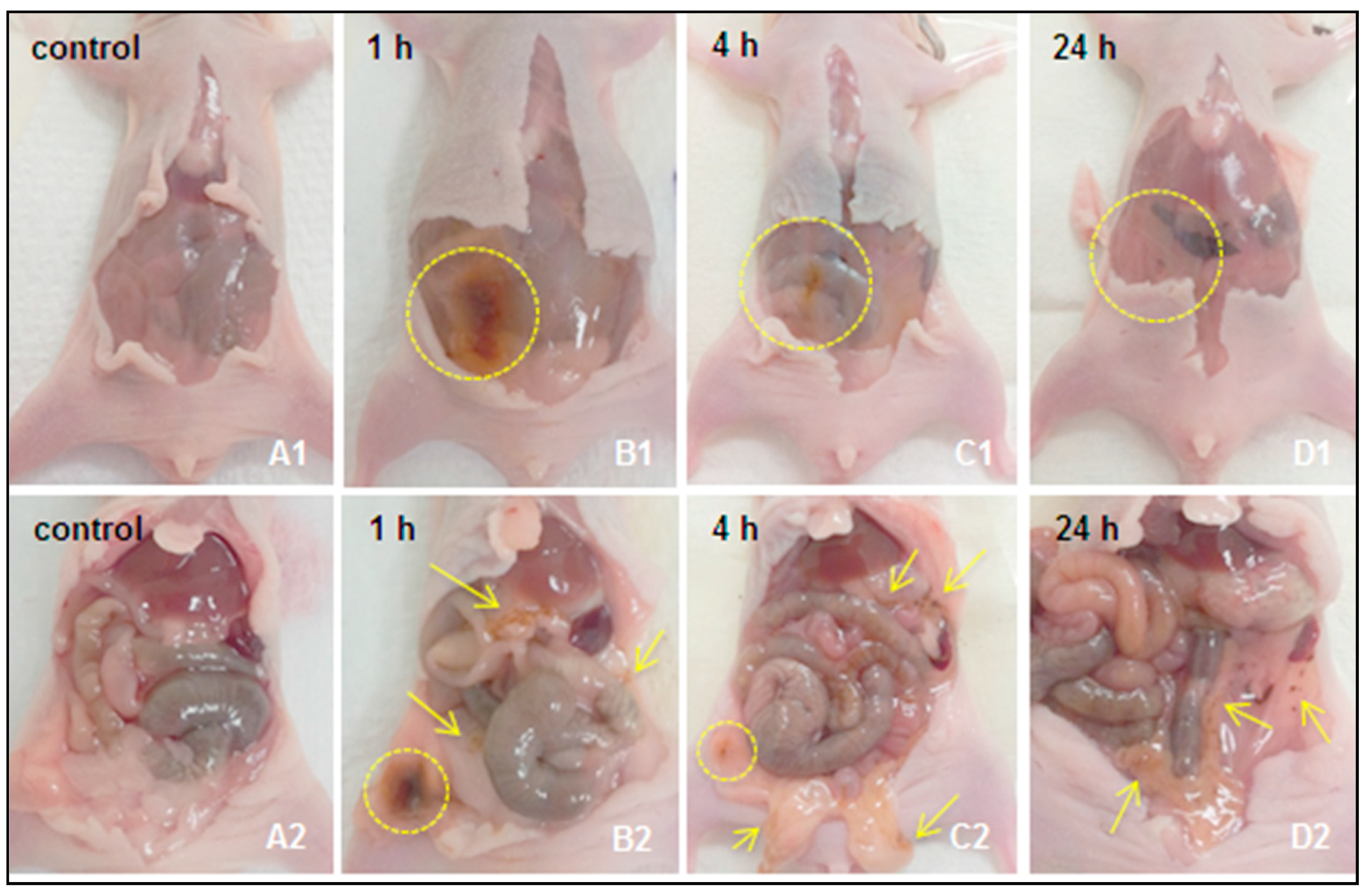
2.2.1. Macroscopic Observations
2.2.2. Quantitative Measurement of Iron Levels in the Peritoneum Wash, Blood Samples, and Collected Tissue Samples
Biodistribution of 10 nm s-SPIONs
Biodistribution of 25 nm s-SPIONs
2.2.3. Mapping the Distribution of s-SPIONs in Tissues through Iron Staining with Prussian Blue
2.2.4. Examination of Tissues for Signs of Toxicity
Histological Assessment
Immunostaining for Macrophages
2.2.5. Clearance Pathway of s-SPIONs
Excretion of s-SPIONs via Feces
Excretion of s-SPIONs via Macrophages
2.2.6. Biodistribution of s-SPIONs in Tumor-Bearing Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Characterization Methods
4.2.1. NMR Spectroscopy
4.2.2. Dynamic Light Scattering (DLS) and Transmission Electron Microscopy (TEM)
4.2.3. Thermogravimetric Analysis (TGA)
4.2.4. Atomic Absorption Spectroscopy (AAS)
4.3. Synthesis of Sterically Stabilized SPIONs
4.3.1. The Raw Magnetic Ferrofluids
4.3.2. Synthesis of N-Boc-ethylenediamine C4-RAFT Agent (tert-butyl (2-(2-(((butylthio)carbonothioyl)thiopropanamido)ethyl)carbamate)
4.3.3. Synthesis of NH2 End Functionalized Macro-RAFT Diblocks: Poly(monoacryloxyethyl phosphate)10-block-poly(acrylamide)20 (RAFT-MAEP10-AAm20-NH2) and poly(monoacryloxyethyl phosphate)10-block-poly(acrylamide)60 (RAFT-MAEP10-AAm60-NH2) Using the N-Boc-Ethylenediamine C4-RAFT Agent
4.3.4. Synthesis of MPEG End Functionalized Macro-RAFT Diblocks: Poly(ethylene oxide)17-block-poly(monoacryloxyethyl phosphate)10 Macro-RAFT Agent (RAFT-MAEP10-MPEG) and Poly(ethylene oxide)17-block-poly(acrylamide)40-block-poly(monoacryloxyethyl phosphate)10 Macro-RAFT Agent (RAFT-MAEP10-AAm40-MPEG)
4.3.5. Stabilization of the γ-Fe2O3 with the Steric Stabilizers
4.4. In Vivo Experiments
4.4.1. Ethics Statement
4.4.2. Animals
4.4.3. Biodistribution in Healthy Mice
4.4.4. Biodistribution in Tumor-Bearing Animals
4.4.5. Preparation of Samples for Iron Quantification
4.4.6. Histology and Immunohistochemistry
4.5. In Vitro Studies
4.5.1. Cell Culture
4.5.2. Preparation of Cell Samples for Iron Quantification
4.5.3. Preparation of Cell Samples for TEM
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SPION | superparamagnetic iron oxide nanoparticles |
| s-SPIONs | sterically stabilized superparamagnetic iron oxide nanoparticles |
| IONP | iron oxide nanoparticle |
| IP | intraperitoneal injection |
| IV | intravenous injection |
| AAS | atomic absorption spectroscopy |
| GF-AAS | graphite furnace—atomic absorption spectroscopy |
| RAFT | reversible addition fragmentation chain transfer |
| MPEG | methoxypolyethylene glycol |
| AAm | acrylamide |
| MAEP | monoacryloxyethyl phosphate |
| NMR | nuclear magnetic resonance |
| TEM | transmission electron microscopy |
| TGA | thermogravimetric analysis |
| DLS | dynamic light scattering |
| ROS | reactive oxygen species |
| PBS | phosphate-buffered saline |
| H&E | haematoxylin and eosin |
References
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef]
- Xie, J.; Chen, K.; Huang, J.; Lee, S.; Wang, J.; Gao, J.; Li, X.; Chen, X. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials 2010, 31, 3016–3022. [Google Scholar] [CrossRef] [PubMed]
- Keliher, E.J.; Yoo, J.; Nahrendorf, M.; Lewis, J.S.; Marinelli, B.; Newton, A.; Pittet, M.J.; Weissleder, R. 89Zr-Labeled Dextran Nanoparticles Allow in Vivo Macrophage Imaging. Bioconjug. Chem. 2011, 22, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.E.; Golovko, D.; Ruffell, B.; Denardo, D.G.; Castaneda, R.; Ansari, C.; Rao, J.; Tikhomirov, G.A.; Wendland, M.F.; Corot, C.; et al. MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clin. Cancer Res. 2011, 17, 5695–5704. [Google Scholar] [CrossRef] [PubMed]
- Tassa, C.; Shaw, S.Y.; Weissleder, R. Dextran-Coated Iron Oxide Nanoparticles: A Versatile Platform for Targeted Molecular Imaging, Molecular Diagnostics, and Therapy. Acc. Chem. Res. 2011, 44, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Kusakabe, Y.; Fujii, H.; Murase, K.; Yamazaki, Y.; Murase, K. Inflammatory imaging with ultrasmall superparamagnetic iron oxide. Magn. Reson. Imaging 2011, 29, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Storey, P.; Lim, R.P.; Chandarana, H.; Rosenkrantz, A.B.; Kim, D.; Stoffel, D.R.; Lee, V.S. MRI assessment of hepatic iron clearance rates after USPIO administration in healthy adults. Investig. Radiol. 2012, 47, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Prodan, A.M.; Iconaru, S.L.; Ciobanu, C.S.; Chifiriuc, M.C.; Stoicea, M.; Predoi, D. Iron Oxide Magnetic Nanoparticles: Characterization and Toxicity Evaluation by In Vitro and In Vivo Assays. J. Nanomater. 2013. [Google Scholar] [CrossRef]
- Thomas, R.; Park, I.K.; Jeong, Y.Y. Magnetic iron oxide nanoparticles for multimodal imaging and therapy of cancer. Int. J. Mol. Sci. 2013, 14, 15910–15930. [Google Scholar] [CrossRef] [PubMed]
- Thorek, D.L.J.; Ulmert, D.; Diop, N.-F.M.; Lupu, M.E.; Doran, M.G.; Huang, R.; Abou, D.S.; Larson, S.M.; Grimm, J. Non-invasive mapping of deep-tissue lymph nodes in live animals using a multimodal PET/MRI nanoparticle. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, A.; Sidhu, N.; Hu, C.A.; Mlady, G.; Eberhardt, S.C.; Sillerud, L.O. Iron-based superparamagnetic nanoparticle contrast agents for MRI of infection and inflammation. Am. J. Roentgenol. 2015, 204, W302–W313. [Google Scholar] [CrossRef] [PubMed]
- Normandin, M.D.; Yuan, H.; Wilks, M.Q.; Chen, H.H.; Kinsella, J.M.; Cho, H.; Guehl, N.J.; Absi-Halabi, N.; Hosseini, S.M.; El Fakhri, G.; et al. Heat-Induced Radiolabeling of Nanoparticles for Monocyte Tracking by PET. Angew. Chem. Int. Ed. Engl. 2015, 54, 13002–13006. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging macrophages with nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Li, J.; Luo, Q.; Hei, Y.; Wang, K.; Tian, Y.; Yang, J.; Lei, H.; Qiu, B.; Liu, W. MRI tracking of bone marrow mesenchymal stem cells labeled with ultra-small superparamagnetic iron oxide nanoparticles in a rat model of temporal lobe epilepsy. Neurosci. Lett. 2015, 606, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Aswendt, M.; Henn, N.; Michalk, S.; Schneider, G.; Steiner, M.-S.; Bissa, U.; Dose, C.; Hoehn, M. Novel bimodal iron oxide particles for efficient tracking of human neural stem cells in vivo. Nanomedicine 2015, 10, 2499–2512. [Google Scholar] [CrossRef] [PubMed]
- Fink, J.R.; Muzi, M.; Peck, M.; Krohn, K.A. Multimodality Brain Tumor Imaging: MR Imaging, PET, and PET/MR Imaging. J. Nucl. Med. 2015, 56, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Wabler, M.; Zhu, W.L.; Hedayati, M.; Attaluri, A.; Zhou, H.M.; Mihalic, J.; Geyh, A.; DeWeese, T.L.; Ivkov, R.; Artemov, D. Magnetic resonance imaging contrast of iron oxide nanoparticles developed for hyperthermia is dominated by iron content. Int. J. Hyperther. 2014, 30, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Puppi, J.; Modo, M.; Dhawan, A.; Lehec, S.C.; Mitry, R.R.; Hughes, R.D. Ex Vivo Magnetic Resonance Imaging of Transplanted Hepatocytes in a Rat Model of Acute Liver Failure. Cell Transplant. 2014, 23, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Luo, Q.; Chen, J.E.; Gan, Y.P.; Du, J.; Ding, S.M.; Xi, Z.G.; Yang, X. Intraperitoneal injection of magnetic Fe3O4-nanoparticle induces hepatic and renal tissue injury via oxidative stress in mice. Int. J. Nanomed. 2012, 7, 4809–4818. [Google Scholar] [CrossRef]
- Storey, P.; Arbini, A.A. Bone marrow uptake of ferumoxytol: A preliminary study in healthy human subjects. J. Magn. Reson. Imaging 2014, 39, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, A.R.; Rakhlin, E.; Weissleder, R.; Thayer, S.P. Magnetic Resonance Imaging Monitors Physiological Changes With Antihedgehog Therapy in Pancreatic Adenocarcinoma Xenograft Model. Pancreas 2008, 37, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Liu, J.; Zhong, Y.J.; Zhang, J.; Wang, Z.Y.; Wang, L.; An, Y.L.; Lin, M.; Gao, Z.Q.; Zhang, D.S. Biocompatibility of Fe3O4@Au composite magnetic nanoparticles in vitro and in vivo. Int. J. Nanomed. 2011, 6, 2805–2819. [Google Scholar] [CrossRef] [PubMed]
- Basel, M.T.; Balivada, S.; Wang, H.W.; Shrestha, T.B.; Seo, G.M.; Pyle, M.; Abayaweera, G.; Dani, R.; Koper, O.B.; Tamura, M.; et al. Cell-delivered magnetic nanoparticles caused hyperthermia-mediated increased survival in a murine pancreatic cancer model. Int. J. Nanomed. 2012, 7, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Toraya-Brown, S.; Sheen, M.R.; Baird, J.R.; Barry, S.; Demidenko, E.; Turk, M.J.; Hoopes, P.J.; Conejo-Garcia, J.R.; Fiering, S. Phagocytes mediate targeting of iron oxide nanoparticles to tumors for cancer therapy. Integr. Biol. 2013, 5, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Kaul, M.G.; Bruns, O.T.; Ducic, T.; Freund, B.; Heine, M.; Reimer, R.; Meents, A.; Salmen, S.C.; Weller, H.; et al. Intraperitoneal Injection Improves the Uptake of Nanoparticle-Labeled HDL to Atherosclerotic Plaques Compared With Intravenous Injection A Multimodal Imaging Study in ApoE−/− Mice. Circ. Cardiovasc. Imaging 2014, 7, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.L.; Andronescu, E.; Stoicea, M.; Le Coustumer, P.; Galaup, S.; Beuran, M.; Iordache, F.M.; Telcian, A.; Bleotu, C.; Prodan, A.M. Fabrication, Characterization, Toxicity and Biocompatibility Evaluation of Iron Oxide Nanoparticles. Dig. J. Nanomater. Biostruct. 2014, 9, 19–25. [Google Scholar]
- Bashir, M.R.; Bhatti, L.; Marin, D.; Nelson, R.C. Emerging applications for ferumoxytol as a contrast agent in MRI. J. Magn. Reson. Imaging 2015, 41, 884–898. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.; Coussens, L.M. MR imaging of tumor-associated macrophages. Oncoimmunology 2012, 1, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015, 88, 20150207. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Elizondo, G.; Stark, D.D.; Hahn, P.F.; Marfil, J.; Gonzalez, J.F.; Saini, S.; Todd, L.E.; Ferrucci, J.T. The diagnosis of splenic lymphoma by MR imaging: Value of superparamagnetic iron oxide. Am. J. Roentgenol. 1989, 152, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Pisani, L.J.; Lee, Y.K.; Messing, S.; Ansari, C.; Bhaumik, S.; Lowery, L.; Lee, B.D.; Meyer, D.E.; Daldrup-Link, H.E. Evaluation of the novel USPIO GEH121333 for MR imaging of cancer immune responses. Contrast Media Mol. Imaging 2013, 8, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (part I): Products on the market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.J. Current status of superparamagnetic iron oxide contrast agents for liver magnetic resonance imaging. J. Gastroenterol. 2015, 21, 13400–13402. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Bowen, A.M.; Josephson, L.; Vasdev, N.; Holland, J.P. Chelate-free metal ion binding and heat-induced radiolabeling of iron oxide nanoparticles. Chem. Sci. 2015, 6, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Bryce, N.S.; Pham, B.T.T.; Fong, N.W.S.; Jain, N.; Pan, E.H.; Whan, R.M.; Hambley, T.W.; Hawkett, B.S. The composition and end-group functionality of sterically stabilized nanoparticles enhances the effectiveness of co-administered cytotoxins. Biomater. Sci. 2013, 1, 1260–1272. [Google Scholar] [CrossRef]
- Eamegdool, S.S.; Weible, M.W., II; Pham, B.T.T.; Hawkett, B.S.; Grieve, S.M.; Chan-ling, T. Ultrasmall superparamagnetic iron oxide nanoparticle prelabelling of human neural precursor cells. Biomaterials 2014, 35, 5549–5564. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Wang, Y.; Jones, S.K.; Hawkett, B.S.; Warr, G.G. Optimized Steric Stabilization of Aqueous Ferrofluids and Magnetic Nanoparticles. Langmuir 2010, 26, 4465–4472. [Google Scholar] [CrossRef] [PubMed]
- Pham, B.T.T.; Jain, N.; Kuchel, P.W.; Chapman, B.E.; Bickley, S.A.; Jones, S.K.; Hawkett, B.S. The interaction of sterically stabilized magnetic nanoparticles with fresh human red blood cells. Int. J. Nanomed. 2015, 10, 6645–6655. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Pham, B.T.T.; Huynh, V.; Kim, B.J.; Pham, N.T.H.; Bickley, S.A.; Jones, S.K.; Serelis, A.; Davey, T.; Such, C.; et al. Monodispersed polymer encapsulated superparamagnetic iron oxide nanoparticles for cell labeling. Polymer 2016, 106, 238–248. [Google Scholar] [CrossRef]
- Kjellander, R.; Florin, E. Water structure and changes in thermal stability of the system poly(ethylene oxide)-water. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1981, 77, 2053–2077. [Google Scholar] [CrossRef]
- Di̇nç, C.Ö.; Ki̇barer, G.; Güner, A. Solubility profiles of poly(ethylene glycol)/solvent systems. II. comparison of thermodynamic parameters from viscosity measurements. J. Appl. Polym. Sci. 2010, 117, 1100–1119. [Google Scholar] [CrossRef]
- Greaves, P. 9—Liver and Pancreas. In Histopathology of Preclinical Toxicity Studies, 3rd ed.; Academic Press: New York, NY, USA, 2007; pp. 457–569. [Google Scholar] [CrossRef]
- Pham, B.T.T.; Such, C.H.; Hawkett, B.S. Synthesis of polymeric janus nanoparticles and their application in surfactant-free emulsion polymerizations. Polym. Chem. 2015, 6, 426–435. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance Properties of Nano-sized Particles and Molecules as Imaging Agents: Considerations and Caveats. Nanomedicine (Lond.) 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Recalcati, S.; Locati, M.; Gammella, E.; Invernizzi, P.; Cairo, G. Iron levels in polarized macrophages: Regulation of immunity and autoimmunity. Autoimmun. Rev. 2012, 11, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Laskar, A.; Eilertsen, J.; Li, W.; Yuan, X.M. SPION primes THP1 derived M2 macrophages towards M1-like macrophages. Biochem. Biophys. Res. Commun. 2013, 441, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]








| Time Point | Total Iron in Feces (µg) from Mice Injected with | |
|---|---|---|
| 10 nm s-SPIONs | 25 nm s-SPIONs | |
| Control | 10.8 ± 2.5 | 10.8 ± 2.5 |
| 1 h | 28.4 ± 17.7 | 20.6 ± 13.9 |
| 4 h | 28.0 ± 14.0 | 11.0 ± 3.7 |
| 24 h | 7.2 ± 2.4 | 11.8 ± 1.5 |
| 48 h | 9.5 ± 1.1 | 13.4 ± 2.8 |
| 1 week | 11.5 ± 0.3 | 8.6 ± 2.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, B.T.T.; Colvin, E.K.; Pham, N.T.H.; Kim, B.J.; Fuller, E.S.; Moon, E.A.; Barbey, R.; Yuen, S.; Rickman, B.H.; Bryce, N.S.; et al. Biodistribution and Clearance of Stable Superparamagnetic Maghemite Iron Oxide Nanoparticles in Mice Following Intraperitoneal Administration. Int. J. Mol. Sci. 2018, 19, 205. https://doi.org/10.3390/ijms19010205
Pham BTT, Colvin EK, Pham NTH, Kim BJ, Fuller ES, Moon EA, Barbey R, Yuen S, Rickman BH, Bryce NS, et al. Biodistribution and Clearance of Stable Superparamagnetic Maghemite Iron Oxide Nanoparticles in Mice Following Intraperitoneal Administration. International Journal of Molecular Sciences. 2018; 19(1):205. https://doi.org/10.3390/ijms19010205
Chicago/Turabian StylePham, Binh T. T., Emily K. Colvin, Nguyen T. H. Pham, Byung J. Kim, Emily S. Fuller, Elizabeth A. Moon, Raphael Barbey, Samuel Yuen, Barry H. Rickman, Nicole S. Bryce, and et al. 2018. "Biodistribution and Clearance of Stable Superparamagnetic Maghemite Iron Oxide Nanoparticles in Mice Following Intraperitoneal Administration" International Journal of Molecular Sciences 19, no. 1: 205. https://doi.org/10.3390/ijms19010205
APA StylePham, B. T. T., Colvin, E. K., Pham, N. T. H., Kim, B. J., Fuller, E. S., Moon, E. A., Barbey, R., Yuen, S., Rickman, B. H., Bryce, N. S., Bickley, S., Tanudji, M., Jones, S. K., Howell, V. M., & Hawkett, B. S. (2018). Biodistribution and Clearance of Stable Superparamagnetic Maghemite Iron Oxide Nanoparticles in Mice Following Intraperitoneal Administration. International Journal of Molecular Sciences, 19(1), 205. https://doi.org/10.3390/ijms19010205