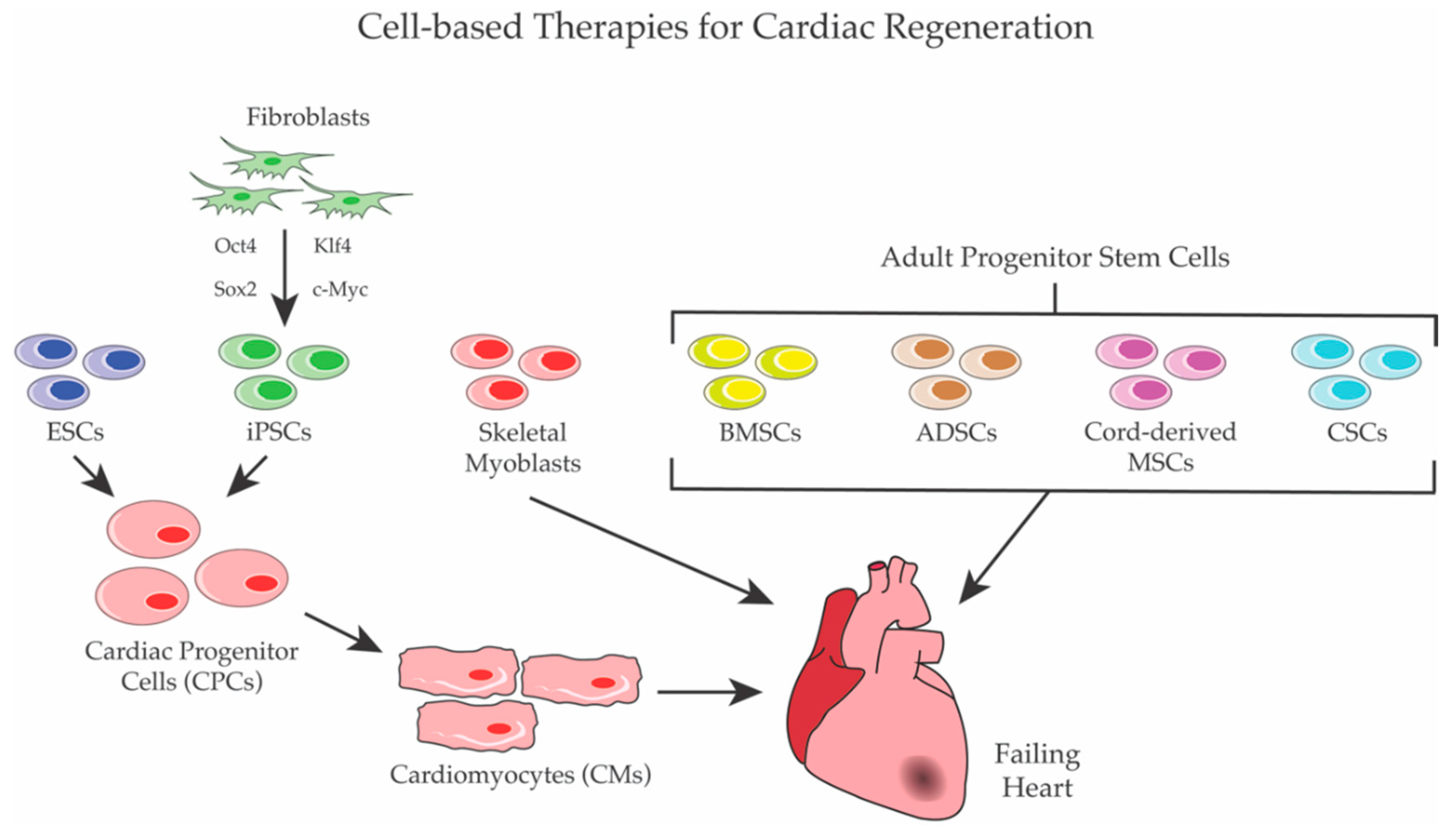
Cell-Based Therapies for Cardiac Regeneration: A Comprehensive Review of Past and Ongoing Strategies
Abstract
:1. Introduction
2. Cell Therapy
2.1. Initial Studies with Committed Cells
2.2. Stem Cells
2.2.1. Embryonic Stem Cells
2.2.2. Adult Stem Cells
2.2.3. Induced-Pluripotent Stem Cells (iPSCs)
3. Considerations on Cell Therapy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization: Annual Report on Cardiovascular Diseases. 2017. Available online: http://www.webcitation.org/72fSeoOIs (accessed on 4 September 2018).
- Reimer, K.A.; Ideker, R.E. Myocardial ischemia and infarction: Anatomic and biochemical substrates for ischemic cell death and ventricular arrhythmias. Hum. Pathol. 1987, 18, 462–475. [Google Scholar] [CrossRef]
- Writing Group Member; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; et al. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef] [PubMed]
- Buja, L.M. Myocardial ischemia and reperfusion injury. Cardiovasc. Pathol. 2005, 14, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef]
- Lesyuk, W.; Kriza, C.; Kolominsky-Rabas, P. Cost-of-illness studies in heart failure: A systematic review 2004–2016. BMC Cardiovasc. Disord. 2018, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabe-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.J. Long-term outcome following heart transplantation: Current perspective. J. Thorac. Dis. 2015, 7, 549–551. [Google Scholar] [PubMed]
- Stehlik, J.; Edwards, L.B.; Kucheryavaya, A.Y.; Benden, C.; Christie, J.D.; Dobbels, F.; Kirk, R.; Rahmel, A.O.; Hertz, M.I. The registry of the international society for heart and lung transplantation: Twenty-eighth adult heart transplant report—2011. J. Heart Lung Transplant. 2011, 30, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, M.J.; Boersma, E.; Reimer, W.J.; Balk, A.H.; Komajda, M.; Swedberg, K.; Follath, F.; Jimenez-Navarro, M.; Simoons, M.L.; Cleland, J.G. Under-utilization of evidence-based drug treatment in patients with heart failure is only partially explained by dissimilarity to patients enrolled in landmark trials: A report from the euro heart survey on heart failure. Eur. Heart J. 2005, 26, 2706–2713. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P.; Vanneaux, V. Stem cells for the treatment of heart failure. Curr. Res. Transl. Med. 2016, 64, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Ito, H.; Sano, S. Challenges to success in heart failure: Cardiac cell therapies in patients with heart diseases. J. Cardiol. 2016, 68, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Anastasia, L.; Piccoli, M.; Garatti, A.; Conforti, E.; Scaringi, R.; Bergante, S.; Castelvecchio, S.; Venerando, B.; Menicanti, L.; Tettamanti, G. Cell reprogramming: A new chemical approach to stem cell biology and tissue regeneration. Curr. Pharm. Biotechnol. 2011, 12, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Plonowska, K.; Wu, S.M. Somatic cell reprogramming into cardiovascular lineages. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, M.; Cirillo, F.; Tettamanti, G.; Anastasia, L. A chemical approach to myocardial protection and regeneration. Eur. Heart J. 2016, 18, E1–E7. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Huang, Y.; Spencer, C.I.; Foley, A.; Vedantham, V.; Liu, L.; Conway, S.J.; Fu, J.D.; Srivastava, D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 2012, 485, 593–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islas, J.F.; Liu, Y.; Weng, K.C.; Robertson, M.J.; Zhang, S.; Prejusa, A.; Harger, J.; Tikhomirova, D.; Chopra, M.; Iyer, D.; et al. Transcription factors ets2 and mesp1 transdifferentiate human dermal fibroblasts into cardiac progenitors. Proc. Natl. Acad. Sci. USA 2012, 109, 13016–13021. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, N.; Yamakawa, H.; Miyamoto, K.; Sadahiro, T.; Umei, T.; Isomi, M.; Nakashima, H.; Akiyama, M.; Wada, R.; Inagawa, K.; et al. Mir-133 promotes cardiac reprogramming by directly repressing snai1 and silencing fibroblast signatures. EMBO J. 2014, 33, 1565–1581. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.J.; Lubczyk, C.; Bhakta, M.; Zang, T.; Fernandez-Perez, A.; McAnally, J.; Bassel-Duby, R.; Olson, E.N.; Munshi, N.V. Induction of diverse cardiac cell types by reprogramming fibroblasts with cardiac transcription factors. Development 2014, 141, 4267–4278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathison, M.; Gersch, R.P.; Nasser, A.; Lilo, S.; Korman, M.; Fourman, M.; Hackett, N.; Shroyer, K.; Yang, J.; Ma, Y.; et al. In vivo cardiac cellular reprogramming efficacy is enhanced by angiogenic preconditioning of the infarcted myocardium with vascular endothelial growth factor. J. Am. Heart Assoc. 2012, 1, e005652. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.M.; Finch, E.A.; Zhang, L.; Zhang, H.; Hodgkinson, C.P.; Pratt, R.E.; Rosenberg, P.B.; Mirotsou, M.; Dzau, V.J. Microrna induced cardiac reprogramming in vivo: Evidence for mature cardiac myocytes and improved cardiac function. Circ. Res. 2015, 116, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Ghiroldi, A.; Piccoli, M.; Ciconte, G.; Pappone, C.; Anastasia, L. Regenerating the human heart: Direct reprogramming strategies and their current limitations. Basic Res. Cardiol. 2017, 112, 68. [Google Scholar] [CrossRef] [PubMed]
- Stem Cell-Based Clinical Trials for Heart Failure 2018. Available online: http://www.webcitation.org/72fVc36rP (accessed on 14 September 2018).
- Gyongyosi, M.; Haller, P.M.; Blake, D.J.; Martin Rendon, E. Meta-analysis of cell therapy studies in heart failure and acute myocardial infarction. Circ. Res. 2018, 123, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P. Cell therapy trials for heart regeneration—Lessons learned and future directions. Nat. Rev. Cardiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Koh, G.Y.; Klug, M.G.; Soonpaa, M.H.; Field, L.J. Differentiation and long-term survival of c2c12 myoblast grafts in heart. J. Clin. Investig. 1993, 92, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, H.; Poppa, V.; Murry, C.E. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J. Mol. Cell. Cardiol. 2002, 34, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; DerSimonian, H.; Brenner, D.A.; Ngoy, S.; Teller, P.; Edge, A.S.; Zawadzka, A.; Wetzel, K.; Sawyer, D.B.; Colucci, W.S.; et al. Cell therapy attenuates deleterious ventricular remodeling and improves cardiac performance after myocardial infarction. Circulation 2001, 103, 1920–1927. [Google Scholar] [CrossRef] [PubMed]
- Scorsin, M.; Hagege, A.; Vilquin, J.T.; Fiszman, M.; Marotte, F.; Samuel, J.L.; Rappaport, L.; Schwartz, K.; Menasche, P. Comparison of the effects of fetal cardiomyocyte and skeletal myoblast transplantation on postinfarction left ventricular function. J. Thorac. Cardiovasc. Surg. 2000, 119, 1169–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, D.A.; Atkins, B.Z.; Hungspreugs, P.; Jones, T.R.; Reedy, M.C.; Hutcheson, K.A.; Glower, D.D.; Kraus, W.E. Regenerating functional myocardium: Improved performance after skeletal myoblast transplantation. Nat. Med. 1998, 4, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P.; Hagege, A.A.; Scorsin, M.; Pouzet, B.; Desnos, M.; Duboc, D.; Schwartz, K.; Vilquin, J.T.; Marolleau, J.P. Myoblast transplantation for heart failure. Lancet 2001, 357, 279–280. [Google Scholar] [CrossRef]
- Pagani, F.D.; DerSimonian, H.; Zawadzka, A.; Wetzel, K.; Edge, A.S.; Jacoby, D.B.; Dinsmore, J.H.; Wright, S.; Aretz, T.H.; Eisen, H.J.; et al. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J. Am. Coll. Cardiol. 2003, 41, 879–888. [Google Scholar] [CrossRef]
- Siminiak, T.; Kalawski, R.; Fiszer, D.; Jerzykowska, O.; Rzezniczak, J.; Rozwadowska, N.; Kurpisz, M. Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: Phase i clinical study with 12 months of follow-up. Am. Heart J. 2004, 148, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Povsic, T.J.; O’Connor, C.M.; Henry, T.; Taussig, A.; Kereiakes, D.J.; Fortuin, F.D.; Niederman, A.; Schatz, R.; Spencer, R.t.; Owens, D.; et al. A double-blind, randomized, controlled, multicenter study to assess the safety and cardiovascular effects of skeletal myoblast implantation by catheter delivery in patients with chronic heart failure after myocardial infarction. Am. Heart J. 2011, 162, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Gavira, J.J.; Herreros, J.; Perez, A.; Garcia-Velloso, M.J.; Barba, J.; Martin-Herrero, F.; Canizo, C.; Martin-Arnau, A.; Marti-Climent, J.M.; Hernandez, M.; et al. Autologous skeletal myoblast transplantation in patients with nonacute myocardial infarction: 1-year follow-up. J. Thorac. Cardiovasc. Surg. 2006, 131, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Hagege, A.A.; Marolleau, J.P.; Vilquin, J.T.; Alheritiere, A.; Peyrard, S.; Duboc, D.; Abergel, E.; Messas, E.; Mousseaux, E.; Schwartz, K.; et al. Skeletal myoblast transplantation in ischemic heart failure: Long-term follow-up of the first phase i cohort of patients. Circulation 2006, 114, I108–I113. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P.; Alfieri, O.; Janssens, S.; McKenna, W.; Reichenspurner, H.; Trinquart, L.; Vilquin, J.T.; Marolleau, J.P.; Seymour, B.; Larghero, J.; et al. The myoblast autologous grafting in ischemic cardiomyopathy (magic) trial: First randomized placebo-controlled study of myoblast transplantation. Circulation 2008, 117, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Li, R.K.; Jia, Z.Q.; Weisel, R.D.; Mickle, D.A.; Zhang, J.; Mohabeer, M.K.; Rao, V.; Ivanov, J. Cardiomyocyte transplantation improves heart function. Ann. Thorac. Surg. 1996, 62, 654–660, discussion 660–651. [Google Scholar] [CrossRef]
- Reinecke, H.; Zhang, M.; Bartosek, T.; Murry, C.E. Survival, integration, and differentiation of cardiomyocyte grafts: A study in normal and injured rat hearts. Circulation 1999, 100, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Huwer, H.; Winning, J.; Vollmar, B.; Welter, C.; Lohbach, C.; Menger, M.D.; Schafers, H.J. Long-term cell survival and hemodynamic improvements after neonatal cardiomyocyte and satellite cell transplantation into healed myocardial cryoinfarcted lesions in rats. Cell Transplant. 2003, 12, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Muller-Ehmsen, J.; Peterson, K.L.; Kedes, L.; Whittaker, P.; Dow, J.S.; Long, T.I.; Laird, P.W.; Kloner, R.A. Rebuilding a damaged heart: Long-term survival of transplanted neonatal rat cardiomyocytes after myocardial infarction and effect on cardiac function. Circulation 2002, 105, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Muller-Ehmsen, J.; Whittaker, P.; Kloner, R.A.; Dow, J.S.; Sakoda, T.; Long, T.I.; Laird, P.W.; Kedes, L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J. Mol. Cell. Cardiol. 2002, 34, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Methot, D.; Poppa, V.; Fujio, Y.; Walsh, K.; Murry, C.E. Cardiomyocyte grafting for cardiac repair: Graft cell death and anti-death strategies. J. Mol. Cell. Cardiol. 2001, 33, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638. [Google Scholar] [CrossRef] [PubMed]
- Graves, K.H.; Moreadith, R.W. Derivation and characterization of putative pluripotential embryonic stem cells from preimplantation rabbit embryos. Mol. Reprod. Dev. 1993, 36, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, P.M.; Taborn, G.U.; Garton, R.L.; Caplice, M.D.; Brenin, D.R. Pluripotent embryonic stem cells from the rat are capable of producing chimeras. Dev. Biol. 1994, 163, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Kalishman, J.; Golos, T.G.; Durning, M.; Harris, C.P.; Becker, R.A.; Hearn, J.P. Isolation of a primate embryonic stem cell line. Proc. Natl. Acad. Sci. USA 1995, 92, 7844–7848. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Kehat, I.; Kenyagin-Karsenti, D.; Snir, M.; Segev, H.; Amit, M.; Gepstein, A.; Livne, E.; Binah, O.; Itskovitz-Eldor, J.; Gepstein, L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Investig. 2001, 108, 407–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehat, I.; Khimovich, L.; Caspi, O.; Gepstein, A.; Shofti, R.; Arbel, G.; Huber, I.; Satin, J.; Itskovitz-Eldor, J.; Gepstein, L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat. Biotechnol. 2004, 22, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Mummery, C.; Ward-van Oostwaard, D.; Doevendans, P.; Spijker, R.; van den Brink, S.; Hassink, R.; van der Heyden, M.; Opthof, T.; Pera, M.; de la Riviere, A.B.; et al. Differentiation of human embryonic stem cells to cardiomyocytes: Role of coculture with visceral endoderm-like cells. Circulation 2003, 107, 2733–2740. [Google Scholar] [CrossRef] [PubMed]
- Passier, R.; Oostwaard, D.W.; Snapper, J.; Kloots, J.; Hassink, R.J.; Kuijk, E.; Roelen, B.; de la Riviere, A.B.; Mummery, C. Increased cardiomyocyte differentiation from human embryonic stem cells in serum-free cultures. Stem Cells 2005, 23, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Kattman, S.J.; Adler, E.D.; Keller, G.M. Specification of multipotential cardiovascular progenitor cell during embryonic stem cell differentiation and embryonic development. Trends Cardiovas. Med. 2007, 17, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Kattman, S.J.; Witty, A.D.; Gagliardi, M.; Dubois, N.C.; Niapour, M.; Hotta, A.; Ellis, J.; Keller, G. Stage-specific optimization of activin/nodal and bmp signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 2011, 8, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, M.A.; Gold, J.; Xu, C.H.; Hassanipour, M.; Rosler, E.; Police, S.; Muskheli, V.; Murry, C.E. Formation of human myocardium in the rat heart from human embryonic stem cells. Am. J. Pathol. 2005, 167, 663–671. [Google Scholar] [CrossRef]
- Kolossov, E.; Bostani, T.; Roell, W.; Breitbach, M.; Pillekamp, F.; Nygren, J.M.; Sasse, P.; Rubenchik, O.; Fries, J.W.U.; Wenzel, D.; et al. Engraftment of engineered es cell-derived cardiomyocytes but not bm cells restores contractile function to the infarcted myocardium. J. Exp. Med. 2006, 203, 2315–2327. [Google Scholar] [CrossRef] [PubMed]
- Min, J.Y.; Yang, Y.K.; Converso, K.L.; Liu, L.X.; Huang, Q.; Morgan, J.P.; Xiao, Y.F. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J. Appl. Physiol. 2002, 92, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Singla, D.K.; Hacker, T.A.; Ma, L.N.; Douglas, P.S.; Sullivan, R.; Lyons, G.E.; Kamp, T.J. Transplantation of embryonic stem cells into the infarcted mouse heart: Formation of multiple cell types. J. Mol. Cell. Cardiol. 2006, 40, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.J.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014, 510, 273–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.W.; Chen, B.; Yang, X.; Fugate, J.A.; Kalucki, F.A.; Futakuchi-Tsuchida, A.; Couture, L.; Vogel, K.W.; Astley, C.A.; Baldessari, A.; et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018, 36, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P.; Vanneaux, V.; Hagege, A.; Bel, A.; Cholley, B.; Cacciapuoti, I.; Parouchev, A.; Benhamouda, N.; Tachdjian, G.; Tosca, L.; et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: First clinical case report. Eur. Heart J. 2015, 36, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P.; Vanneaux, V.; Hagege, A.; Bel, A.; Cholley, B.; Parouchev, A.; Cacciapuoti, I.; Al-Daccak, R.; Benhamouda, N.; Blons, H.; et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 2018, 71, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, J.; Minami, E.; Laflamme, M.A.; Virag, J.A.I.; Ware, C.B.; Masino, A.; Muskheli, V.; Pabon, L.; Reinecke, H.; Murry, C.E. Transplantation of undifferentiated murine embryonic stem cells in the heart: Teratoma formation and immune response. FASEB J. 2007, 21, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.J.; Tuan, R.S.; Cheung, K.M.; Leung, V.Y. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Assmus, B.; Schachinger, V.; Teupe, C.; Britten, M.; Lehmann, R.; Dobert, N.; Grunwald, F.; Aicher, A.; Urbich, C.; Martin, H.; et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (topcare-ami). Circulation 2002, 106, 3009–3017. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Meyer, G.P.; Lotz, J.; Ringes-Lichtenberg, S.; Lippolt, P.; Breidenbach, C.; Fichtner, S.; Korte, T.; Hornig, B.; Messinger, D.; et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The boost randomised controlled clinical trial. Lancet 2004, 364, 141–148. [Google Scholar] [CrossRef]
- Schachinger, V.; Erbs, S.; Elsasser, A.; Haberbosch, W.; Hambrecht, R.; Holschermann, H.; Yu, J.; Corti, R.; Mathey, D.G.; Hamm, C.W.; et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: Final 1-year results of the repair-ami trial. Eur. Heart J. 2006, 27, 2775–2783. [Google Scholar] [CrossRef] [PubMed]
- Surder, D.; Schwitter, J.; Moccetti, T.; Astori, G.; Rufibach, K.; Plein, S.; Lo Cicero, V.; Soncin, S.; Windecker, S.; Moschovitis, A.; et al. Cell-based therapy for myocardial repair in patients with acute myocardial infarction: Rationale and study design of the swiss multicenter intracoronary stem cells study in acute myocardial infarction (swiss-ami). Am. Heart J. 2010, 160, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Traverse, J.H.; Henry, T.D.; Pepine, C.J.; Willerson, J.T.; Zhao, D.X.; Ellis, S.G.; Forder, J.R.; Anderson, R.D.; Hatzopoulos, A.K.; Penn, M.S.; et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: The time randomized trial. JAMA 2012, 308, 2380–2389. [Google Scholar] [CrossRef] [PubMed]
- Strauer, B.E.; Brehm, M.; Zeus, T.; Kostering, M.; Hernandez, A.; Sorg, R.V.; Kogler, G.; Wernet, P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 2002, 106, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Meyer, G.P.; Muller-Ehmsen, J.; Tschope, C.; Bonarjee, V.; Larsen, A.I.; May, A.E.; Empen, K.; Chorianopoulos, E.; Tebbe, U.; et al. Intracoronary autologous bone marrow cell transfer after myocardial infarction: The boost-2 randomised placebo-controlled clinical trial. Eur. Heart J. 2017, 38, 2936–2943. [Google Scholar] [CrossRef] [PubMed]
- Hamano, K.; Nishida, M.; Hirata, K.; Mikamo, A.; Li, T.S.; Harada, M.; Miura, T.; Matsuzaki, M.; Esato, K. Local implantation of autologous bone marrow cells for therapeutic angiogenesis in patients with ischemic heart disease: Clinical trial and preliminary results. Jpn. Circ. J. 2001, 65, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Leistner, D.M.; Fischer-Rasokat, U.; Honold, J.; Seeger, F.H.; Schachinger, V.; Lehmann, R.; Martin, H.; Burck, I.; Urbich, C.; Dimmeler, S.; et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (topcare-ami): Final 5-year results suggest long-term safety and efficacy. Clin. Res. Cardiol. 2011, 100, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Lunde, K.; Solheim, S.; Aakhus, S.; Arnesen, H.; Abdelnoor, M.; Forfang, K.; investigators, A. Autologous stem cell transplantation in acute myocardial infarction: The astami randomized controlled trial. Intracoronary transplantation of autologous mononuclear bone marrow cells, study design and safety aspects. Scand. Cardiovasc. J. 2005, 39, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Suerder, D.; Manka, R.; Moccetti, T.; Lo Cicero, V.; Emmert, M.Y.; Klersy, C.; Soncin, S.; Turchetto, L.; Radrizzani, M.; Zuber, M.; et al. The effect of bone marrow derived mononuclear cell treatment, early or late after acute myocardial infarction: Twelve months cmr and long-term clinical results. Circ. Res. 2016, 119, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Wohrle, J.; Merkle, N.; Mailander, V.; Nusser, T.; Schauwecker, P.; von Scheidt, F.; Schwarz, K.; Bommer, M.; Wiesneth, M.; Schrezenmeier, H.; et al. Results of intracoronary stem cell therapy after acute myocardial infarction. Am. J. Cardiol. 2010, 105, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Strauer, B.E.; Yousef, M.; Schannwell, C.M. The acute and long-term effects of intracoronary stem cell transplantation in 191 patients with chronic heart failure: The star-heart study. Eur. J. Heart Fail. 2010, 12, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Stamm, C.; Kleine, H.D.; Choi, Y.H.; Dunkelmann, S.; Lauffs, J.A.; Lorenzen, B.; David, A.; Liebold, A.; Nienaber, C.; Zurakowski, D.; et al. Intramyocardial delivery of cd133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: Safety and efficacy studies. J. Thorac. Cardiovasc. Surg. 2007, 133, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, B.A.; Ebell, W.; Dandel, M.; Kukucka, M.; Gebker, R.; Doltra, A.; Knosalla, C.; Choi, Y.H.; Hetzer, R.; Stamm, C. Autologous cd133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: The cardio133 trial. Eur. Heart J. 2014, 35, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Soonpaa, M.H.; Reinecke, H.; Nakajima, H.; Nakajima, H.O.; Rubart, M.; Pasumarthi, K.B.; Virag, J.I.; Bartelmez, S.H.; Poppa, V.; et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 2004, 428, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Duong Van Huyen, J.P.; Smadja, D.M.; Bruneval, P.; Gaussem, P.; Dal-Cortivo, L.; Julia, P.; Fiessinger, J.N.; Cavazzana-Calvo, M.; Aiach, M.; Emmerich, J. Bone marrow-derived mononuclear cell therapy induces distal angiogenesis after local injection in critical leg ischemia. Mod. Pathol. 2008, 21, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, J.; Luo, R.; Jiang, J.; Wang, J.A. Bone marrow mesenchymal stem cells induce angiogenesis and attenuate the remodeling of diabetic cardiomyopathy. Exp. Clin. Endocrinol. Diabetes 2008, 116, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhou, S.; Liu, Y.; Hu, H. Efficacy and safety of bone marrow cell transplantation for chronic ischemic heart disease: A meta-analysis. Med. Sci. Monit. 2014, 20, 1768–1777. [Google Scholar] [PubMed]
- Seeger, F.H.; Tonn, T.; Krzossok, N.; Zeiher, A.M.; Dimmeler, S. Cell isolation procedures matter: A comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur. Heart J. 2007, 28, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Tendera, M.; Wojakowski, W.; Ruzyllo, W.; Chojnowska, L.; Kepka, C.; Tracz, W.; Musialek, P.; Piwowarska, W.; Nessler, J.; Buszman, P.; et al. Intracoronary infusion of bone marrow-derived selected cd34+cxcr4+ cells and non-selected mononuclear cells in patients with acute stemi and reduced left ventricular ejection fraction: Results of randomized, multicentre myocardial regeneration by intracoronary infusion of selected population of stem cells in acute myocardial infarction (regent) trial. Eur. Heart J. 2009, 30, 1313–1321. [Google Scholar] [PubMed]
- Povsic, T.J.; Henry, T.D.; Traverse, J.H.; Fortuin, F.D.; Schaer, G.L.; Kereiakes, D.J.; Schatz, R.A.; Zeiher, A.M.; White, C.J.; Stewart, D.J.; et al. The renew trial: Efficacy and safety of intramyocardial autologous cd34(+) cell administration in patients with refractory angina. JACC Cardiovasc. Interv. 2016, 9, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Noiseux, N.; Mansour, S.; Weisel, R.; Stevens, L.M.; Der Sarkissian, S.; Tsang, K.; Crean, A.M.; Larose, E.; Li, S.H.; Wintersperger, B.; et al. The impact-cabg trial: A multicenter, randomized clinical trial of cd133(+) stem cell therapy during coronary artery bypass grafting for ischemic cardiomyopathy. J. Thorac. Cardiovasc. Surg. 2016, 152, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A.A.; Mathiasen, A.B.; Mygind, N.D.; Kuhl, J.T.; Jorgensen, E.; Helqvist, S.; Elberg, J.J.; Kofoed, K.F.; Vejlstrup, N.G.; Fischer-Nielsen, A.; et al. Adipose-derived stromal cells for treatment of patients with chronic ischemic heart disease (mystromalcell trial): A randomized placebo-controlled study. Stem Cells Int. 2017, 2017, 5237063. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Suzuki, K. Bone marrow-derived mesenchymal stem cells for the treatment of heart failure. Heart Fail. Rev. 2015, 20, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Davani, S.; Marandin, A.; Mersin, N.; Royer, B.; Kantelip, B.; Herve, P.; Etievent, J.P.; Kantelip, J.P. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation 2003, 108 (Suppl. 1), II253–II258. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Peng, P.; Cheng, L.; Chen, S.; Li, K.; Li, Z.Y.; Mo, Y.H.; Zhou, Z.; Li, M. A natural compound induced cardiogenic differentiation of endogenous mscs for repair of infarcted heart. Differ. Res. Biol. Divers. 2012, 83, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Liu, X.C.; Kong, F.; Qi, T.G.; Cheng, G.H.; Wang, J.; Sun, C.; Luan, Y. Bone marrow mesenchymal stem cells improve myocardial function in a swine model of acute myocardial infarction. Mol. Med. Rep. 2014, 10, 1448–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hare, J.M.; Fishman, J.E.; Gerstenblith, G.; DiFede Velazquez, D.L.; Zambrano, J.P.; Suncion, V.Y.; Tracy, M.; Ghersin, E.; Johnston, P.V.; Brinker, J.A.; et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The poseidon randomized trial. JAMA 2012, 308, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Heldman, A.W.; DiFede, D.L.; Fishman, J.E.; Zambrano, J.P.; Trachtenberg, B.H.; Karantalis, V.; Mushtaq, M.; Williams, A.R.; Suncion, V.Y.; McNiece, I.K.; et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The tac-hft randomized trial. JAMA 2014, 311, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Tano, N.; Narita, T.; Kaneko, M.; Ikebe, C.; Coppen, S.R.; Campbell, N.G.; Shiraishi, M.; Shintani, Y.; Suzuki, K. Epicardial placement of mesenchymal stromal cell-sheets for the treatment of ischemic cardiomyopathy; in vivo proof-of-concept study. Mol. Ther. 2014, 22, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Bartunek, J.; Behfar, A.; Dolatabadi, D.; Vanderheyden, M.; Ostojic, M.; Dens, J.; El Nakadi, B.; Banovic, M.; Beleslin, B.; Vrolix, M.; et al. Cardiopoietic stem cell therapy in heart failure: The c-cure (cardiopoietic stem cell therapy in heart failure) multicenter randomized trial with lineage-specified biologics. J. Am. Coll. Cardiol. 2013, 61, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, S.H.; Youn, Y.J.; Ahn, M.S.; Kim, J.Y.; Yoo, B.S.; Yoon, J.; Kwon, W.; Hong, I.S.; Lee, K.; et al. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J. Korean Med. Sci. 2014, 29, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Mathiasen, A.B.; Qayyum, A.A.; Jorgensen, E.; Helqvist, S.; Fischer-Nielsen, A.; Kofoed, K.F.; Haack-Sorensen, M.; Ekblond, A.; Kastrup, J. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: A randomized placebo-controlled trial (msc-hf trial). Eur. Heart J. 2015, 36, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Fang, W.W.; Ye, F.; Liu, Y.H.; Qian, J.; Shan, S.J.; Zhang, J.J.; Chunhua, R.Z.; Liao, L.M.; Lin, S.; et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am. J. Cardiol. 2004, 94, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Z.; Tian, N.; Zhang, J.; Yei, F.; Duan, B.; Zhu, Z.; Lin, S.; Kwan, T.W. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J. Invasive Cardiol. 2006, 18, 552–556. [Google Scholar] [PubMed]
- Hare, J.M.; Traverse, J.H.; Henry, T.D.; Dib, N.; Strumpf, R.K.; Schulman, S.P.; Gerstenblith, G.; DeMaria, A.N.; Denktas, A.E.; Gammon, R.S.; et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 2009, 54, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, F.; Ma, W.; Chen, B.; Zhou, F.; Xu, Z.; Zhang, Y.; Zhang, D.; Zhu, T.; Wang, L.; et al. A novel approach to transplanting bone marrow stem cells to repair human myocardial infarction: Delivery via a noninfarct-relative artery. Cardiovasc. Ther. 2010, 28, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.R.; Pei, X.T.; Ding, Q.A.; Chen, Y.; Zhang, N.K.; Chen, H.Y.; Wang, Z.G.; Wang, Y.F.; Zhu, Z.M.; Li, T.C.; et al. A critical challenge: Dosage-related efficacy and acute complication intracoronary injection of autologous bone marrow mesenchymal stem cells in acute myocardial infarction. Int. J. Cardiol. 2013, 168, 3191–3199. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, S.F.; van Ramshorst, J.; Hoogslag, G.E.; Boden, H.; Velders, M.A.; Cannegieter, S.C.; Roelofs, H.; Al Younis, I.; Dibbets-Schneider, P.; Fibbe, W.E.; et al. Intramyocardial injection of autologous bone marrow-derived ex vivo expanded mesenchymal stem cells in acute myocardial infarction patients is feasible and safe up to 5 years of follow-up. J. Cardiovasc. Transl. Res. 2013, 6, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Karantalis, V.; DiFede, D.L.; Gerstenblith, G.; Pham, S.; Symes, J.; Zambrano, J.P.; Fishman, J.; Pattany, P.; McNiece, I.; Conte, J.; et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The prospective randomized study of mesenchymal stem cell therapy in patients undergoing cardiac surgery (prometheus) trial. Circ. Res. 2014, 114, 1302–1310. [Google Scholar] [PubMed]
- Ascheim, D.D.; Gelijns, A.C.; Goldstein, D.; Moye, L.A.; Smedira, N.; Lee, S.; Klodell, C.T.; Szady, A.; Parides, M.K.; Jeffries, N.O.; et al. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation 2014, 129, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Chullikana, A.; Majumdar, A.S.; Gottipamula, S.; Krishnamurthy, S.; Kumar, A.S.; Prakash, V.S.; Gupta, P.K. Randomized, double-blind, phase i/ii study of intravenous allogeneic mesenchymal stromal cells in acute myocardial infarction. Cytotherapy 2015, 17, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.C.; Borow, K.M.; Silva, G.V.; DeMaria, A.N.; Marroquin, O.C.; Huang, P.P.; Traverse, J.H.; Krum, H.; Skerrett, D.; Zheng, Y.; et al. A phase ii dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ. Res. 2015, 117, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Guijarro, D.; Lebrin, M.; Lairez, O.; Bourin, P.; Piriou, N.; Pozzo, J.; Lande, G.; Berry, M.; Le Tourneau, T.; Cussac, D.; et al. Intramyocardial transplantation of mesenchymal stromal cells for chronic myocardial ischemia and impaired left ventricular function: Results of the mesami 1 pilot trial. Int. J. Cardiol. 2016, 209, 258–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, W.; Guo, S.; Gao, C.; Dai, G.; Gao, Y.; Li, M.; Wang, X.; Hu, D. A randomized comparative study on the efficacy of intracoronary infusion of autologous bone marrow mononuclear cells and mesenchymal stem cells in patients with dilated cardiomyopathy. Int. Heart J. 2017, 58, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Florea, V.; Rieger, A.C.; DiFede, D.L.; El-Khorazaty, J.; Natsumeda, M.; Banerjee, M.N.; Tompkins, B.A.; Khan, A.; Schulman, I.H.; Landin, A.M.; et al. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (the trident study). Circ. Res. 2017, 121, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Epstein, S.E.; Greene, S.J.; Quyyumi, A.A.; Sikora, S.; Kim, R.J.; Anderson, A.S.; Wilcox, J.E.; Tankovich, N.I.; Lipinski, M.J.; et al. Intravenous allogeneic mesenchymal stem cells for nonischemic cardiomyopathy: Safety and efficacy results of a phase ii-a randomized trial. Circ. Res. 2017, 120, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Bartunek, J.; Terzic, A.; Davison, B.A.; Filippatos, G.S.; Radovanovic, S.; Beleslin, B.; Merkely, B.; Musialek, P.; Wojakowski, W.; Andreka, P.; et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: Results at 39 weeks of the prospective, randomized, double blind, sham-controlled chart-1 clinical trial. Eur. Heart J. 2017, 38, 648–660. [Google Scholar] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Rigol, M.; Solanes, N.; Roura, S.; Roque, M.; Novensa, L.; Dantas, A.P.; Martorell, J.; Sitges, M.; Ramirez, J.; Bayes-Genis, A.; et al. Allogeneic adipose stem cell therapy in acute myocardial infarction. Eur. J. Clin. Investig. 2014, 44, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, Y.; Nagaya, N.; Kataoka, M.; Yanagawa, B.; Tanaka, K.; Hao, H.; Ishino, K.; Ishida, H.; Shimizu, T.; Kangawa, K.; et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat. Med. 2006, 12, 459–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazo, M.; Planat-Benard, V.; Abizanda, G.; Pelacho, B.; Leobon, B.; Gavira, J.J.; Penuelas, I.; Cemborain, A.; Penicaud, L.; Laharrague, P.; et al. Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. Eur. J. Heart Fail. 2008, 10, 454–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houtgraaf, J.H.; den Dekker, W.K.; van Dalen, B.M.; Springeling, T.; de Jong, R.; van Geuns, R.J.; Geleijnse, M.L.; Fernandez-Aviles, F.; Zijlsta, F.; Serruys, P.W.; et al. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with st-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2012, 59, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.C.; Sanz-Ruiz, R.; Sanchez, P.L.; Lasso, J.; Perez-Cano, R.; Alonso-Farto, J.C.; Perez-David, E.; Fernandez-Santos, M.E.; Serruys, P.W.; Duckers, H.J.; et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The precise trial. Am. Heart J. 2014, 168, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Kastrup, J.; Schou, M.; Gustafsson, I.; Nielsen, O.W.; Mogelvang, R.; Kofoed, K.F.; Kragelund, C.; Hove, J.D.; Fabricius-Bjerre, A.; Heitman, M.; et al. Rationale and design of the first double-blind, placebo-controlled trial with allogeneic adipose tissue-derived stromal cell therapy in patients with ischemic heart failure: A phase ii danish multicentre study. Stem Cells Int. 2017, 2017, 8506370. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.D.; Pepine, C.J.; Lambert, C.R.; Traverse, J.H.; Schatz, R.; Costa, M.; Povsic, T.J.; David Anderson, R.; Willerson, J.T.; Kesten, S.; et al. The athena trials: Autologous adipose-derived regenerative cells for refractory chronic myocardial ischemia with left ventricular dysfunction. Catheter. Cardiovasc. Interv. 2017, 89, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Okura, H.; Matsuyama, A.; Lee, C.M.; Saga, A.; Kakuta-Yamamoto, A.; Nagao, A.; Sougawa, N.; Sekiya, N.; Takekita, K.; Shudo, Y.; et al. Cardiomyoblast-like cells differentiated from human adipose tissue-derived mesenchymal stem cells improve left ventricular dysfunction and survival in a rat myocardial infarction model. Tissue Eng. Part C Methods 2010, 16, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.E.; Yang, D.; Li, L.; Wang, W.; Peng, Y.; Chen, C.; Chen, P.; Xia, X.; Wang, H.; Jiang, J.; et al. Prolyl hydroxylase domain protein 2 silencing enhances the survival and paracrine function of transplanted adipose-derived stem cells in infarcted myocardium. Circ. Res. 2013, 113, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Lee, H.C.; Park, J.H.; Kim, B.W.; Ahn, J.; Kim, J.H.; Park, J.S.; Oh, J.H.; Choi, J.H.; Cha, K.S.; et al. Effects of intracoronary administration of autologous adipose tissue-derived stem cells on acute myocardial infarction in a porcine model. Yonsei Med. J. 2015, 56, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, S.; Wenisch, S. Adipose tissue derived mesenchymal stem cells for musculoskeletal repair in veterinary medicine. Am. J. Stem Cells 2015, 4, 1–12. [Google Scholar] [PubMed]
- Timmers, L.; Lim, S.K.; Hoefer, I.E.; Arslan, F.; Lai, R.C.; van Oorschot, A.A.; Goumans, M.J.; Strijder, C.; Sze, S.K.; Choo, A.; et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011, 6, 206–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, C.Y.; Chak, L.L.; Biswas, A.; Tan, J.H.; Gauthaman, K.; Chan, W.K.; Bongso, A. Human wharton’s jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev. 2011, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.R.; Zhang, N.K.; Ding, Q.A.; Chen, H.Y.; Hu, X.; Jiang, S.; Li, T.C.; Chen, Y.; Wang, Z.G.; Ye, Y.; et al. Common expression of stemness molecular markers and early cardiac transcription factors in human wharton’s jelly-derived mesenchymal stem cells and embryonic stem cells. Cell Transplant. 2013, 22, 1883–1900. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.J.; Kim, H.; Lee, S.K.; Kim, J.; Shen, Y.; Jung, S.; Kang, K.S.; Im, S.G.; Lee, S.Y.; Choi, M.; et al. Umbilical-cord-blood-derived mesenchymal stem cells seeded onto fibronectin-immobilized polycaprolactone nanofiber improve cardiac function. Acta Biomater. 2014, 10, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Choi, E.K.; Kang, S.K.; Kim, G.H.; Park, J.Y.; Kang, H.J.; Lee, S.W.; Kim, K.H.; Kwon, J.S.; Lee, K.H.; et al. N-cadherin determines individual variations in the therapeutic efficacy of human umbilical cord blood-derived mesenchymal stem cells in a rat model of myocardial infarction. Mol. Ther. 2012, 20, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Lilyanna, S.; Martinez, E.C.; Vu, T.D.; Ling, L.H.; Gan, S.U.; Tan, A.L.; Phan, T.T.; Kofidis, T. Cord lining-mesenchymal stem cells graft supplemented with an omental flap induces myocardial revascularization and ameliorates cardiac dysfunction in a rat model of chronic ischemic heart failure. Tissue Eng. Part A 2013, 19, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, X.C.; Yang, L.; Zhu, D.L.; Zhang, Y.D.; Chen, Y.; Zhang, H.Y. Wharton’s jelly-derived mesenchymal stem cells promote myocardial regeneration and cardiac repair after miniswine acute myocardial infarction. Coronary Artery Dis. 2013, 24, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Yin, X.; Wang, J.; Tian, N.; Ao, Q.; Gu, Y.; Liu, Y. Functional characterization of human umbilical cord-derived mesenchymal stem cells for treatment of systolic heart failure. Exp. Ther. Med. 2016, 12, 3328–3332. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, Y.D.; Guo, Y.; Chen, Y.; Guo, D.X.; Zhou, H.L.; Zhang, F.L.; Zhao, Q.N. Safety and efficacy of intracoronary human umbilical cord-derived mesenchymal stem cell treatment for very old patients with coronary chronic total occlusion. Curr. Pharm. Des. 2015, 21, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Musialek, P.; Mazurek, A.; Jarocha, D.; Tekieli, L.; Szot, W.; Kostkiewicz, M.; Banys, R.P.; Urbanczyk, M.; Kadzielski, A.; Trystula, M.; et al. Myocardial regeneration strategy using wharton’s jelly mesenchymal stem cells as an off-the-shelf ‘unlimited’ therapeutic agent: Results from the acute myocardial infarction first-in-man study. Adv. Int. Cardiol. 2015, 11, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, J.; Verdugo, F.J.; Gonzalez, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A.; et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: A phase 1/2 randomized controlled trial (rimecard trial [randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]). Circ. Res. 2017, 121, 1192–1204. [Google Scholar] [PubMed]
- Gao, L.R.; Chen, Y.; Zhang, N.K.; Yang, X.L.; Liu, H.L.; Wang, Z.G.; Yan, X.Y.; Wang, Y.; Zhu, Z.M.; Li, T.C.; et al. Intracoronary infusion of wharton’s jelly-derived mesenchymal stem cells in acute myocardial infarction: Double-blind, randomized controlled trial. BMC Med. 2015, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.F.; Xu, Y.; Zhu, Z.Y.; Gao, C.Y.; Shi, Y.N. Clinical observation of umbilical cord mesenchymal stem cell treatment of severe systolic heart failure. Genet. Mol. Res. 2015, 14, 3010–3017. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Celikkan, F.T.; Cinar, O. Umbilical cord mesenchymal stromal cell transplantations: A systemic analysis of clinical trials. Cytotherapy 2017, 19, 1351–1382. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.E.; Partridge, T.A. Muscle satellite cells. Int. J. Biochem. Cell. Biol. 2003, 35, 1151–1156. [Google Scholar] [CrossRef]
- Chiu, R.C.; Zibaitis, A.; Kao, R.L. Cellular cardiomyoplasty: Myocardial regeneration with satellite cell implantation. Ann. Thorac. Surg. 1995, 60, 12–18. [Google Scholar] [CrossRef]
- Marelli, D.; Desrosiers, C.; el-Alfy, M.; Kao, R.L.; Chiu, R.C. Cell transplantation for myocardial repair: An experimental approach. Cell Transplant. 1992, 1, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Herreros, J.; Prosper, F.; Perez, A.; Gavira, J.J.; Garcia-Velloso, M.J.; Barba, J.; Sanchez, P.L.; Canizo, C.; Rabago, G.; Marti-Climent, J.M.; et al. Autologous intramyocardial injection of cultured skeletal muscle-derived stem cells in patients with non-acute myocardial infarction. Eur. Heart J. 2003, 24, 2012–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menasche, P. Stem cell therapy for heart failure: Are arrhythmias a real safety concern? Circulation 2009, 119, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Bolli, R.; Chugh, A.R.; D’Amario, D.; Loughran, J.H.; Stoddard, M.F.; Ikram, S.; Beache, G.M.; Wagner, S.G.; Leri, A.; Hosoda, T.; et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet 2011, 378, 1847–1857. [Google Scholar] [CrossRef]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.; Berman, D.; Czer, L.S.; Marban, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (caduceus): A prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef]
- Malliaras, K.; Makkar, R.R.; Smith, R.R.; Cheng, K.; Wu, E.; Bonow, R.O.; Marban, L.; Mendizabal, A.; Cingolani, E.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells after myocardial infarction: Evidence of therapeutic regeneration in the final 1-year results of the caduceus trial (cardiosphere-derived autologous stem cells to reverse ventricular dysfunction). J. Am. Coll. Cardiol. 2014, 63, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Bolli, R.; Hare, J.M.; March, K.L.; Pepine, C.J.; Willerson, J.T.; Perin, E.C.; Yang, P.C.; Henry, T.D.; Traverse, J.H.; Mitrani, R.D.; et al. Rationale and design of the concert-hf trial (combination of mesenchymal and c-kit(+) cardiac stem cells as regenerative therapy for heart failure). Circ. Res. 2018, 122, 1703–1715. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauritz, C.; Schwanke, K.; Reppel, M.; Neef, S.; Katsirntaki, K.; Maier, L.S.; Nguemo, F.; Menke, S.; Haustein, M.; Hescheler, J.; et al. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation 2008, 118, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Montserrat, N.; Bahima, E.G.; Batlle, L.; Hafner, S.; Rodrigues, A.M.; Gonzalez, F.; Izpisua Belmonte, J.C. Generation of pig ips cells: A model for cell therapy. J. Cardiovasc. Transl. Res. 2011, 4, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wilson, G.F.; Soerens, A.G.; Koonce, C.H.; Yu, J.; Palecek, S.P.; Thomson, J.A.; Kamp, T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009, 104, e30–41. [Google Scholar] [CrossRef] [PubMed]
- Beqqali, A.; Kloots, J.; Ward-van Oostwaard, D.; Mummery, C.; Passier, R. Genome-wide transcriptional profiling of human embryonic stem cells differentiating to cardiomyocytes. Stem Cells 2006, 24, 1956–1967. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.P.; van den Berg, C.W.; Casini, S.; Braam, S.R.; Mummery, C.L. Pluripotent stem cell models of cardiac disease and their implication for drug discovery and development. Trends Mol. Med. 2011, 17, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Wada, M.; Konishi, K.; Sato, M.; Iwamoto, U.; Sato, Y.; Tachibana, A.; Kikuchi, T.; Iwamiya, T.; Shimizu, T.; et al. Fabrication of mouse embryonic stem cell-derived layered cardiac cell sheets using a bioreactor culture system. PLoS ONE 2012, 7, e52176. [Google Scholar] [CrossRef] [PubMed]
- So, K.H.; Han, Y.J.; Park, H.Y.; Kim, J.G.; Sung, D.J.; Bae, Y.M.; Yang, B.C.; Park, S.B.; Chang, S.K.; Kim, E.Y.; et al. Generation of functional cardiomyocytes from mouse induced pluripotent stem cells. Int. J. Cardiol. 2011, 153, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Gai, H.; Leung, E.L.; Costantino, P.D.; Aguila, J.R.; Nguyen, D.M.; Fink, L.M.; Ward, D.C.; Ma, Y. Generation and characterization of functional cardiomyocytes using induced pluripotent stem cells derived from human fibroblasts. Cell Biol. Int. 2009, 33, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Quattrocelli, M.; Palazzolo, G.; Agnolin, I.; Martino, S.; Bouche, M.; Anastasia, L.; Sampaolesi, M. Synthetic sulfonyl-hydrazone-1 positively regulates cardiomyogenic microrna expression and cardiomyocyte differentiation of induced pluripotent stem cells. J. Cell. Biochem. 2011, 112, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Tan, G.; Manasi; Qiu, S.; Kong, G.; Yong, P.; Koh, C.; Ooi, T.H.; Lim, S.Y.; Wong, P.; et al. One-step derivation of cardiomyocytes and mesenchymal stem cells from human pluripotent stem cells. Stem Cell Res. 2012, 9, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating wnt/beta-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Tulloch, N.L.; Muskheli, V.; Razumova, M.V.; Korte, F.S.; Regnier, M.; Hauch, K.D.; Pabon, L.; Reinecke, H.; Murry, C.E. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ. Res. 2011, 109, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Miyagawa, S.; Miki, K.; Saito, A.; Fukushima, S.; Higuchi, T.; Kawamura, T.; Kuratani, T.; Daimon, T.; Shimizu, T.; et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 2012, 126, S29–S37. [Google Scholar] [CrossRef] [PubMed]
- Ja, K.P.; Miao, Q.; Zhen Tee, N.G.; Lim, S.Y.; Nandihalli, M.; Ramachandra, J.A.; Mehta, A.; Shim, W. Ipsc-derived human cardiac progenitor cells improve ventricular remodelling via angiogenesis and interstitial networking of infarcted myocardium. J. Cell. Mol. Med. 2016, 20, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Robinton, D.A.; Daley, G.Q. The promise of induced pluripotent stem cells in research and therapy. Nature 2012, 481, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abyzov, A.; Mariani, J.; Palejev, D.; Zhang, Y.; Haney, M.S.; Tomasini, L.; Ferrandino, A.F.; Rosenberg Belmaker, L.A.; Szekely, A.; Wilson, M.; et al. Somatic copy number mosaicism in human skin revealed by induced pluripotent stem cells. Nature 2012, 492, 438–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, M.A.; Larson, D.E.; Sun, C.W.; George, D.R.; Ding, L.; Miller, C.A.; Lin, L.; Pawlik, K.M.; Chen, K.; Fan, X.; et al. Background mutations in parental cells account for most of the genetic heterogeneity of induced pluripotent stem cells. Cell Stem Cell 2012, 10, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, Z.N.; Rong, Z.; Xu, Y. Immunogenicity of induced pluripotent stem cells. Nature 2011, 474, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Malliaras, K.; Marban, E. Moving beyond surrogate endpoints in cell therapy trials for heart disease. Stem Cells Transl. Med. 2014, 3, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Garcia, A.A.; Anker, S.D.; Armstrong, P.W.; Calvo, G.; Cleland, J.G.; Cohn, J.N.; Dickstein, K.; Domanski, M.J.; Ekman, I.; et al. Clinical outcome endpoints in heart failure trials: A european society of cardiology heart failure association consensus document. Eur. J. Heart Fail. 2013, 15, 1082–1094. [Google Scholar] [CrossRef] [PubMed]
- Gyongyosi, M.; Wojakowski, W.; Lemarchand, P.; Lunde, K.; Tendera, M.; Bartunek, J.; Marban, E.; Assmus, B.; Henry, T.D.; Traverse, J.H.; et al. Meta-analysis of cell-based cardiac studies (accrue) in patients with acute myocardial infarction based on individual patient data. Circ. Res. 2015, 116, 1346–1360. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, Z.; Sun, Z. The potential and challenges of using stem cells for cardiovascular repair and regeneration. Genes Dis. 2014, 1, 113–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.O.; Han, J.W.; Kim, J.M.; Cho, H.J.; Park, C.; Lee, N.; Kim, D.W.; Yoon, Y.S. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ. Res. 2011, 108, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Ptaszek, L.M.; Mansour, M.; Ruskin, J.N.; Chien, K.R. Towards regenerative therapy for cardiac disease. Lancet 2012, 379, 933–942. [Google Scholar] [CrossRef]
- Sheng, C.C.; Zhou, L.; Hao, J. Current stem cell delivery methods for myocardial repair. BioMed Res. Int. 2013, 2013, 547902. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Youssef, E.A.; Brinton, T.J.; Zhang, P.; Rogers, P.; Price, E.T.; Yeung, A.C.; Johnstone, B.H.; Yock, P.G.; March, K.L. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: Implications for current clinical trials. Circulation 2005, 112, I150–156. [Google Scholar] [PubMed]
- Freyman, T.; Polin, G.; Osman, H.; Crary, J.; Lu, M.M.; Cheng, L.; Palasis, M.; Wilensky, R.L. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur. Heart J. 2006, 27, 1114–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.H.; Guo, Y.; Ou, Q.H.; Chen, N.; Wu, W.J.; Yuan, F.P.; O’Brien, E.; Wang, T.; Luo, L.; Hunt, G.N.; et al. Intracoronary administration of cardiac stem cells in mice: A new, improved technique for cell therapy in murine models. Basic Res. Cardiol. 2011, 106, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.V.; Sasano, T.; Mills, K.; Evers, R.; Lee, S.T.; Smith, R.R.; Lardo, A.C.; Lai, S.H.; Steenbergen, C.; Gerstenblith, G.; et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation 2009, 120, U1075–U1095. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, F.S.; Steinhauser, M.L.; Gannon, J.; Lee, R.T. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell 2011, 8, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Mirotsou, M.; Jayawardena, T.M.; Schmeckpeper, J.; Gnecchi, M.; Dzau, V.J. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J. Mol. Cell. Cardiol. 2011, 50, 280–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Pan, Y.; Li, X.H.; Yang, X.Y.; Feng, Y.L.; Tan, H.H.; Jiang, L.; Feng, J.; Yu, X.Y. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal mir-21 by targeting pdcd4. Cell Death Dis. 2016, 7, e2277. [Google Scholar] [CrossRef] [PubMed]
- Cambier, L.; de Couto, G.; Ibrahim, A.; Echavez, A.K.; Valle, J.; Liu, W.; Kreke, M.; Smith, R.R.; Marban, L.; Marban, E. Y rna fragment in extracellular vesicles confers cardioprotection via modulation of il-10 expression and secretion. EMBO Mol. Med. 2017, 9, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Gallet, R.; Dawkins, J.; Valle, J.; Simsolo, E.; de Couto, G.; Middleton, R.; Tseliou, E.; Luthringer, D.; Kreke, M.; Smith, R.R.; et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 2017, 38, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Tseliou, E.; Fouad, J.; Reich, H.; Slipczuk, L.; de Couto, G.; Aminzadeh, M.; Middleton, R.; Valle, J.; Weixin, L.; Marban, E. Fibroblasts rendered antifibrotic, antiapoptotic, and angiogenic by priming with cardiosphere-derived extracellular membrane vesicles. J. Am. Coll. Cardiol. 2015, 66, 599–611. [Google Scholar] [CrossRef] [PubMed]
| Reference | Clinical Trial | Disease | Delivery Method | Subjects | LVEF Improvement |
|---|---|---|---|---|---|
| Pagani [32] | Phase I | ICM | SI | Treated: 5 | Not shown |
| Siminiak [33] | Phase I | AMI | SI | Treated: 10 | Yes |
| Menasche [37] | MAGIC | CHF | SI | Treated: 97 Control: 30 | Not shown |
| Povsic [34] | MARVEL-I | CHF | SI | Treated: 15 Control: 8 | Not shown |
| Reference | Clinical Trial | Disease | Delivery Method | Subjects | LVEF Improvement |
|---|---|---|---|---|---|
| Menasche [61] | Case Report | HF | FS | 1 | Yes |
| Menasche [62] | Phase I | ICM | FS | Treated: 6 | Not shown |
| Reference | Clinical Trial | Disease | Delivery Method | Subjects | LVEF Improvement |
|---|---|---|---|---|---|
| Hamano [72] | Phase I | ICM | IM | Treated: 5 | Not shown |
| Strauer [70] | Phase I | AMI | IC | Treated: 1 0Control: 10 | Yes |
| Assmus [65] Leistner [73] | TOPCARE-AMI | AMI | IC | Treated: 59 | Yes |
| Wollert [66] | BOOST | AMI | IC | Treated: 3 0Control: 30 | Yes |
| Lunde [74] | ASTAMI | AMI | IC | Treated: 24 Control: 25 | Not shown |
| Schachinger [67] | REPAIR-AMI | AMI | IC | Treated: 101 Control: 103 | Yes |
| Surder [68] Suerder [75] | SWISS-AMI | AMI | IC | Treated: 128 Control: 64 | No |
| Wohrle [76] | SCAMY | AMI | IC | Treated: 29 Control: 13 | No |
| Strauer [77] | STAR-heart | ICM | IC | Treated: 191 Control: 200 | Yes |
| Traverse [69] | The TIME Study | AMI | IC | Treated: 79 Control: 41 | No |
| Reference | Clinical Trial | Disease | Delivery Method | Subjects | LVEF Improvement |
|---|---|---|---|---|---|
| Stamm [78] | Phase I | ICM | IM | Treated: 35 Control: 20 | Yes |
| Tendera [85] | REGENT | AMI | IC | Treated: 16 0Control: 40 | No |
| Povsic [86] | RENEW | RA | IM | Treated: 57 Control: 55 | Not shown |
| Noiseux [87] | IMPACT-CABG | ICM | IM | Treated: 2 0Control: 20 | Not shown |
| Quyyum [88] | PreSERVE-AMI | AMI | IC | Treated: 78 Control: 83 | Yes |
| Reference | Clinical Trial | Disease | Delivery Method | Subjects | LVEF Improvement |
|---|---|---|---|---|---|
| Chen [99] | Phase II | AMI | IC | Treated: 34 Control: 35 | Yes |
| Chen [100] | Phase II | AMI | IC | Treated: 24 Control: 24 | No |
| Hare [101] | Phase I | AMI | IV | Treated: 39 Control: 21 | No |
| Yang [102] | Phase I | AMI | IC | Treated: 16 | No control |
| Hare [93] | POSEIDON | ICM | IM | Treated: 31 | No control |
| Bartunek [96] | C-CURE | ICM | IM | Treated: 32 Control: 15 | Yes |
| Gao [103] | Phase II | AMI | IC | Treated: 21 Control: 22 | No |
| Rodrigo [104] | Phase I | AMI | IM | Treated: 9 Control: 45 | No |
| Karantalis [105] | PROMETHEUS | ICM | IM | Treated: 6 | No control |
| Heldman [94] | TAC-HFT | ICM | IM | Treated: 22 Control: 11 | No |
| Lee [97] | SEED-MSC | AMI | IC | Treated: 33 Control: 36 | Yes |
| Ascheim [106] | Phase II | ICM | IM | Treated: 2 0Control: 10 | No |
| Chullikana [107] | Phase I/II | AMI | IV | Treated: 1 0Control: 10 | No |
| Perin [108] | Phase II | ICM | IM | Treated: 45 Control: 15 | No |
| Mathiasen [98] | MSC-HF | ICM | IM | Treated: 4 0Control: 20 | Yes |
| Guijarro [109] | MESAMI | ICM | IM | Treated: 10 | No control |
| Xiao [110] | - | DC | IC | Treated: 17 Control: 20 | Yes |
| Florea [111] | TRIDENT | ICM | IM | Treated: 30 | No control |
| Butler [112] | Phase II | Non-ICM | IV | Treated: 11 Control: 12 | No |
| Bartunek [113] | CHART-I | ICM | IM | Treated: 12 0Control: 151 | No |
| Ref. | Clinical Trial | Disease | Delivery Method | Subjects | LVEF Improvement |
|---|---|---|---|---|---|
| Houtgraaf [118] | APOLLO | AMI | IC | Treated: 1 0Control: 4 | No |
| Perin [119] | PRECISE | ICM | IM | Treated: 21 Control: 6 | No |
| Henry [121] | ATHENA I | ICM | IM | Treated: 17 Control: 14 | No |
| Kastrup [120] | ATHENA II | ICM | IM | Treated: 10 | No control |
| Qayyum [88] | MyStromalCell | ICM | IM | Treated: 41 Control: 20 | Not shown |
| Reference | Clinical Trial | Disease | Delivery Method | Subjects | LVEF Improvement |
|---|---|---|---|---|---|
| Li [134] | - | ICM | IC | Treated: 15 | No control |
| Musialek [135] | - | AMI | IC | Treated: 10 | No control |
| Fang [133] | - | ICM | IV | Treated: 3 | No control |
| Zhao [138] | - | ICM | IM | Treated: 3 0Control: 29 | Yes |
| Gao [137] | Phase II | AMI | IC | Treated: 58 Control: 58 | Yes |
| Can [139] | HUC-HEART | ICM | IM | Treated: 18 Control: 4 | Not shown |
| Bartolucci [136] | RIMECARD | ICM | IV | Treated: 15 Control: 15 | Yes |
| Reference | Clinical Trial | Disease | Delivery Method | Subjects | LVEF Improvement |
|---|---|---|---|---|---|
| Herreros [143] | Phase 1 | Non-AMI | SI | Treated: 12 | Yes |
| Reference | Clinical Trial | Disease | Delivery Method | Subjects | LVEF Improvement |
|---|---|---|---|---|---|
| Bolli [145] | SCIPIO | ICM | IC | Treated: 16 Control: 5 | Yes |
| Makkar [146] Malliaras [147] | CADUCEUS | AMI | IC | Treated: 17 Control: 8 | Yes |
| Bolli [148] | CONCERT-HF | ICM | IM | Treated: 9 Control: 9 | Not shown |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghiroldi, A.; Piccoli, M.; Cirillo, F.; Monasky, M.M.; Ciconte, G.; Pappone, C.; Anastasia, L. Cell-Based Therapies for Cardiac Regeneration: A Comprehensive Review of Past and Ongoing Strategies. Int. J. Mol. Sci. 2018, 19, 3194. https://doi.org/10.3390/ijms19103194
Ghiroldi A, Piccoli M, Cirillo F, Monasky MM, Ciconte G, Pappone C, Anastasia L. Cell-Based Therapies for Cardiac Regeneration: A Comprehensive Review of Past and Ongoing Strategies. International Journal of Molecular Sciences. 2018; 19(10):3194. https://doi.org/10.3390/ijms19103194
Chicago/Turabian StyleGhiroldi, Andrea, Marco Piccoli, Federica Cirillo, Michelle M. Monasky, Giuseppe Ciconte, Carlo Pappone, and Luigi Anastasia. 2018. "Cell-Based Therapies for Cardiac Regeneration: A Comprehensive Review of Past and Ongoing Strategies" International Journal of Molecular Sciences 19, no. 10: 3194. https://doi.org/10.3390/ijms19103194
APA StyleGhiroldi, A., Piccoli, M., Cirillo, F., Monasky, M. M., Ciconte, G., Pappone, C., & Anastasia, L. (2018). Cell-Based Therapies for Cardiac Regeneration: A Comprehensive Review of Past and Ongoing Strategies. International Journal of Molecular Sciences, 19(10), 3194. https://doi.org/10.3390/ijms19103194