Screening of Additive Manufactured Scaffolds Designs for Triple Negative Breast Cancer 3D Cell Culture and Stem-Like Expansion
Abstract
1. Introduction
2. Results
2.1. PLA Scaffolds Production and Characterization
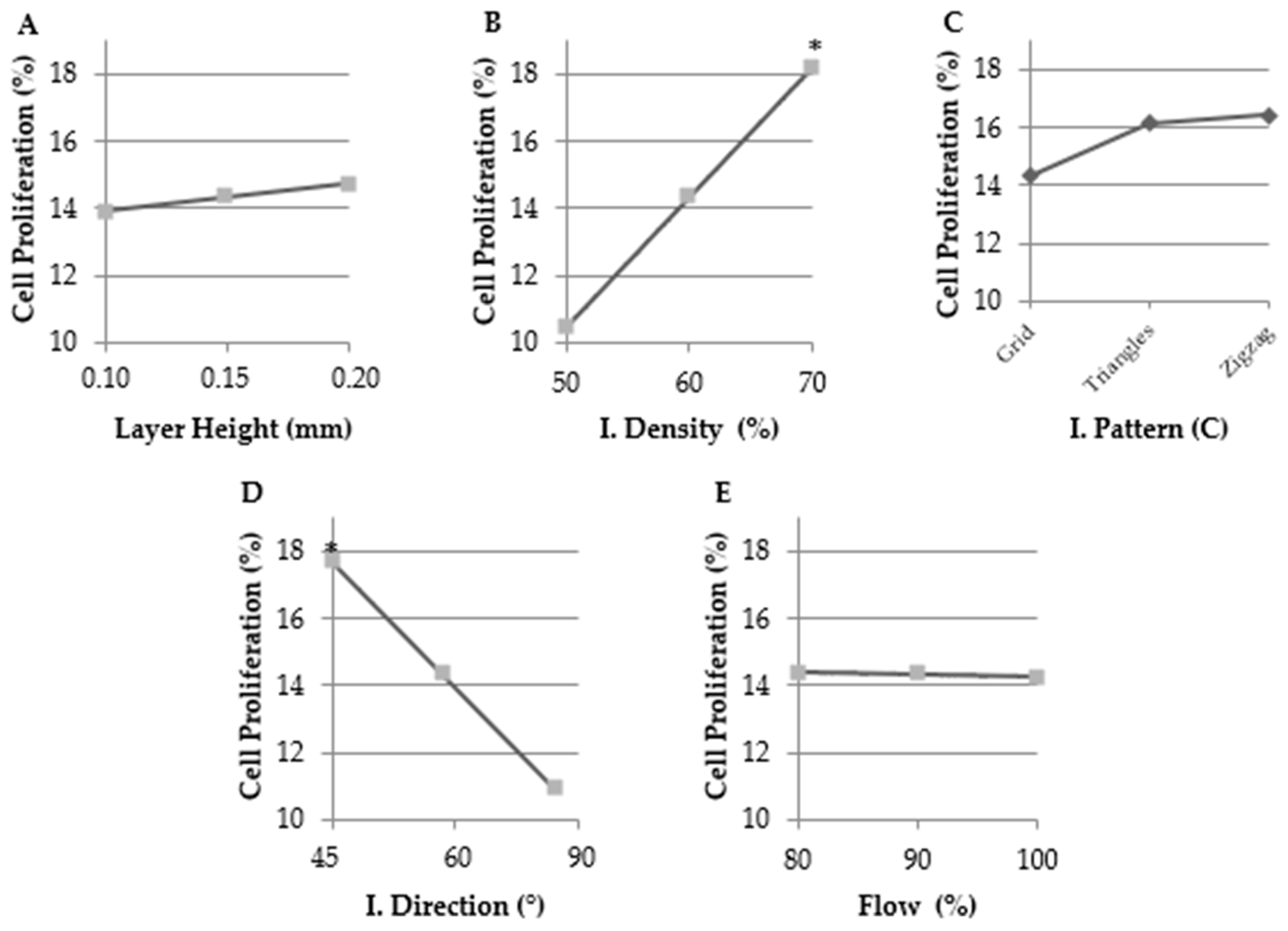
2.2. Cell Proliferation Assay
2.2.1. Selecting the Optimal Values for Each Parameter Tested
2.2.2. Selected Value Verification and Final Selection of the Optimal Designs
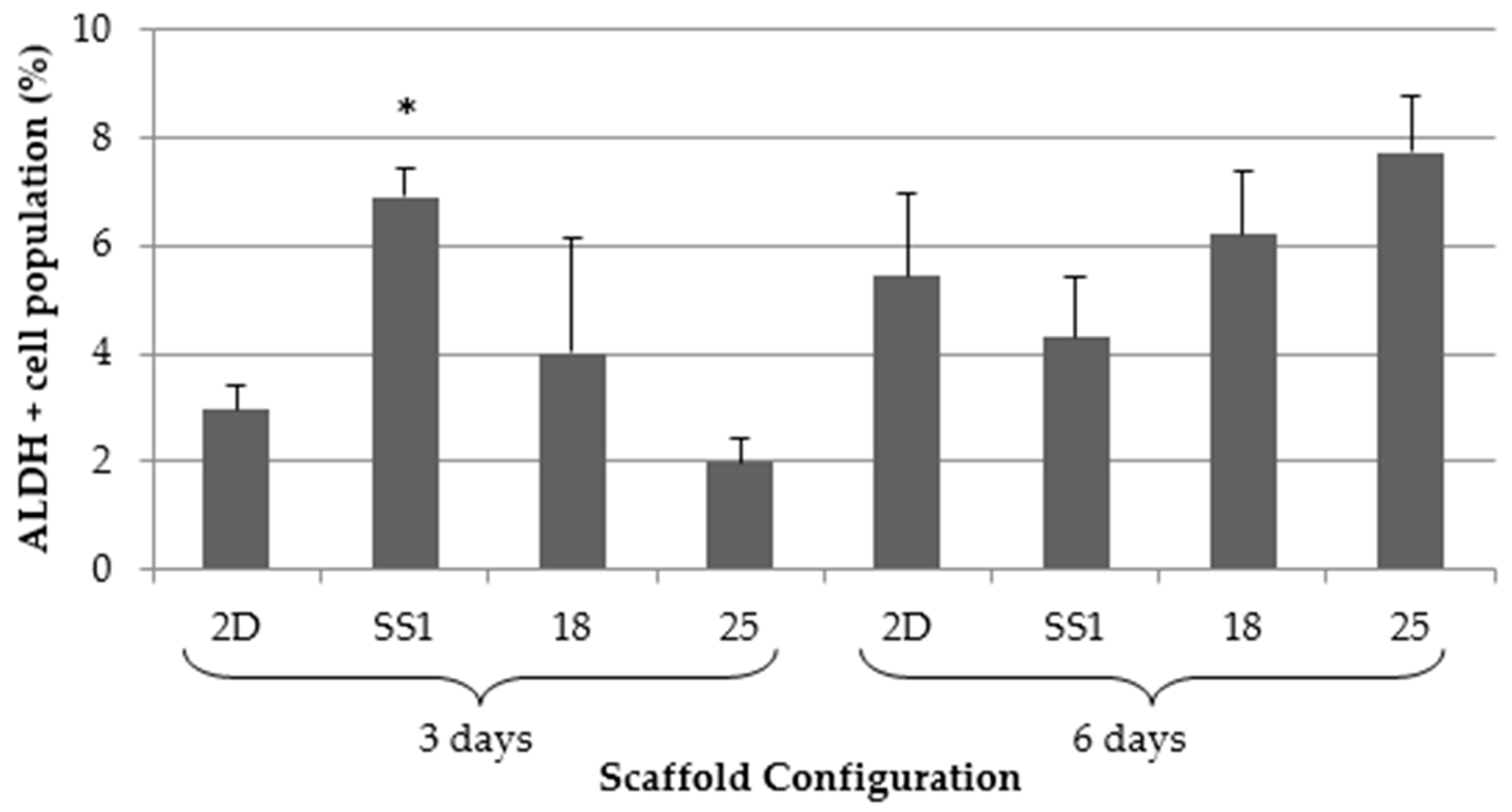
2.3. Aldehyde Dehydrogenase Activity
3. Discussion
4. Materials and Methods
4.1. Scaffolds Design and Manufacture Process
4.2. Design of Experiment
4.3. Material
4.4. Cell Line
4.5. Three-Dimensional Cell Culture
4.6. Scaffold Dimensional Characterization
4.7. Cell Proliferation Assay
4.8. Aldefluor Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| ALDH | Aldehyde Dehydrogenase |
| AM | Additive Manufacturing |
| BCSC | Breast cancer stem cell |
| CAD | Computer-aided design |
| CAM | Computer-aided manufacturing |
| CSC | Cancer stem cell |
| DMEM | Dulbecco’s modified eagle’s medium |
| ECM | Extracellular matrix |
| FBS | Fetal bovine serum |
| FFF | Fused filament fabrication |
| MTT | 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide |
| PBS | Phosphate-buffered saline |
| PCL | Poly(ε-caprolactone) |
| PLA | Poly (lactic acid) |
| SS | Selected scaffold |
| STL | Stereolithography |
| TNBC | Triple negative breast cancer |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.R.; Brown, M.; Cress, R.D.; Parise, C.A.; Caggiano, V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype. Cancer 2007, 109, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M. Molecular Stratification of Triple-Negative Breast Cancers. Oncologist 2011, 16 (Suppl. 1), 61–70. [Google Scholar] [CrossRef]
- Matsui, W.; Wang, Q.; Barber, J.P.; Brennan, S.; Smith, B.D.; Borrello, I.; McNiece, I.; Lin, L.; Ambinder, R.F.; Peacock, C.; et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008, 68, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Iinuma, H.; Aoyagi, Y.; Shibuya, H.; Watanabe, T. Predictive value of cancer stem-like cells and cancer-associated genetic markers for peritoneal recurrence of colorectal cancer in patients after curative surgery. Oncology 2010, 78, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Kyjacova, L.; Hubackova, S.; Krejcikova, K.; Strauss, R.; Hanzlikova, H.; Dzijak, R.; Imrichova, T.; Simova, J.; Reinis, M.; Bartek, J.; et al. Radiotherapy-induced plasticity of prostate cancer mobilizes stem-like non-adherent, Erk signaling-dependent cells. Cell Death Differ. 2015, 22, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar] [PubMed]
- Dontu, G.; Abdallah, W.M.; Foley, J.M.; Jackson, K.W.; Clarke, M.F.; Kawamura, M.J.; Wicha, M.S. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003, 17, 1253–1270. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.H.; Collier, J.H.; Sfeir, C.S.; Healy, K.E. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc. Natl. Acad. Sci. USA 2002, 99, 1972–1977. [Google Scholar] [CrossRef] [PubMed]
- Vergani, L.; Grattarola, M.; Nicolini, C. Modifications of chromatin structure and gene expression following induced alterations of cellular shape. Int. J. Biochem. Cell Biol. 2004, 36, 1447–1461. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.; Przyborski, S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J. Anat. 2015, 747, 746–756. [Google Scholar] [CrossRef] [PubMed]
- De Ciurana, J.; Serenó, L.; Vallès, È. Selecting process parameters in RepRap additive manufacturing system for PLA scaffolds manufacture. Procedia CIRP 2013, 5, 152–157. [Google Scholar] [CrossRef]
- Mikos, A.G.; Sarakinos, G.; Leite, S.M.; Vacanti, J.P.; Langer, R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials 1993, 14, 323–330. [Google Scholar] [CrossRef]
- Conn, R.; Kolstad, J.; Borzelleca, J.; Dixler, D.; Filer, L.J.; LaDu, B.J.; Pariza, M.W. Safety assessment of polylactide (PLA) for use as a food-contact polymer. Food Chem. Toxicol. 1995, 33, 273–283. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Tsuyada, A.; Chow, A.; Wu, J.; Somlo, G.; Chu, P.; Loera, S.; Luu, T.; Li, A.X.; Wu, X.; Ye, W.; et al. CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res. 2012, 72, 2768–2779. [Google Scholar] [CrossRef] [PubMed]
- Sims-Mourtada, J.; Niamat, R.A.; Samuel, S.; Eskridge, C.; Kmiec, E.B. Enrichment of breast cancer stem-like cells by growth on electrospun polycaprolactone-chitosan nanofiber scaffolds. Int. J. Nanomed. 2014, 9, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Charafe-Jauffret, E.; Ginestier, C.; Iovino, F.; Wicinski, J.; Cervera, N.; Finetti, P.; Hur, M.H.; Diebel, M.E.; Monville, F.; Dutcher, J.; et al. Breast Cancer Cell Lines Contain Functional Cancer Stem Cells with Metastatic Capacity and a Distinct Molecular Signature. Cancer Res. 2009, 69, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Burg, K.J. Three-dimensional polymeric systems for cancer cell studies. Cytotechnology 2007, 54, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Palomeras, S.; Rabionet, M.; Ferrer, I.; Sarrats, A.; Garcia-Romeu, M.; Puig, T.; Ciurana, J. Breast Cancer Stem Cell Culture and Enrichment Using Poly(ε-Caprolactone) Scaffolds. Molecules 2016, 21, 537. [Google Scholar] [CrossRef] [PubMed]
- Rabionet, M.; Yeste, M.; Puig, T.; Ciurana, J. Electrospinning PCL Scaffolds Manufacture for Three-Dimensional Breast Cancer Cell Culture. Polymers 2017, 9, 328. [Google Scholar] [CrossRef]
- Marchitti, S.A.; Brocker, C.; Stagos, D.; Vasiliou, V. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert. Opin. Drug Metab. Toxicol. 2008, 4, 697–720. [Google Scholar] [CrossRef] [PubMed]
- Douville, J.; Beaulieu, R.; Balicki, D. ALDH1 as a functional marker of cancer stem and progenitor cells. Stem Cells Dev. 2009, 18, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.P.; Fleming, J.B.; Wang, H.; Abbruzzese, J.L.; Choi, W.; Kopetz, S.; McConkey, D.J.; Evans, D.B.; Gallick, G.E. ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS ONE 2011, 6, e20636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Yo, Y.-T.; Lee, H.-Y.; Liao, Y.-P.; Chao, T.-K.; Su, P.-H.; Lai, H.-C. ALDH1-Bright Epithelial Ovarian Cancer Cells Are Associated with CD44 Expression, Drug Resistance, and Poor Clinical Outcome. Am. J. Pathol. 2012, 180, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, S.; Vieira, A.F.; Gerhard, R.; Leitao, D.; Pinto, R.; Cameselle-Teijeiro, J.F.; Milanezi, F.; Schmitt, F.; Paredes, J. Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. J. Clin. Pathol. 2011, 64, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Luo, H.; Zhu, Y.; Raman, S.; Wan, Y. Creation of macropores in three-dimensional bacterial cellulose scaffold for potential cancer cell culture. Carbohydr. Polym. 2014, 114, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Florczyk, S.J.; Kievit, F.M.; Wang, K.; Erickson, A.E.; Ellenbogen, R.G.; Zhang, M. 3D Porous Chitosan-Alginate Scaffolds Promote Proliferation and Enrichment of Cancer Stem-Like Cells. J. Mater. Chem. B 2016, 4, 6326–6334. [Google Scholar] [CrossRef] [PubMed]
- Rijal, G.; Bathula, C.; Li, W. Application of Synthetic Polymeric Scaffolds in Breast Cancer 3D Tissue Cultures and Animal Tumor Models. Int. J. Biomater. 2017, 2017, 8074890. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Duan, X.; Lo, P.-K.; Liu, S.; Liu, X.; Chen, H.; Wang, Q. Expansion of breast cancer stem cells with fibrous scaffolds. Integr. Biol. 2013, 5, 768. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Mohanty, P.; Ghosh, G. Biomimetic apatite-coated porous PVA scaffolds promote the growth of breast cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Gleadall, A.; Visscher, D.; Yang, J.; Thomas, D.; Segal, J. Review of additive manufactured tissue engineering scaffolds: relationship between geometry and performance. Burn Trauma 2018, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Sobral, J.M.; Caridade, S.G.; Sousa, R.A.; Mano, J.F.; Reis, R.L. Three-dimensional plotted scaffolds with controlled pore size gradients: Effect of scaffold geometry on mechanical performance and cell seeding efficiency. Acta Biomater. 2011, 7, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Noreikaitė, A.; Antanavičiūtė, I.; Mikalayeva, V.; Darinskas, A.; Tamulevičius, T.; Adomavičiūtė, E.; Šimatonis, L.; Akramienė, D.; Stankevičius, E. Scaffold design for artificial tissue with bone marrow stem cells. Medicina 2017, 53, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Thankappan, S.K.; Lee, P.; Fard, S.E.; Harmon, M.D.; Tran, K.; Yu, X. Natural polymers in tissue engineering and regenerative medicine. In Natural and Synthetic Biomedical Polymers; Kumar, S.G., Laurencin, C.T., Eng, M.D., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2014; pp. 352–358. [Google Scholar]
- Nair, N.R.; Nampoothiri, K.M.; Pandey, A. Preparation of poly(L-lactide) blends and biodegradation by Lentzea waywayandensis. Biotechnol. Lett. 2012, 34, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Takayama, T.; Todo, M.; Tsuji, H. Effect of annealing on the mechanical properties of PLA/PCL and PLA/PCL/LTI polymer blends. J. Mech. Behav. Biomed. Mater. 2011, 4, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.-Y.; Salick, M.R.; Jing, X.; Jacques, B.R.; Crone, W.C.; Peng, X.-F.; Turng, L.S. Characterization of thermoplastic polyurethane/polylactic acid (TPU/PLA) tissue engineering scaffolds fabricated by microcellular injection molding. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4767–4776. [Google Scholar] [CrossRef] [PubMed]
- Achille, C.; Sundaresh, S.; Chu, B.; Hadjiargyrou, M. Cdk2 silencing via a DNA/PCL electrospun scaffold suppresses proliferation and increases death of breast cancer cells. PLoS ONE 2012, 7, e52356. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.J.; Cano, P.; Rabionet, M.; Puig, T.; Ciurana, J. Effects of different sterilization processes on the properties of a novel 3D-printed polycaprolactone stent. Polym. Adv. Technol. 2018, 29, 2327–2335. [Google Scholar] [CrossRef]




| Configuration | Layer Height (mm) | Infill Density (%) | Infill Pattern | Infill Direction (°) | Flow (%) |
|---|---|---|---|---|---|
| 1 | 0.10 | 50 | Zigzag | 45 | 80 |
| 2 | 0.10 | 50 | Zigzag | 45 | 90 |
| 3 | 0.10 | 50 | Zigzag | 45 | 100 |
| 4 | 0.10 | 60 | Grid | 60 | 80 |
| 5 | 0.10 | 60 | Grid | 60 | 90 |
| 6 | 0.10 | 60 | Grid | 60 | 100 |
| 7 | 0.10 | 70 | Triangles | 90 | 80 |
| 8 | 0.10 | 70 | Triangles | 90 | 90 |
| 9 | 0.10 | 70 | Triangles | 90 | 100 |
| 10 | 0.15 | 50 | Grid | 90 | 80 |
| 11 | 0.15 | 50 | Grid | 90 | 90 |
| 12 | 0.15 | 50 | Grid | 90 | 100 |
| 13 | 0.15 | 60 | Triangles | 45 | 80 |
| 14 | 0.15 | 60 | Triangles | 45 | 90 |
| 15 | 0.15 | 60 | Triangles | 45 | 100 |
| 16 | 0.15 | 70 | Zigzag | 60 | 80 |
| 17 | 0.15 | 70 | Zigzag | 60 | 90 |
| 18 | 0.15 | 70 | Zigzag | 60 | 100 |
| 19 | 0.20 | 50 | Triangles | 60 | 80 |
| 20 | 0.20 | 50 | Triangles | 60 | 90 |
| 21 | 0.20 | 50 | Triangles | 60 | 100 |
| 22 | 0.20 | 60 | Zigzag | 90 | 80 |
| 23 | 0.20 | 60 | Zigzag | 90 | 90 |
| 24 | 0.20 | 60 | Zigzag | 90 | 100 |
| 25 | 0.20 | 70 | Grid | 45 | 80 |
| 26 | 0.20 | 70 | Grid | 45 | 90 |
| 27 | 0.20 | 70 | Grid | 45 | 100 |
| Configuration | Pore Area (mm2) | Filament Diameter (mm) |
|---|---|---|
| 1 | 0.353 ± 0.010 | 0.243 ± 0.006 |
| 2 | 0.337 ± 0.009 | 0.301 ± 0.009 |
| 3 | 0.301 ± 0.006 | 0.331 ± 0.007 |
| 4 | 0.402 ± 0.025 (Irregular Pores; From 0.055 to 0.753) | 0.287 ± 0.007 |
| 5 | 0.384 ± 0.032 (Irregular Pores; From 0.065 to 0.794) | 0.294 ± 0.010 |
| 6 | 0.361 ± 0.031 (Irregular Pores; From 0.047 to 0.748) | 0.338 ± 0.012 |
| 7 | 0.263 ± 0.021 (Irregular Pores; From 0.029 to 0.550) | 0.348 ± 0.011 |
| 8 | 0.240 ± 0.017 (Irregular Pores; From 0.081 to 0.505) | 0.359 ± 0.010 |
| 9 | 0.197 ± 0.018 (Irregular Pores; From 0.044 to 0.546) | 0.367 ± 0.007 |
| 10 | 1.376 ± 0.013 | 0.298 ± 0.008 |
| 11 | 1.322 ± 0.009 | 0.341 ± 0.007 |
| 12 | 1.216 ± 0.010 | 0.378 ± 0.008 |
| 13 | 0.365 ± 0.034 (Irregular Pores; From 0.065 to 0.825) | 0.334 ± 0.013 |
| 14 | 0.335 ± 0.031 (Irregular Pores; From 0.054 to 0.786) | 0.354 ± 0.015 |
| 15 | 0.296 ± 0.032 (Irregular Pores; From 0.054 to 0.715) | 0.370 ± 0.011 |
| 16 | 0.074 ± 0.002 | 0.332 ± 0.007 |
| 17 | 0.069 ± 0.002 | 0.348 ± 0.007 |
| 18 | 0.041 ± 0.002 | 0.408 ± 0.007 |
| 19 | 1.736 ± 0.029 | 0.329 ± 0.007 |
| 20 | 1.714 ± 0.027 | 0.340 ± 0.004 |
| 21 | 1.611 ± 0.025 | 0.374 ± 0.008 |
| 22 | 0.125 ± 0.003 | 0.295 ± 0.005 |
| 23 | 0.098 ± 0.002 | 0.312 ± 0.005 |
| 24 | 0.090 ± 0.002 | 0.338 ± 0.005 |
| 25 | 0.202 ± 0.019 (Irregular Pores; From 0.039 to 0.437) | 0.364 ± 0.011 |
| 26 | 0.187 ± 0.018 (Irregular Pores; From 0.029 to 0.390) | 0.373 ± 0.014 |
| 27 | 0.180 ± 0.016 (Irregular Pores; From 0.046 to 0.381) | 0.394 ± 0.014 |
| Configuration | Selected Values | Pore Area (mm2) | Filament Diameter (mm) | Microscopic Image |
|---|---|---|---|---|
| SS1 | Layer Height: 0.2 mm Infill Density: 70% Infill Pattern: Zigzag Infill Direction: 45° Flow: 100% | 0.054 ± 0.002 | 0.483 ± 0.009 |  |
| SS2 | Layer Height: 0.2 mm Infill Density: 70% Infill Pattern: Triangles Infill Direction: 45° Flow: 100% | 0.224 ± 0.020 (Irregular pores; From 0.041 to 0.491) | 0.387 ± 0.010 |  |
| Parameter | Tested Values |
|---|---|
| Layer Height | 0.1, 0.15 and 0.2 mm |
| Infill Density | 50, 60 and 70% |
| Infill Pattern | Grid, Triangles and Zigzag |
| Infill Direction | 45, 60 and 90° |
| Flow | 80, 90 and 100% |
| Material(#) | Molecular Weight (g/mol) | Young’s Modulus (MPa) | Strain at Break (%) | Degradation Time (Months) |
|---|---|---|---|---|
| PLA | 30,000 | 108 | 3.5 | ≈12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polonio-Alcalá, E.; Rabionet, M.; Guerra, A.J.; Yeste, M.; Ciurana, J.; Puig, T. Screening of Additive Manufactured Scaffolds Designs for Triple Negative Breast Cancer 3D Cell Culture and Stem-Like Expansion. Int. J. Mol. Sci. 2018, 19, 3148. https://doi.org/10.3390/ijms19103148
Polonio-Alcalá E, Rabionet M, Guerra AJ, Yeste M, Ciurana J, Puig T. Screening of Additive Manufactured Scaffolds Designs for Triple Negative Breast Cancer 3D Cell Culture and Stem-Like Expansion. International Journal of Molecular Sciences. 2018; 19(10):3148. https://doi.org/10.3390/ijms19103148
Chicago/Turabian StylePolonio-Alcalá, Emma, Marc Rabionet, Antonio J. Guerra, Marc Yeste, Joaquim Ciurana, and Teresa Puig. 2018. "Screening of Additive Manufactured Scaffolds Designs for Triple Negative Breast Cancer 3D Cell Culture and Stem-Like Expansion" International Journal of Molecular Sciences 19, no. 10: 3148. https://doi.org/10.3390/ijms19103148
APA StylePolonio-Alcalá, E., Rabionet, M., Guerra, A. J., Yeste, M., Ciurana, J., & Puig, T. (2018). Screening of Additive Manufactured Scaffolds Designs for Triple Negative Breast Cancer 3D Cell Culture and Stem-Like Expansion. International Journal of Molecular Sciences, 19(10), 3148. https://doi.org/10.3390/ijms19103148








