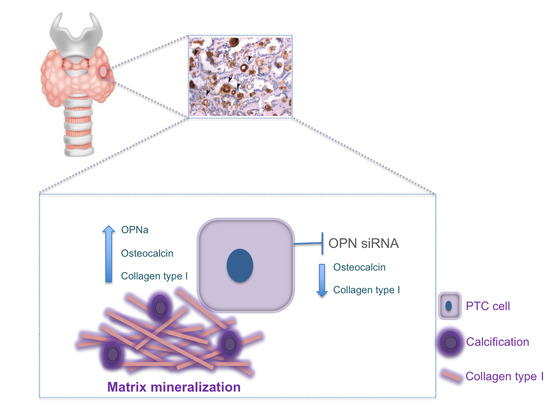
OPNa Overexpression Is Associated with Matrix Calcification in Thyroid Cancer Cell Lines
Abstract
:1. Introduction
2. Results
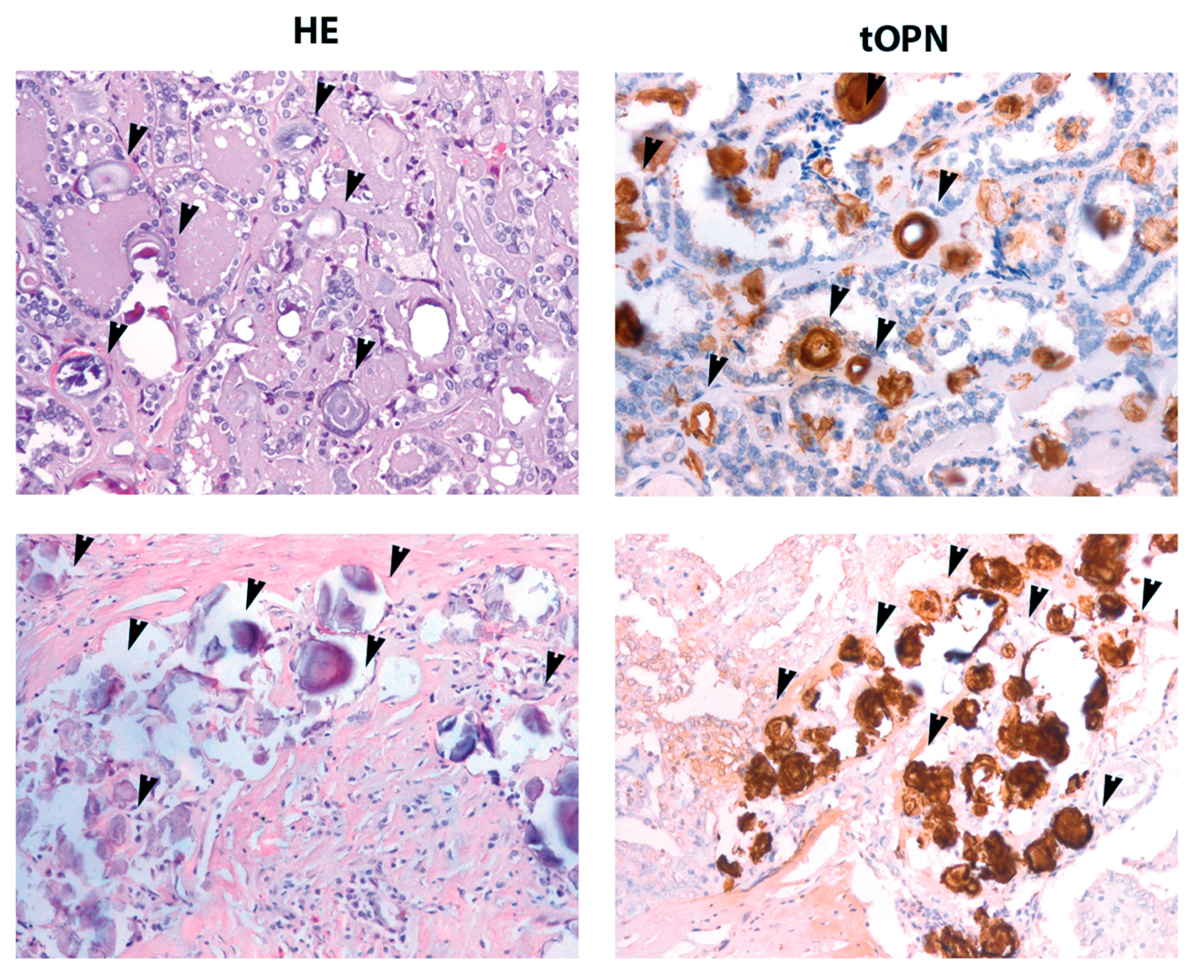
2.1. cPTC Samples Containing PB Exhibit Higher tOPN Staining Intensity
2.2. OPNa Transcript Overexpression Is Associated with Presence of PB in cPTC Samples
2.3. OPNa Overexpression Induces Matrix Calcification in c643 Thyroid Cells
2.4. OPNa Overexpression Promotes Collagen Synthesis in c643 Thyroid Cancer Cell Line
2.5. TPC-1 Thyroid Cancer Cell Line Produce Matrix Calcification and Collagen Synthesis
2.6. OPNa and Calcification Markers Are Upregulated in TPC-1 Cell Line during Culture Time Course
2.7. OPN Silencing Decreases Matrix Calcification and the Expression of Calcification Markers in TPC-1 Cell Line
3. Discussion
4. Materials and Methods
4.1. Tumor Specimens
4.2. Cell Culture
4.3. Immunohistochemistry
4.4. Matrix Calcification Assessment
4.5. Matrix Collagen Synthesis Assay
4.6. OPN Silencing
4.7. RNA Extraction and RT-qPCR
4.8. Immunoblot Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baloch, Z.W.; LiVolsi, V.A. Pathology of the Thyroid Gland; Livingston, C., Ed.; Endocrine Pathology: Philadelphia, PA, USA, 2002. [Google Scholar]
- Davies, L.; Welch, H.G. Epidemiology of head and neck cancer in the United States. Otolaryngol. Head Neck Surg. 2006, 135, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Sprague, B.L.; Warren Andersen, S.; Trentham-Dietz, A. Thyroid cancer incidence and socioeconomic indicators of health care access. Cancer Causes Control 2008, 19, 585–593. [Google Scholar] [CrossRef] [PubMed]
- De Biase, D.; Visani, M.; Pession, A.; Tallini, G. Molecular diagnosis of carcinomas of the thyroid gland. Front. Biosci. 2014, 6, 1–14. [Google Scholar]
- LiVolsi, V.A.A.-S.J.; Asa, S.L.; Baloch, Z.W.; Sobrinho-Simões, M.; Wenig, B.; DeLellis, R.A.; Cady, B.; Mazzaferri, E.L.; Hay, I.; Fagin, J.A.; et al. Pathology and Genetics of Tumours of Endocrine Organs (World Health Organization Classification of Tumours), 1st ed.; DeLellis, R.A., Lloyd, R.V., Heitz, P.U., Eng, C., Eds.; ARC Press: Lyon, France, 2004; pp. 57–66. [Google Scholar]
- Kakudo, K.; Tang, W.; Ito, Y.; Mori, I.; Nakamura, Y.; Miyauchi, A. Papillary carcinoma of the thyroid in Japan: Subclassification of common type and identification of low risk group. J. Clin. Pathol. 2004, 57, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Kakudo, K.; Li, Y.; Liu, Z.; Ozaki, T.; Ito, Y.; Kihara, M.; Miyauchi, A. Subclassification of non-solid-type papillary thyroid carcinoma identification of high-risk group in common type. Cancer Sci. 2008, 99, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Consorti, F.; Anello, A.; Benvenuti, C.; Boncompagni, A.; Giovannone, G.; Moles, N.; Scardella, L.; Antonaci, A. Clinical value of calcifications in thyroid carcinoma and multinodular goiter. Anticancer Res. 2003, 23, 3089–3092. [Google Scholar] [PubMed]
- Seiberling, K.A.; Dutra, J.C.; Grant, T.; Bajramovic, S. Role of intrathyroidal calcifications detected on ultrasound as a marker of malignancy. Laryngoscope 2004, 114, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Triggiani, V.; Guastamacchia, E.; Licchelli, B.; Tafaro, E. Microcalcifications and psammoma bodies in thyroid tumors. Thyroid 2008, 18, 1017–1018. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xu, Y.; Ge, C.; Guo, R.; Guo, K. Association of sonographically detected calcification with thyroid carcinoma. Head Neck 2006, 28, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K.; Haviv, I.; Campbell, I.G. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009, 25, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhou, G.; Nakamura, M.; Ozaki, T.; Mori, I.; Taniguchi, E.; Miyauchi, A.; Ito, Y.; Kakudo, K. Survival impact of psammoma body, stromal calcification, and bone formation in papillary thyroid carcinoma. Mod. Pathol. 2009, 22, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, J.V.; Sobrinho-Simoes, M. The origin and significance of thyroid psammoma bodies. Lab. Investig. 1980, 43, 287–296. [Google Scholar] [PubMed]
- Das, D.K.; Sheikh, Z.A.; George, S.S.; Al-Baquer, T.; Francis, I.M. Papillary thyroid carcinoma: Evidence for intracytoplasmic formation of precursor substance for calcification and its release from well-preserved neoplastic cells. Diagn. Cytopathol. 2008, 36, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K. Psammoma body: A product of dystrophic calcification or of a biologically active process that aims at limiting the growth and spread of tumor? Diagn. Cytopathol. 2009, 37, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, G.R.; Cartmell, S.H. Genes and Proteins Involved in the Regulation of Osteogenesis; Oulu University: Oulu, Finland, 2007; Volume 3. [Google Scholar]
- Lian, J.B.; Stein, G.S. Runx2/Cbfa1: A multifunctional regulator of bone formation. Curr. Pharm. Des. 2003, 9, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.; Karsenty, G.; Gundberg, C.; Vashishth, D. Osteocalcin and osteopontin influence bone morphology and mechanical properties. Ann. N. Y. Acad. Sci. 2017, 1409, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Butler, W.T. The nature and significance of osteopontin. Connect. Tissue Res. 1989, 23, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.; Blunck, C.B.; Emerenciano, M.; Gimba, E.R.P. Osteopontin and their roles in hematological malignancies: Splice variants on the new avenues. Cancer Lett. 2017, 408, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Tunio, G.M.; Hirota, S.; Nomura, S.; Kitamura, Y. Possible relation of osteopontin to development of psammoma bodies in human papillary thyroid cancer. Arch. Pathol. Lab. Med. 1998, 122, 1087–1090. [Google Scholar] [PubMed]
- Wu, G.; Guo, J.J.; Ma, Z.Y.; Wang, J.; Zhou, Z.W.; Wang, Y. Correlation between calcification and bone sialoprotein and osteopontin in papillary thyroid carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 2010–2017. [Google Scholar] [PubMed]
- Ferreira, L.B.; Tavares, C.; Pestana, A.; Pereira, C.L.; Eloy, C.; Pinto, M.T.; Castro, P.; Batista, R.; Rios, E.; Sobrinho-Simões, M.; et al. Osteopontin-a splice variant is overexpressed in papillary thyroid carcinoma and modulates invasive behavior. Oncotarget 2016, 7, 52003. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.B.; Eloy, C.; Pestana, A.; Lyra, J.; Moura, M.; Prazeres, H.; Tavares, C.; Sobrinho-Simoes, M.; Gimba, E.; Soares, P. Osteopontin expression is correlated with differentiation and good prognosis in medullary thyroid carcinoma. Eur. J. Endocrinol. 2016, 174, 551–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerda, M.N. Meningiomas: Morphologic and ultrastructural characteristics of psammoma bodies. Arch. Neurobiol. 1992, 55, 256–261. [Google Scholar]
- Hirota, S.; Nakajima, Y.; Yoshimine, T.; Kohri, K.; Nomura, S.; Taneda, M.; Hayakawa, T.; Kitamura, Y. Expression of bone-related protein messenger RNA in human meningiomas: Possible involvement of osteopontin in development of psammoma bodies. J. Neuropathol. Exp. Neurol. 1995, 54, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.S.; Kang, G.; Kim, D.H.; Park, C.; Kim, J.H.; Sohn, J.H. The prognostic relevance of psammoma bodies and ultrasonographic intratumoral calcifications in papillary thyroid carcinoma. World J. Surg. 2013, 37, 2330–2335. [Google Scholar] [CrossRef] [PubMed]
- Paloian, N.J.; Leaf, E.M.; Giachelli, C.M. Osteopontin protects against high phosphate-induced nephrocalcinosis and vascular calcification. Kidney Int. 2016, 89, 1027–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.J.; Zhang, J.; Cai, Y.; Zhou, Y.B.; Wen, G.B.; Tang, C.S.; Qi, Y.F.; Jiang, Z.S. C-type natriuretic peptide inhibiting vascular calcification might involve decreasing bone morphogenic protein 2 and osteopontin levels. Mol. Cell. Biochem. 2014, 392, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.L.; Foster, B.L.; Silverio, K.G.; Martins, L.; Casati, M.Z.; Sallum, E.A.; Somerman, M.J.; Nociti, F.H., Jr. Hypophosphatasia-associated deficiencies in mineralization and gene expression in cultured dental pulp cells obtained from human teeth. J. Endod. 2012, 38, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, Y.; Yoshida, K.; Ohba, H.; Seto, H.; Kido, J.; Haneji, T.; Nagata, T. High glucose levels increase osteopontin production and pathologic calcification in rat dental pulp tissues. J. Endod. 2010, 36, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Huang, G.; Pan, J.; Li, J.; Zhang, X.; Fang, L.; Liu, B.; Meng, W.; Zhang, Y.; Liu, X. Involvement of osteopontin as a core protein in craniopharyngioma calcification formation. J. Neurooncol. 2010, 98, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Prieto, R.M.; Sanchis, P.; Saus, C.; De Francisco, T. Role of phytate and osteopontin in the mechanism of soft tissue calcification. J. Nephrol. 2008, 21, 768–775. [Google Scholar] [PubMed]
- Hayashi, N.; Tamaki, N.; Yamamoto, K.; Senda, M.; Yonekura, Y.; Misaki, T.; Iida, Y.; Kasagi, K.; Endo, K.; Konishi, J.; et al. Real-time ultrasonography of thyroid nodules. Acta Radiol. Diagn. 1986, 27, 403–408. [Google Scholar] [CrossRef]
- Watters, D.A.; Ahuja, A.T.; Evans, R.M.; Chick, W.; King, W.W.; Metreweli, C.; Li, A.K. Role of ultrasound in the management of thyroid nodules. Am. J. Surg. 1992, 164, 654–657. [Google Scholar] [CrossRef]
- Khoo, M.L.; Asa, S.L.; Witterick, I.J.; Freeman, J.L. Thyroid calcification and its association with thyroid carcinoma. Head Neck 2002, 24, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Komolafe, F. Radiological patterns and significance of thyroid calcification. Clin. Radiol. 1981, 32, 571–575. [Google Scholar] [CrossRef]
- Katz, J.F.; Kane, R.A.; Reyes, J.; Clarke, M.P.; Hill, T.C. Thyroid nodules: Sonographic-pathologic correlation. Radiology 1984, 151, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Saiselet, M.; Floor, S.; Tarabichi, M.; Dom, G.; Hebrant, A.; van Staveren, W.C.; Maenhaut, C. Thyroid cancer cell lines: An overview. Front. Endocrinol. 2012, 3, 133. [Google Scholar] [CrossRef] [PubMed]
- Castellone, M.D.; Celetti, A.; Guarino, V.; Cirafici, A.M.; Basolo, F.; Giannini, R.; Medico, E.; Kruhoffer, M.; Orntoft, T.F.; Curcio, F.; et al. Autocrine stimulation by osteopontin plays a pivotal role in the expression of the mitogenic and invasive phenotype of RET/PTC-transformed thyroid cells. Oncogene 2004, 23, 2188–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Levenson, A.S.; Satcher, R.L., Jr. Identification of a unique set of genes altered during cell-cell contact in an in vitro model of prostate cancer bone metastasis. Int. J. Mol. Med. 2006, 17, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Gericke, A.; Qin, C.; Spevak, L.; Fujimoto, Y.; Butler, W.T.; Sorensen, E.S.; Boskey, A.L. Importance of phosphorylation for osteopontin regulation of biomineralization. Calcif. Tissue Int. 2005, 77, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guan, X.; Tang, R.; Hoyer, J.R.; Wierzbicki, A.; De Yoreo, J.J.; Nancollas, G.H. Phosphorylation of osteopontin is required for inhibition of calcium oxalate crystallization. J. Phys. Chem. B 2008, 112, 9151–9157. [Google Scholar] [CrossRef] [PubMed]
- Gimba, E.R.; Tilli, T.M. Human osteopontin splicing isoforms: Known roles, potential clinical applications and activated signaling pathways. Cancer Lett. 2013, 331, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Reinholt, F.P.; Hultenby, K.; Oldberg, A.; Heinegard, D. Osteopontin--a possible anchor of osteoclasts to bone. Proc. Natl. Acad. Sci. USA 1990, 87, 4473–4475. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; House, C.M.; Udagawa, N.; Kazama, J.J.; McFarland, R.J.; Martin, T.J.; Findlay, D.M. Casein kinase 2 phosphorylation of recombinant rat osteopontin enhances adhesion of osteoclasts but not osteoblasts. J. Cell. Physiol. 1998, 176, 179–187. [Google Scholar] [CrossRef]
- Nagata, T.; Todescan, R.; Goldberg, H.A.; Zhang, Q.; Sodek, J. Sulphation of secreted phosphoprotein I (SPPI, osteopontin) is associated with mineralized tissue formation. Biochem. Biophys. Res. Commun. 1989, 165, 234–240. [Google Scholar] [CrossRef]
- Sodek, J.; Chen, J.; Nagata, T.; Kasugai, S.; Todescan, R., Jr.; Li, I.W.; Kim, R.H. Regulation of osteopontin expression in osteoblasts. Ann. N. Y. Acad. Sci. 1995, 760, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Rosai, J.; DeLellis, R.A.; Carcangiu, M.L.; Frable, W.J.; Tallini, G. Tumors of the Thyroid & Parathyroid Glands, 4th ed.; Pathology, A.R.O., Ed.; American Registry of Pathology: Rockville, MD, USA, 2015. [Google Scholar]
- He, B.; Mirza, M.; Weber, G.F. An osteopontin splice variant induces anchorage independence in human breast cancer cells. Oncogene 2006, 25, 2192–2202. [Google Scholar] [CrossRef] [PubMed]



| Variable | cPTC n (%) |
|---|---|
| Sex * | |
| Male | 6 (15) |
| Female | 41 (85) |
| Age (yr) (mean ± S.D.) * | 39.87 (±16.42) |
| <45 | 26 (56.5) |
| ≥45 | 20 (43.4) |
| Stroma | |
| Absent | 21 (44) |
| Present | 27 (56) |
| Tumor size (cm) * | |
| (mean ± S.D.) | 2.4 (±1.1) |
| <2 | 17 (37) |
| ≥2 | 29 (63) |
| Capsule* | |
| Absent | 24 (56) |
| Present | 19 (44) |
| Psammoma Bodies * | |
| Absent | 21 (47) |
| Present | 24 (53) |
| Extrathyroid Extension * | |
| Absent | 24 (57) |
| Present | 18 (43) |
| Invasion Lympho (vascular and/or capsular) * | |
| Absent | 15 (35) |
| Present | 28 (65) |
| Lymph Node Metastases * | |
| Absent | 21 (60) |
| Present | 14 (40) |
| RET/PTC1 translocation * | |
| Absent | 37 (84) |
| Present | 7 (16) |
| BRAFV600E mutation * | |
| Absent | 22 (48) |
| Present | 24 (52) |
| RAS mutation * | |
| Absent | 44 (96) |
| Present | 2 (4) |
| Psammoma Bodies | |||
|---|---|---|---|
| Variable | Absent (n = 19) | Present (n = 26) | p-Value |
| tOPN mRNA expression (Median) | 0.15 | 0.18 | 0.73 |
| OPNa mRNA expression (Median) | 0.12 | 0.14 | 0.01 |
| OPNb mRNA expression (Median) | 0.10 | 0.11 | 0.04 |
| OPNc mRNA expression (Median) | 0.05 | 0.05 | 0.07 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, L.B.; Lima, R.T.; Bastos, A.C.S.d.F.; Silva, A.M.; Tavares, C.; Pestana, A.; Rios, E.; Eloy, C.; Sobrinho-Simões, M.; Gimba, E.R.P.; et al. OPNa Overexpression Is Associated with Matrix Calcification in Thyroid Cancer Cell Lines. Int. J. Mol. Sci. 2018, 19, 2990. https://doi.org/10.3390/ijms19102990
Ferreira LB, Lima RT, Bastos ACSdF, Silva AM, Tavares C, Pestana A, Rios E, Eloy C, Sobrinho-Simões M, Gimba ERP, et al. OPNa Overexpression Is Associated with Matrix Calcification in Thyroid Cancer Cell Lines. International Journal of Molecular Sciences. 2018; 19(10):2990. https://doi.org/10.3390/ijms19102990
Chicago/Turabian StyleFerreira, Luciana B., Raquel T. Lima, Ana Clara Santos da Fonseca Bastos, Andreia M. Silva, Catarina Tavares, Ana Pestana, Elisabete Rios, Catarina Eloy, Manuel Sobrinho-Simões, Etel R. P. Gimba, and et al. 2018. "OPNa Overexpression Is Associated with Matrix Calcification in Thyroid Cancer Cell Lines" International Journal of Molecular Sciences 19, no. 10: 2990. https://doi.org/10.3390/ijms19102990
APA StyleFerreira, L. B., Lima, R. T., Bastos, A. C. S. d. F., Silva, A. M., Tavares, C., Pestana, A., Rios, E., Eloy, C., Sobrinho-Simões, M., Gimba, E. R. P., & Soares, P. (2018). OPNa Overexpression Is Associated with Matrix Calcification in Thyroid Cancer Cell Lines. International Journal of Molecular Sciences, 19(10), 2990. https://doi.org/10.3390/ijms19102990