Involvement of Bax and Bcl-2 in Induction of Apoptosis by Essential Oils of Three Lebanese Salvia Species in Human Prostate Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Cell Growth Inhibitory Effect of the Essential Oils
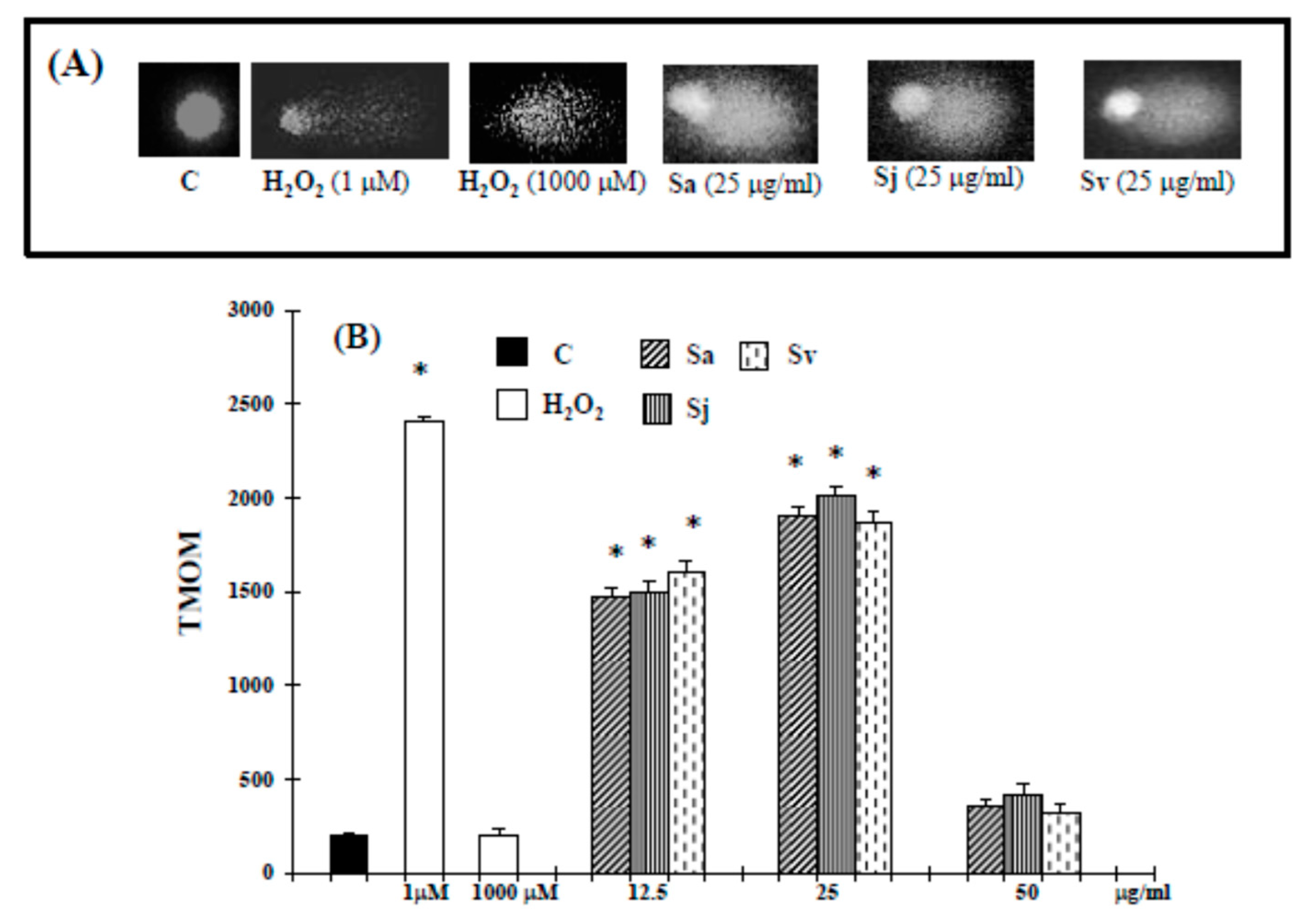
2.2. Induction of Cell Death
3. Discussion
4. Materials and Methods
4.1. Essential Oils
4.2. Study on Human Tumor Cell Line
4.2.1. Cell Culture and Treatments
4.2.2. MTT Bioassay
4.2.3. Lactate Dehydrogenase (LDH) Release
4.2.4. Activity of Caspase-3
4.2.5. DNA Analysis by COMET Assay
4.2.6. TUNEL Assay (ApoAlert® DNA Fragmentation Assay)
4.2.7. Western Blot Analysis
4.2.8. Reactive Oxygen Species Assay
4.2.9. Measurement of GSH Levels
4.2.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| EMEM | Earle minimal essential medium |
| PBS | Phosphate buffer saline |
| Bcl-2 | B-cell lymphoma 2 |
| Bax | Bcl-2 associated X protein |
| MTT | 3(4,5-dimethyl-thiazol-2-yl)2,5-diphenyl-tetrazolium bromide |
| LDH | Lactate dehydrogenase |
| Ac-DEVD-pNA | Acetyl-Asp-Glu-Val-Asp p-nitroanilide |
| HEPES | 1-piperazineethane sulfonic acid, 4-(2-hydroxyethyl)-monosodium salt |
| CHAPS | 3[(3-cholamidopropyl)dimethylammonio]-propanesulfonic acid |
| DTT | 1,4 dithio-dl-threitol |
| TUNEL | Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end-labeling |
| DCFH-DA | 2′,7′-dichlorofluorescein diacetate |
| DCF | 2′,7′-dichlorofluorescein |
| GSH | Glutathione |
| DTNB | 5,5′-dithio-bis(2-nitrobenzoic acid) |
| TNB | 5′-thio-2-nitrobenzoic acid |
| Sa | Salvia aurea |
| Sj | Salvia judaica |
| Sv | Salvia viscose |
References
- American Cancer Society. Available online: http://www.cancer.org/ (accessed on 27 July 2017).
- Stavridi, F.; Karapanagiotou, E.M.; Syrigos, K.N. Targeted therapeutic approaches for hormone-refractory prostate cancer. Cancer Treat. Rev. 2010, 36, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Chuu, C.P.; Kokontis, J.M.; Hiipakka, R.A.; Fukuchi, J.; Lin, H.P.; Lin, C.Y.; Huo, C.; Su, L.C. Androgens as therapy for androgen receptor-positive castration-resistant prostate cancer. J. Biomed. Sci. 2011, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Censits, J.; Fu, M. Chemotherapy and targeted therapies: Are we making progress in castrate-resistant prostate cancer? Semin. Oncol. 2013, 40, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Higano, C.S.; Small, E.J.; Schellhammer, P.; Yasothan, U.; Gubernick, S.; Kirkpatrick, P.; Kantoff, P.W. Sipuleucel-T. Nat. Rev. Drug Discov. 2010, 9, 513–514. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; Pal, S.K. Abiraterone and its place in the treatment of metastatic CRPC. Nat. Rev. Clin. Oncol. 2013, 10, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, P.; Mainwaring, K.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Yakes, F.M.; Chen, J.; Tan, J.; Yamaguchi, K.; Shi, Y.; Yu, P.; Qian, F.; Chu, F.; Bentzien, F.; Cancilla, B.; et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 2011, 10, 2298–2308. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C.; Smith, M.R.; Sweeney, C.; Elfiky, A.A.; Logothetis, C.; Corn, P.G.; Vogelzang, N.J.; Small, E.J.; Harzstark, A.L.; Gordon, M.S.; et al. Cabozantinib in patients with advanced prostate cancer: Results of a phase II randomized discontinuation trial. J. Clin. Oncol. 2013, 31, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Modulation of apoptosis by natural products for cancer therapy. Planta Med. 2010, 76, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, E.; Adhami, V.M.; Khan, N.; Mukhtar, H. Apoptosis and autophagy induction as mechanism of cancer prevention by naturally occurring dietary agents. Curr. Drug Targets 2012, 13, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Cree, I.A. High-throughput screening of natural products for cancer therapy. Planta Med. 2010, 76, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Cardile, V.; Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Arnold, N.A.; Piozzi, F. Essential oils of Salvia bracteata and Salvia rubifolia from Lebanon. Chemical composition, antimicrobial activity and inhibitory effect on human melanoma cells. J. Ethnopharmacol. 2009, 126, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.B.; Sytsma, K.J.; Treutlein, J.; Wink, M. Salvia (Lamiaceae) is not monophyletic: Implications for the systematics, radiation, and ecological specializations of Salvia and tribe Mentheae. Am. J. Bot. 2004, 91, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.P.; van Zyl, R.L.; van Vuuren, S.F.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Viljoen, A.M. Seasonal variation in essential oil composition, oil toxicity and the biological activity of solvent extracts of three South African Salvia species. S. Afr. J. Bot. 2008, 74, 230–237. [Google Scholar] [CrossRef]
- Boszormenyi, A.; Hethelyi, E.; Farkas, A.; Horvath, G.; Papp, N.; Lemberkovics, E.; Szoke, E. Chemical and genetic relationships among Sage (Salvia officinalis L.) cultivars and Judean Sage (Salvia judaica Boiss.). J. Agric. Food Chem. 2009, 57, 4663–4667. [Google Scholar] [CrossRef] [PubMed]
- Mouterde, P. Nouvelle Flore du Liban et de la Syrie; Dar-el-Machreq: Beyrouth, Lebanon, 1983. [Google Scholar]
- Kamatou, G.P.P.; Makunga, N.P.; Ramogola, W.P.N.; Viljoen, A.M. South African Salvia species: A review of biological activities and phytochemistry. J. Ethnopharmacol. 2008, 119, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Mancini, E.; Arnold, N.A.; De Martino, L.; De Feo, V.; Formisano, C.; Rigano, D.; Senatore, F. Chemical composition and phytotoxic effects of essential oils of Salvia hierosolymitana Boiss. and Salvia multicaulis Vahl. var. simplicifolia Boiss. growing wild in Lebanon. Molecules 2009, 14, 4725–4736. [Google Scholar] [PubMed]
- Canzoneri, M.; Bruno, M.; Rosselli, S.; Russo, A.; Cardile, V.; Formisano, C.; Rigano, D.; Senatore, F. Chemical composition and biological activity of Salvia verbenaca essential oil. Nat. Prod. Commun. 2011, 6, 1023–1026. [Google Scholar] [PubMed]
- Tenore, G.C.; Ciampaglia, R.; Arnold, N.A.; Piozzi, F.; Napolitano, F.; Rigano, D.; Senatore, F. Antimicrobial and antioxidant properties of the essential oil of Salvia lanigera from Cyprus. Food Chem. Toxicol. 2011, 49, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Formisano, C.; Rigano, D.; Cardile, V.; Arnold, N.A.; Senatore, F. Comparative phytochemical profile and antiproliferative activity on human melanoma cells of essential oils of three Lebanese Salvia species. Ind. Crops Prod. 2016, 83, 492–499. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55C, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; Russo, A.; Cardile, V.; Galvano, G.; Vanella, A.; Renis, M. DNA damage in human fibroblasts exposed to fumonisin B(1). Food Chem. Toxicol. 2002, 40, 25–31. [Google Scholar] [CrossRef]
- Sekine, T.; Takahashi, J.; Nishishiro, M.; Arai, A.; Wakabayashi, H.; Kurihara, T.; Kobayashi, M.; Hashimoto, K.; Kikuchi, H.; Katayama, T.; et al. Tumor-specific and type of cell death induced by trihaloacetylazulenes in human tumor cell lines. Anticancer Res. 2007, 27, 133–143. [Google Scholar] [PubMed]
- Baigi, M.G.; Brault, L.; Nequesque, A.; Beley, M.; Hilali, R.E.; Gauzere, F.; Bagrel, D. Apoptosis/necrosis switch in two different cancer cell lines: Influence of benzoquinone-and hydrogen peroxide-induced oxidative stress intensity, and glutathione. Toxicol. In Vitro 2008, 22, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.W.; Ruefli, A.A.; Lowe, S.W. Apoptosis: A link between cancergenetics and chemotherapy. Cell 2002, 108, 153–164. [Google Scholar] [CrossRef]
- Shi, Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef]
- Godard, T.; Deslandes, E.; Lebailly, P.; Vigreux, C.; Sichel, F.; Poul, J.M.; Gauduchon, P. Early detection of staurosporine-induced apoptosis by comet and annexin V assays. Histochem. Cell Biol. 1999, 112, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Callagy, G.M.; Webber, M.J.; Pharoah, P.D.; Caldas, C. Meta-analysis confirms Bcl-2 is an independent prognostic marker in breast cancer. BMC Cancer 2008, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H. Structural features of caspase-activating complexes. Int. J. Mol. Sci. 2012, 13, 4807–4818. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Srivastava, S.K.; Kim, S.H. Caspase-9 as a therapeutic target for treating cancer. Expert Opin. Ther. Targets 2015, 19, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; You, Z. In vitro and in vivo model systems used in prostate cancer research. J. Biol. Methods 2015, 2, e17. [Google Scholar] [CrossRef] [PubMed]
- Shirahama, T.; Sakakura, C.; Sweeney, E.A.; Ozawa, M.; Takemoto, M.; Nishiyama, K.; Ohi, Y.; Igarashi, Y. Sphingosine induces apoptosis in androgen-independent human prostatic carcinoma DU-145 cells by suppression of bcl-XL gene expression. FEBS Lett. 1997, 407, 97–100. [Google Scholar] [CrossRef]
- Mu, D.; Zhuo, G.; Guo, H.; Zhou, G.; Sun, B. Sodium butyrate induces growth inhibition and apoptosis in human prostate cancer DU145 cells by up-regulation of the expression of annexin A1. PLoS ONE 2013, 8, e74922. [Google Scholar] [CrossRef] [PubMed]
- Nutt, L.K.; Chandra, J.; Pataer, A.; Fang, B.; Roth, J.A.; Swisher, S.G.; O’Neil, R.G.; McConkey, D.J. Bax-mediated Ca2+ mobilization promotes cytochromec release during apoptosis. J. Biol. Chem. 2002, 277, 20301–20308. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Caggia, S.; Piovano, M.; Garbarino, J.; Cardile, V. Effect of vicanicin and protolichesterinic acid on human prostate cancer cells: Role of Hsp70 protein. Chem. Biol. Interact. 2012, 195, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Espinoza, C.L.; Caggia, S.; Garbarino, J.A.; Peña-Cortés, H.; Carvajal, T.M.; Cardile, V. A new jasmonic acid stereoisomeric derivative induces apoptosis via reactive oxygen species in human prostate cancer cells. Cancer Lett. 2012, 326, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Buttke, T.M.; Sandstrom, P.A. Oxidative stress as a mediator of apoptosis. Immunol. Today 1994, 15, 7–10. [Google Scholar] [CrossRef]
- Loo, G. Redox-sensitive mechanisms of phytochemical-mediated inhibition of cancer cell proliferation. J. Nutr. Biochem. 2003, 14, 64–73. [Google Scholar] [CrossRef]
- Nimmanapalli, R.; Perkins, C.L.; Orlando, M.; O’Bryan, E.; Nguyen, D.; Bhalla, K.N. Pretreatment with paclitaxel enhances Apo-2 ligand/tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis of prostate cancer cells by inducing death receptors 4 and 5 protein levels. Cancer Res. 2001, 61, 759–763. [Google Scholar] [PubMed]
- Ambrož, M.; Boušová, I.; Skarka, A.; Hanušová, V.; Králová, V.; Matoušková, P.; Szotáková, B.; Skálová, L. The influence of sesquiterpenes from Myrica rubra on the antiproliferative and pro-oxidative effects of doxorubicin and its accumulation in cancer cells. Molecules 2015, 20, 15343–15358. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cho, S.K.; Kapoor, S.; Kumar, A.; Vali, S.; Abbasi, T.; Kim, S.H.; Sethi, G.; Ahn, K.S. Caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase. Mol. Carcinog. 2014, 53, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-Caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-R.; Nam, D.; Yun, H.-M.; Lee, S.-G.; Jang, H.-J.; Sethi, G.; Cho, S.K.; Ahn, K.S. Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Ryu, N.H.; Park, K.-R.; Kim, S.-M.; Yun, H.-M.; Nam, D.; Lee, S.-G.; Janq, H.J.; Ahn, K.S.; Kim, S.H.; Shim, B.S.; et al. A hexane fraction of guava leaves (Psidium guajava L.) induces anticancer activity by suppressing AKT/mammalian target of rapamycin/ribosomal p70 S6 kinase in human prostate cancer cells. J. Med. Food 2012, 15, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; Chen, J.Y.F.; Din, Z.H.; Su, J.H.; Yang, Z.Y.; Chen, Y.J.; Wang, R.Y.; Wu, Y.J. 13-acetoxysarcocrassolide induces apoptosis on human gastric carcinoma cells through mitochondria-related apoptotic pathways: P38/JNK activation and PI3K/AKT suppression. Mar. Drugs 2014, 12, 5295–5315. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Osthoff, K.; Bakker, A.C.; Vanhaesebroek, B.; Beyaert, R.; Jacob, W.A.; Fiers, W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions: Evidence for the involvement of mitochondrial radical generation. J. Biol. Chem. 1992, 267, 5317–5323. [Google Scholar] [PubMed]
- Gautam, N.; Mantha, A.K.; Mittal, S. Essential oils and their constituents as anticancer agents: A mechanistic view. BioMed Res. Int. 2014, 2014, 154106. [Google Scholar] [CrossRef] [PubMed]
- Pathania, A.S.; Guru, S.K.; Verma, M.K.; Sharma, C.; Abdullah, S.T.; Malik, F.; Chandra, S.; Katoch, M.; Bhushan, S. Disruption of the PI3K/AKT/mTOR signaling cascade and induction of apoptosis in HL-60 cells by an essential oil from Monarda citriodora. Food Chem. Toxicol. 2013, 62, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F.; Dodaro, D.; Passalacqua, N.G.; Statti, G.; Menichini, F. In vitro cytotoxic effects of Senecio stabianus Lacaita (Asteraceae) on human cancer cell lines. Nat. Prod. Res. 2009, 23, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singh, N.P.; Tice, R.R.; Stephens, R.E.; Scheneider, E.L. A microgel electrophoresis technique for the direct quantitation of DNA damage and repair in individual fibroblasts cultured on microscope slides. Mutat. Res. 1991, 252, 289–296. [Google Scholar] [CrossRef]
- Cardile, V.; Graziano, A.C.; Avola, R.; Piovano, M.; Russo, A. Potential anticancer activity of lichen secondary metabolite physodic acid. Chem. Biol. Interact. 2017, 263, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]






| Treatments | DU-145 % LDH Released |
|---|---|
| Vehicle DMSO (control) | 3.05 ± 0.7 |
| H2O2 | |
| 1 µM | 2.9 ± 0.7 |
| 1000 µM | 49 ± 0.4 * |
| Sa | |
| 12.5 µg/mL | 4.23 ± 0.8 |
| 25 µg/mL | 4.97 ± 0.6 |
| 50 µg/mL | 16.4 ± 0.9 * |
| Sj | |
| 12.5 µg/mL | 4.95 ± 0.8 |
| 25 µg/mL | 4.91 ± 0.6 |
| 50 µg/mL | 16.3 ± 0.6 * |
| Sv | |
| 12.5 µg/mL | 4.03 ± 0.4 |
| 25 µg/mL | 5.1 ± 0.7 |
| 50 µg/mL | 17.1 ± 0.8 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, A.; Cardile, V.; Graziano, A.C.E.; Avola, R.; Bruno, M.; Rigano, D. Involvement of Bax and Bcl-2 in Induction of Apoptosis by Essential Oils of Three Lebanese Salvia Species in Human Prostate Cancer Cells. Int. J. Mol. Sci. 2018, 19, 292. https://doi.org/10.3390/ijms19010292
Russo A, Cardile V, Graziano ACE, Avola R, Bruno M, Rigano D. Involvement of Bax and Bcl-2 in Induction of Apoptosis by Essential Oils of Three Lebanese Salvia Species in Human Prostate Cancer Cells. International Journal of Molecular Sciences. 2018; 19(1):292. https://doi.org/10.3390/ijms19010292
Chicago/Turabian StyleRusso, Alessandra, Venera Cardile, Adriana C. E. Graziano, Rosanna Avola, Maurizio Bruno, and Daniela Rigano. 2018. "Involvement of Bax and Bcl-2 in Induction of Apoptosis by Essential Oils of Three Lebanese Salvia Species in Human Prostate Cancer Cells" International Journal of Molecular Sciences 19, no. 1: 292. https://doi.org/10.3390/ijms19010292
APA StyleRusso, A., Cardile, V., Graziano, A. C. E., Avola, R., Bruno, M., & Rigano, D. (2018). Involvement of Bax and Bcl-2 in Induction of Apoptosis by Essential Oils of Three Lebanese Salvia Species in Human Prostate Cancer Cells. International Journal of Molecular Sciences, 19(1), 292. https://doi.org/10.3390/ijms19010292








