Induction of ATP Release, PPIX Transport, and Cholesterol Uptake by Human Red Blood Cells Using a New Family of TSPO Ligands
Abstract
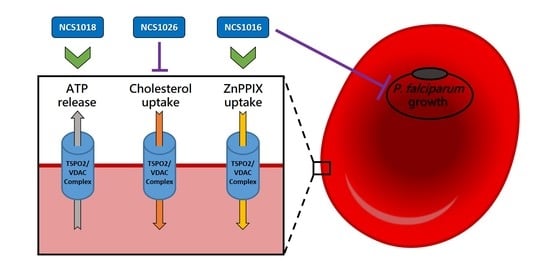
1. Introduction
2. Results
2.1. Binding-Affinity of the Translocator Protein (TSPO) Ligands
2.2. P. falciparum Growth Inhibition
2.3. ZnPPIX Uptake and Reactive Oxygen Species (ROS) Accumulation
2.4. Sorbitol Hemolysis
2.5. Cholesterol Uptake
2.6. ATP Release
3. Discussion
4. Materials and Methods
4.1. Red Blood Cell Samples
4.2. Chemicals
4.3. Radioligand Binding Assays
4.4. ATPe Measurements
4.5. Measurement of Hemolysis and Estimation of Lytic ATPe
4.6. P. falciparum Culture with TSPO Ligands
4.7. Sorbitol Hemolysis Assays
4.8. ZnPPIX Uptake
4.9. ROS Accumulation
4.10. Cholesterol Uptake
4.11. Fluorescence Measurements by Flow Cytometry
4.12. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANT | Adenine nucleotide transporter |
| ATAD3 | ATPase family AAA Domain-containing protein 3 |
| ATP | Adenosine Tri Phosphate |
| ATPe | Extracellular ATP |
| CBX | Carbonexolone |
| DCFDA | 2′,7′-dichlorofluorescein diacetate |
| MFI | Mean fluorescence intensity |
| NPP | New permeability pathway |
| PBC | Probenecid |
| PBR | Peripheral benzodiazepine receptor |
| PBS | Phosphate buffer saline |
| RBC | Red blood cell |
| ROS | Reactive oxygen species |
| RT | Room temperature |
| TSPO | Translocator protein |
| VDAC | Voltage dependent anion channel |
| ZnPPIX | Zinc protoporphyrin |
References
- Braestrup, C.; Albrechtsen, R.; Squires, R.F. High densities of benzodiazepine receptors in human cortical areas. Nature 1977, 269, 702–704. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapere, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharm. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Lindemann, P.; Feuilloley, M.G.; Papadopoulos, V. Structural and functional evolution of the translocator protein (18 kDa). Curr. Mol. Med. 2012, 12, 369–386. [Google Scholar] [PubMed]
- Fan, J.; Rone, M.B.; Papadopoulos, V. Translocator protein 2 is involved in cholesterol redistribution during erythropoiesis. J. Boil. Chem. 2009, 284, 30484–30497. [Google Scholar] [CrossRef] [PubMed]
- Marginedas-Freixa, I.; Hattab, C.; Bouyer, G.; Halle, F.; Chene, A.; Lefevre, S.D.; Cambot, M.; Cueff, A.; Schmitt, M.; Gamain, B.; et al. TSPO ligands stimulate ZnPPIX transport and ROS accumulation leading to the inhibition of P. falciparum growth in human blood. Sci. Rep. 2016, 6, 33516. [Google Scholar] [CrossRef] [PubMed]
- McEnery, M.W.; Snowman, A.M.; Trifiletti, R.R.; Snyder, S.H. Isolation of the mitochondrial benzodiazepine receptor: Association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc. Natl. Acad. Sci. USA 1992, 89, 3170–3174. [Google Scholar] [CrossRef] [PubMed]
- Rone, M.B.; Midzak, A.S.; Issop, L.; Rammouz, G.; Jagannathan, S.; Fan, J.; Ye, X.; Blonder, J.; Veenstra, T.; Papadopoulos, V. Identification of a dynamic mitochondrial protein complex driving cholesterol import, trafficking, and metabolism to steroid hormones. Mol. Endocrinol. 2012, 26, 1868–1882. [Google Scholar] [CrossRef] [PubMed]
- Delavoie, F.; Li, H.; Hardwick, M.; Robert, J.C.; Giatzakis, C.; Peranzi, G.; Yao, Z.X.; Maccario, J.; Lacapere, J.J.; Papadopoulos, V. In vivo and in vitro peripheral-type benzodiazepine receptor polymerization: Functional significance in drug ligand and cholesterol binding. Biochemistry 2003, 42, 4506–4519. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Papadopoulos, V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology 1998, 139, 4991–4997. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yao, Z.; Degenhardt, B.; Teper, G.; Papadopoulos, V. Cholesterol binding at the cholesterol recognition/ interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an HIV TAT-CRAC peptide. Proc. Natl. Acad. Sci. USA 2001, 98, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Taketani, S.; Kohno, H.; Furukawa, T.; Tokunaga, R. Involvement of peripheral-type benzodiazepine receptors in the intracellular transport of heme and porphyrins. J. Biochem. 1995, 117, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Wendler, G.; Lindemann, P.; Lacapere, J.J.; Papadopoulos, V. Protoporphyrin IX binding and transport by recombinant mouse PBR. Biochem. Biophys. Res. Commun. 2003, 311, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Campioli, E.; Midzak, A.; Culty, M.; Papadopoulos, V. Conditional steroidogenic cell-targeted deletion of TSPO unveils a crucial role in viability and hormone-dependent steroid formation. Proc. Natl. Acad. Sci. USA 2015, 112. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zirkin, B.; Papadopoulos, V. Response to Letter to the Editor: “Dubious Conclusions on TSPO Function”. Endocrinology 2018, 159, 2530–2531. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Stocco, D.M. Letter to the Editor: Dubious Conclusions on TSPO Function. Endocrinology 2018, 159, 2528–2529. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Stocco, D.M. The changing landscape in translocator protein (TSPO) function. Trends Endocrinol. Metab. 2015, 26, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Hatty, C.R.; Banati, R.B. Protein-ligand and membrane-ligand interactions in pharmacology: The case of the translocator protein (TSPO). Pharmacol. Res. 2015, 100, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Iacobazzi, R.M.; Lopalco, A.; Cutrignelli, A.; Laquintana, V.; Lopedota, A.; Franco, M.; Denora, N. Bridging Pharmaceutical Chemistry with Drug and Nanoparticle Targeting to Investigate the Role of the 18-kDa Translocator Protein TSPO. ChemMedChem 2017, 12, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Veenman, L.; Vainshtein, A.; Yasin, N.; Azrad, M.; Gavish, M. Tetrapyrroles as Endogenous TSPO Ligands in Eukaryotes and Prokaryotes: Comparisons with Synthetic Ligands. Int. J. Mol. Sci. 2016, 17, 880. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Lee, J.; Lee, S.Y. Recent Progress in the Development of TSPO PET Ligands for Neuroinflammation Imaging in Neurological Diseases. Nucl. Med. Mol. Imaging 2017, 51, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Le Fur, G.; Vaucher, N.; Perrier, M.L.; Flamier, A.; Benavides, J.; Renault, C.; Dubroeucq, M.C.; Gueremy, C.; Uzan, A. Differentiation between two ligands for peripheral benzodiazepine binding sites, [3H]RO5-4864 and [3H]PK 11195, by thermodynamic studies. Life Sci. 1983, 33, 449–457. [Google Scholar] [CrossRef]
- Jamin, N.; Neumann, J.M.; Ostuni, M.A.; Vu, T.K.; Yao, Z.X.; Murail, S.; Robert, J.C.; Giatzakis, C.; Papadopoulos, V.; Lacapere, J.J. Characterization of the cholesterol recognition amino acid consensus sequence of the peripheral-type benzodiazepine receptor. Mol. Endocrinol. 2005, 19, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Jaremko, L.; Jaremko, M.; Giller, K.; Becker, S.; Zweckstetter, M. Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science 2014, 343, 1363–1366. [Google Scholar] [CrossRef] [PubMed]
- Garnier, M.; Dimchev, A.B.; Boujrad, N.; Price, J.M.; Musto, N.A.; Papadopoulos, V. In vitro reconstitution of a functional peripheral-type benzodiazepine receptor from mouse Leydig tumor cells. Mol. Pharm. 1994, 45, 201–211. [Google Scholar]
- Ostuni, M.A.; Peranzi, G.; Ducroc, R.A.; Fasseu, M.; Vidic, B.; Dumont, J.; Papadopoulos, V.; Lacapere, J.J. Distribution, pharmacological characterization and function of the 18 kDa translocator protein in rat small intestine. Boil. Cell 2009, 101, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, F.; Alev, C.; Shin, M.; Nakaya, Y.; Jakt, L.M.; Sheng, G. PBRL, a putative peripheral benzodiazepine receptor, in primitive erythropoiesis. Gene Expr. Patterns 2009, 9, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Marginedas-Freixa, I.; Alvarez, C.L.; Moras, M.; Leal Denis, M.F.; Hattab, C.; Halle, F.; Bihel, F.; Mouro-Chanteloup, I.; Lefevre, S.D.; Le Van Kim, C.; et al. Human erythrocytes release ATP by a novel pathway involving VDAC oligomerization independent of pannexin-1. Sci. Rep. 2018, 8, 11384. [Google Scholar] [CrossRef] [PubMed]
- Hallé, F.; Lejri, I.; Abarghaz, M.; Grimm, A.; Klein, C.; Maitre, M.; Schmitt, M.; Bourguignon, J.J.; Mensah-Nyagan, A.G.; Eckert, A. Discovery of Imidazoquinazolinone Derivatives as TSPO Ligands Modulating Neurosteroidogenesis and Cellular Bioenergetics in Neuroblastoma Cells Expressing Amyloid Precursor Protein. ChemistrySelect 2017, 2, 6452–6457. [Google Scholar] [CrossRef]
- Bouyer, G.; Cueff, A.; Egee, S.; Kmiecik, J.; Maksimova, Y.; Glogowska, E.; Gallagher, P.G.; Thomas, S.L. Erythrocyte peripheral type benzodiazepine receptor/voltage-dependent anion channels are upregulated by Plasmodium falciparum. Blood 2011, 118, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Tanneur, V.; Duranton, C.; Brand, V.B.; Sandu, C.D.; Akkaya, C.; Kasinathan, R.S.; Gachet, C.; Sluyter, R.; Barden, J.A.; Wiley, J.S.; et al. Purinoceptors are involved in the induction of an osmolyte permeability in malaria-infected and oxidized human erythrocytes. FASEB J. 2006, 20, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Sprague, R.S.; Stephenson, A.H.; Ellsworth, M.L. Red not dead: Signaling in and from erythrocytes. Trends Endocrinol. Metab. 2007, 18, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, M.; Bowles, E.A.; Richards, J.P.; Krantic, M.; Davis, K.L.; Dietrich, K.A.; Stephenson, A.H.; Ellsworth, M.L.; Sprague, R.S. Prostacyclin receptor-mediated ATP release from erythrocytes requires the voltage-dependent anion channel. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H553–H559. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, M.L.; Ellis, C.G.; Goldman, D.; Stephenson, A.H.; Dietrich, H.H.; Sprague, R.S. Erythrocytes: Oxygen sensors and modulators of vascular tone. Physiology 2009, 24, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Leal Denis, M.F.; Alvarez, H.A.; Lauri, N.; Alvarez, C.L.; Chara, O.; Schwarzbaum, P.J. Dynamic Regulation of Cell Volume and Extracellular ATP of Human Erythrocytes. PLoS ONE 2016, 11, e0158305. [Google Scholar] [CrossRef] [PubMed]
- Montalbetti, N.; Leal Denis, M.F.; Pignataro, O.P.; Kobatake, E.; Lazarowski, E.R.; Schwarzbaum, P.J. Homeostasis of extracellular ATP in human erythrocytes. J. Biol. Chem. 2011, 286, 38397–38407. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Orlov, S.N.; Furuya, K.; Grygorczyk, R. Hemolysis is a primary ATP-release mechanism in human erythrocytes. Blood 2014, 124, 2150–2157. [Google Scholar] [CrossRef] [PubMed]
- Locovei, S.; Bao, L.; Dahl, G. Pannexin 1 in erythrocytes: Function without a gap. Proc. Natl. Acad. Sci. USA 2006, 103, 7655–7659. [Google Scholar] [CrossRef] [PubMed]
- Lacapere, J.J.; Delavoie, F.; Li, H.; Peranzi, G.; Maccario, J.; Papadopoulos, V.; Vidic, B. Structural and functional study of reconstituted peripheral benzodiazepine receptor. Biochem. Biophys. Res. Commun. 2001, 284, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Dosch, M.; Gerber, J.; Jebbawi, F.; Beldi, G. Mechanisms of ATP Release by Inflammatory Cells. Int. J. Mol. Sci. 2018, 19, 1222. [Google Scholar] [CrossRef] [PubMed]
- Taruno, A. ATP Release Channels. Int. J. Mol. Sci. 2018, 19, 808. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.L.; Schachter, J.; de Sa Pinheiro, A.A.; Silva Lde, S.; Verstraeten, S.V.; Persechini, P.M.; Schwarzbaum, P.J. Regulation of extracellular ATP in human erythrocytes infected with Plasmodium falciparum. PLoS ONE 2014, 9, e96216. [Google Scholar] [CrossRef] [PubMed]
- Canat, X.; Carayon, P.; Bouaboula, M.; Cahard, D.; Shire, D.; Roque, C.; Le Fur, G.; Casellas, P. Distribution profile and properties of peripheral-type benzodiazepine receptors on human hemopoietic cells. Life Sci. 1993, 52, 107–118. [Google Scholar] [CrossRef]
- Olson, J.M.; Ciliax, B.J.; Mancini, W.R.; Young, A.B. Presence of peripheral-type benzodiazepine binding sites on human erythrocyte membranes. Eur J. Pharm. 1988, 152, 47–53. [Google Scholar] [CrossRef]
- Ostuni, M.A.; Ducroc, R.; Peranzi, G.; Tonon, M.C.; Papadopoulos, V.; Lacapere, J.J. Translocator protein (18 kDa) ligand PK 11195 induces transient mitochondrial Ca2+ release leading to transepithelial Cl- secretion in HT-29 human colon cancer cells. Biol. Cell 2007, 99, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kalathur, R.C.; Liu, Q.; Kloss, B.; Bruni, R.; Ginter, C.; Kloppmann, E.; Rost, B.; Hendrickson, W.A. Protein structure. Structure and activity of tryptophan-rich TSPO proteins. Science 2015, 347, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Cleary, J.; Johnson, K.M.; Opipari, A.W., Jr.; Glick, G.D. Inhibition of the mitochondrial F1F0-ATPase by ligands of the peripheral benzodiazepine receptor. Bioorg. Med. Chem. Lett. 2007, 17, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- Hatty, C.R.; Le Brun, A.P.; Lake, V.; Clifton, L.A.; Liu, G.J.; James, M.; Banati, R.B. Investigating the interactions of the 18kDa translocator protein and its ligand PK11195 in planar lipid bilayers. Biochim. Biophys. Acta 2014, 1838, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Ferzaz, B.; Benavides, J.; Marguet, F.; Froissant, J.; Marabout, B.; Evanno, Y.; Sevrin, M.; Janiak, P. Use of pyridazino[4,5-b]indole-1-acetamide Derivatives for Preparing Medicines for Treating Diseases Related to the Dysfunction of Peripheral Benzodiazepin Receptors. US Patent No. 6395729, 28 May 2002. [Google Scholar]
- Leal Denis, M.F.; Incicco, J.J.; Espelt, M.V.; Verstraeten, S.V.; Pignataro, O.P.; Lazarowski, E.R.; Schwarzbaum, P.J. Kinetics of extracellular ATP in mastoparan 7-activated human erythrocytes. Biochim. Biophys. Acta 2013, 1830, 4692–4707. [Google Scholar] [CrossRef] [PubMed]







| Ligand | Ro5-4864 | SSR-180,575 | NCS1016 | NCS1018 | NCS1026 |
|---|---|---|---|---|---|
| IC50 (µM) | 1.5 ± 0.6 | 1.7 ± 0.5 | 0.45 ± 0.05 | 0.59 ± 0.07 | 0.28 ± 0.06 |
| Ligand | Vehicle | PK 11195 | Ro5-4864 | SSR-180,575 | NCS1016 | NCS1018 | NCS1026 |
|---|---|---|---|---|---|---|---|
| % | 0.64 ± 0.02 | 0.60 ± 0.06 | 0.68 ± 0.16 | 0.70 ± 0.08 | 0.65 ± 0.09 | 0.72 ± 0.06 | 0.52 ± 0.06 |
| Ligand | 10 µM | 50 µM |
|---|---|---|
| Vehicle | 5.24 ± 0.34 | 4.04 ± 0.22 |
| PK 11195 | 7.82 ± 0.72 * | 11.88 ± 0.68 * |
| Ro5-4864 | 9.76 ± 0.84 * | 29.49 ± 0.45 ** |
| SSR-180,575 | 9.16 ± 0.64 * | 12.59 ± 0.62 ** |
| NCS1016 | 11.67 ± 0.90 * | 30.23 ± 1.71 ** |
| NCS1018 | 17.87 ± 2.57 ** | 37.15 ± 3.42 ** |
| NCS1026 | 11.67 ± 0.90 * | 10.74 ± 0.30 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marginedas-Freixa, I.; Alvarez, C.L.; Moras, M.; Hattab, C.; Bouyer, G.; Chene, A.; Lefevre, S.D.; Le Van Kim, C.; Bihel, F.; Schwarzbaum, P.J.; et al. Induction of ATP Release, PPIX Transport, and Cholesterol Uptake by Human Red Blood Cells Using a New Family of TSPO Ligands. Int. J. Mol. Sci. 2018, 19, 3098. https://doi.org/10.3390/ijms19103098
Marginedas-Freixa I, Alvarez CL, Moras M, Hattab C, Bouyer G, Chene A, Lefevre SD, Le Van Kim C, Bihel F, Schwarzbaum PJ, et al. Induction of ATP Release, PPIX Transport, and Cholesterol Uptake by Human Red Blood Cells Using a New Family of TSPO Ligands. International Journal of Molecular Sciences. 2018; 19(10):3098. https://doi.org/10.3390/ijms19103098
Chicago/Turabian StyleMarginedas-Freixa, Irene, Cora L. Alvarez, Martina Moras, Claude Hattab, Guillaume Bouyer, Arnaud Chene, Sophie D. Lefevre, Caroline Le Van Kim, Frederic Bihel, Pablo J. Schwarzbaum, and et al. 2018. "Induction of ATP Release, PPIX Transport, and Cholesterol Uptake by Human Red Blood Cells Using a New Family of TSPO Ligands" International Journal of Molecular Sciences 19, no. 10: 3098. https://doi.org/10.3390/ijms19103098
APA StyleMarginedas-Freixa, I., Alvarez, C. L., Moras, M., Hattab, C., Bouyer, G., Chene, A., Lefevre, S. D., Le Van Kim, C., Bihel, F., Schwarzbaum, P. J., & Ostuni, M. A. (2018). Induction of ATP Release, PPIX Transport, and Cholesterol Uptake by Human Red Blood Cells Using a New Family of TSPO Ligands. International Journal of Molecular Sciences, 19(10), 3098. https://doi.org/10.3390/ijms19103098