Abstract
Despite considerable improvements in the treatment of cardiovascular diseases, heart failure (HF) still represents one of the leading causes of death worldwide. Poor prognosis is mostly due to the limited regenerative capacity of the adult human heart, which ultimately leads to left ventricular dysfunction. As a consequence, heart transplantation is virtually the only alternative for many patients. Therefore, novel regenerative approaches are extremely needed, and several attempts have been performed to improve HF patients’ clinical conditions by promoting the replacement of the lost cardiomyocytes and by activating cardiac repair. In particular, cell-based therapies have been shown to possess a great potential for cardiac regeneration. Different cell types have been extensively tested in clinical trials, demonstrating consistent safety results. However, heterogeneous efficacy data have been reported, probably because precise end-points still need to be clearly defined. Moreover, the principal mechanism responsible for these beneficial effects seems to be the paracrine release of antiapoptotic and immunomodulatory molecules from the injected cells. This review covers past and state-of-the-art strategies in cell-based heart regeneration, highlighting the advantages, challenges, and limitations of each approach.
1. Introduction
Cardiovascular diseases (CVDs) represent one of the leading causes of death in the world. An estimated 17.7 million people died from CVD each year, corresponding approximately to 31% of all global deaths [1]. Acute myocardial infarction (AMI), a life-threatening condition resulting from a complete interruption of the blood flow to the heart [2], is considered one of the major components of CVDs, including nearly 580,000 new coronary attacks and 210,000 recurrent attacks every year in the United States [3]. Nonetheless, AMI induces a characteristic pattern of ultrastructural, cellular, molecular, and metabolic alterations, which leads to irreversible cardiac damage, ultimately culminating in heart failure [4]. In the past 10 years, despite improvements and innovations in CVDs treatment, the number of patients diagnosed with HF increased by 23% [5], thus representing a challenging issue for both the scientific community and the health care systems. The estimated costs associated with HF management have globally reached 108 billion dollars per year [6]. These statistics provide a perfect picture of the magnitude of the impact of chronic heart diseases (CHDs) on society, and the development of novel therapies is urgently warranted.
In the human heart, adult cardiomyocytes (CMs) are able to divide and exhibit spontaneous turnover [7]. Despite this, heart transplantation still represents the gold standard for the treatment of end-stage HF, because it is characterized by an extremely high late-survival rate and by a significant improvement of the patients’ quality of life [8]. Unfortunately, the limited number of heart donors, which is not sufficient to cover the increasing organ demand, and the mandatory transplant-associated lifelong immunosuppressant therapy reduce the feasibility of this therapeutic approach, which could not be applied on a large scale [9]. Moreover, many HF patients are ineligible for heart transplantation because of concomitant comorbidities, and they remain without possible medical, surgical, or interventional treatment options [10]. These are the main reasons why alternative regenerative approaches are extremely needed, and great effort has been directed accordingly by the scientific community for the past 25 years.
The primary therapeutic target of all these innovative strategies is to reduce the myocardial scar by generating new functional cardiac tissue and activating endogenous cardiac-associated mechanisms of repair. The first approach that was attempted was to replace dead cardiomyocytes by the exogenous administration of progenitor cells. Several different cell sources have been employed, ranging from bone marrow stem cells to resident cardiac stem cells [11,12]. More recently, direct cardiac reprogramming strategies have been proposed to generate cardiomyocytes starting from terminally committed cells (i.e., dermal fibroblasts), in order to overcome the technical limitations associated with stem cells [13,14,15]. Moreover, a direct reprogramming approach could be potentially directly applied in vivo on the scar tissue, reverting the cardiac myofibroblasts into cardiomyocytes and restoring the cardiac functionality [16]. However, while several research groups have already demonstrated its efficacy both in vitro [17,18,19] and in animal models [16,20,21], several critical issues still need to be addressed, including the extremely low conversion rate, the incomplete maturation of the induced-cardiomyocytes, and the safety concerns related to the use of genetic material and viruses to induce the transdifferentiation process. Therefore, to date, no clinical applications nor clinical trials have been developed with direct reprogramming approaches [22]. On the contrary, in the past two decades, almost 100 clinical trials with adult stem cells of different tissue origin have been performed [23]. However, while no major adverse safety issues have been observed with the administration of most adult stem cells, the real efficacy of these approaches is still controversial [24].
Indeed, an unbiased comparison of the trial outcomes is challenging, because of the use of stem cells from different tissue sources, as well as the extreme variability in the protocols and in the patient populations that were chosen [25]. Nonetheless, in this review, we tried to provide an accurate analysis of the different cell-based regenerative strategies that have been proposed and developed, illustrating the pros and cons of each approach, drawing an overall picture of where we are today and where we are heading.
2. Cell Therapy
2.1. Initial Studies with Committed Cells
The first cell therapy attempt (i.e., injection of exogenous cells) for heart regeneration was performed with skeletal myoblasts [26]. This approach was conceived mainly because of the high availability of these cells from autologous sources, their ability to proliferate in vitro and to regenerate skeletal muscle after an injury, and their somewhat high resistance to ischemic insults. Unfortunately, once cells were transplanted into the heart, results were unsuccessful, as they differentiated only into skeletal muscle and not into CMs [27], although some beneficial effects were observed on the infarcted heart of animal models [28,29,30]. Nonetheless, a series of clinical studies using skeletal myoblasts were performed (Table 1) [31,32,33,34]. Early results showed a general amelioration in cardiac function, especially in left ventricular ejection fraction (LVEF), in regional contractility, and in both viability and perfusion of the treated area [35,36], although other clinical studies failed to reproduce these encouraging results [37].

Table 1.
Clinical trials with committed cells (skeletal myoblasts) for cardiac regeneration.
Later, further attempts were made using fetal cardiomyocytes. However, while initial results engrafting fetal CMs in syngeneic recipients seemed promising, as they showed the formation of new myocardium in injured hearts [38,39] and functional improvement after small grafts, long-term studies revealed massive cell death and limited proliferation of the implanted cells, as expected with differentiated cells [40,41]. Therefore, the use of CMs was abandoned, as it was clear that the number of cells needed to regenerate the damaged area had to be incredibly higher than the few cells that survived transplantation. Thus, the scientific community started to look into other cell sources that would be able to proliferate after injection, thus increasing their number, before differentiating [42,43]. Indeed, as described in the next section, stem cells intrinsically possess the ability to self-renew and differentiate into different cell types. For this reason, they have soon become the ideal choice for regenerative heart approaches.
2.2. Stem Cells
As stem cell biology has developed over the past two decades, progenitor cells of different sources have become available for regenerative medicine, including cardiac repair. Indeed, several types of stem cells have been investigated, and several therapeutic approaches attempted, as detailed below.
2.2.1. Embryonic Stem Cells
Embryonic Stem Cells (ESCs) have been considered ideal candidates to produce CMs for cardiac repair due to their pluripotency and self-renewal. However, while they were initially isolated in the mouse [44] and other species [45,46,47], they have been available from human sources only relatively recently, as they were isolated from a human blastocyst and cultured in vitro only in 1998 [48]. Successively, CMs were generated from both mouse and human ESCs using different differentiation protocols. The first described method is based on the generation of embryoid bodies, which are spherical cell aggregates formed via self-aggregation of ESCs [49]. The CMs generated from ESCs expressed specific cardiac genes, such as GATA4, Nkx2.5, troponin I, troponin T, α-myosin heavy chain, ventricular myosin light chain, and connexin 43 and 45, proteins typical of gap junctions [50]. The efficiency, which was initially low, was successively increased through a co-culture protocol of ESCs and the endoderm-like cell line, END-2 [51,52], and, successively, by modulating the environment with growth factors to reproduce the in vivo early stage of embryonic cardiac development [53,54]. The first in vivo experiments conducted on rat [55] and pig [50] models showed that ESC-derived CMs were able to proliferate, to express cardiac markers, and to form pacemaker cells, coupling electrically with resident CMs, also after myocardial infarction [56,57,58]. Actually, in a non-human primate model, it has been demonstrated that an intramyocardial delivery of 1 billion human ESC-derived CMs gave re-muscularization of the infarcted zone, despite an incomplete CMs maturation. However, adverse arrhythmic complications were observed [59]. A successive study indicated that the re-muscularization by ESC-derived CMs was able to restore cardiac function after transplantation, as demonstrated by the increase of the global left ventricular ejection fraction after one and three months [60]. In the meantime, the first clinical trial on a human patient was performed showing an improved cardiac functional outcome after three months from the cardiac-committed ESCs implantation [61]. More recently, a small phase I trial (n = 6) demonstrated the technical feasibility of producing clinical-grade ESC-derived CMs and their medium-term safety (Table 2) [62].

Table 2.
Clinical trials with ESCs for cardiac regeneration.
Overall, while positive results have been reported, the same original concerns about the employment of ESCs on humans are still actual, especially regarding their tumorigenic potential, the immune rejection [63], and the ethical issues on cell isolation from blastocysts.
2.2.2. Adult Stem Cells
Adult stem cells have been isolated from a variety of human tissues. However, their use in cardiac regeneration is limited to the following sources:
● Bone Marrow Stem Cells (BMSCs)
BMSCs are a heterogeneous population of stem cells isolated from the bone marrow that is often divided into two main subtypes, depending on their surface markers: hematopoietic bone marrow stem cells (BM-HSCs) characterized by the expression of CD31, CD34, CD45, and CD133, and mesenchymal bone marrow stem cells (BM-MSCs), which express CD73, CD90, and CD105 [64]. The isolation procedure of the stem cell population from bone marrow is a well-established protocol based on density gradient centrifugation. The resulting product is defined as bone marrow mononuclear cells (BMMNCs), which includes BM-HSCs, BM-MSCs, and committed cells in the various stages of differentiation. BMMNC represent the first stem cell population employed in clinical trials (Table 3), although it has been demonstrated that they do not directly contribute to the formation of the cardiac cell lineage. The TOPCARE-AMI (Transplantation Of Progenitor Cells And Regenerations Enhancement in Acute Myocardial Infarction) trial was one of the first clinical trials with adult stem cells, and it was conducted on 20 randomized patients, receiving an intracoronary infusion of BMMNCs after 4–5 days from an acute myocardial infarction. Results showed a modest increase of the cardiac function after four months of follow-up in addition to the feasibility and safety of the intracoronary infusion of BMMNCs [65]. Many other clinical trials employed BMMNCs, including, for example, the BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial [66], the REPAIR-AMI (Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction) trial [67], the SWISS-AMI (SWiss multicenter Intracoronary Stem cells Study in Acute Myocardial Infarction) trial [68], or the TIME (Timing In Myocardial infarction Evaluation) trial [69]. The majority of the clinical trials with BMMNCs reported a modest improvement of left ventricular function and cardiac perfusion by enhanced microvascularization [65,66,68,69,70]. Even though the cell population employed was namely the same, there were significant differences among the trials, regarding the number of cells implanted, the delivery method or the patients’ population. These differences made difficult a reliable comparison of the trials’ outcome and also the reproducibility. An example of this aspect is represented by the two versions of the BOOST trial. The former trial was conducted in 2004 on infarcted patients (n = 60) who received an infusion of BMMNCs. After six months from the intervention, the treated group showed an increase of 6.7% of the mean global left ventricle ejection fraction, compared to an increase of 0.7% of the control group [66]. However, in the subsequent randomized placebo-controlled, double-blind BOOST-2 trial, they investigated the effects of a low or a high dose of infused cells and the effects of the γ-irradiation, but they failed to reproduce the positive effects observed before [71].

Table 3.
Clinical trials with BMMNCs for cardiac regeneration.
Since BMMNCs include not only stem cells, but also committed cells, several groups conducted clinical trials with a purified population of bone marrow stem cells. In particular, HSCs (CD34+ and/or CD133+), representing the most abundant stem cell population in BMMNCs (2–4%), were the first purified stem cell population to be used in clinical trials (Table 4). Initially, positive results were obtained by Stamm et al. [78], who described an increase in LVEF and cardiac perfusion six months after transplant. However, in a second study with a more accurate experimental design, they did not reproduce the same results [79]. In this regard, it was demonstrated that HSCs are not able to differentiate into cardiomyocytes once implanted into the heart [80], and that the observed beneficial effects on patients were a consequence of their angiogenic [72,81,82], rather than their differentiation, capacity. Moreover, results from other clinical trials did not show any improvement in cardiac function [69,75,83], highlighting the poor reproducibility of this method, probably due to the different strategies of cell purification, expansion, and concentration [84].

Table 4.
Clinical trials with BM-HSCs for cardiac regeneration.
Considering the issues in HSCs manipulation, many groups focused their attention on BM-MSCs, which were easier to use and standardize due to their capacity to be cultured and expanded with well-defined procedures [89], including the potential to differentiate into a variety of adult cell types. Indeed, promising results, characterized by an amelioration of cardiac function and a reduction of the infarct size, were obtained on rodent and swine models [90,91,92]. For these reasons, more than 20 clinical trials were conducted with BM-MSCs (Table 5). In general, the results showed improvements of the cardiac function, demonstrated by an increase of cardiac perfusion or reduction of the infarcted area, accompanied by signs of angiogenesis and reduced fibrosis and scar formation [93,94,95]. However, only a few of these clinical trials presented a well-organized and detailed experimental design and described a measurable improvement of the cardiac function with specific indicators, such as the comparison of the LVEF to a correct control group. Most of these trials observed the beneficial effects at 6 or 12 months of follow-up, while there were no differences between treated and untreated patients at a longer follow-up. However, the enormous effort spent in these trials strongly increased the knowledge of BM-MSCs biology and stem cells therapy. For example, the POSEIDON (PercutaneOus StEm cell Injection Delivery effect On Neomyogenesis) trial demonstrated that BM-MSCs could be used for allogenic transplant without severe immunological response [93], and that to obtain at least little improvements in the clinical outcome, it was necessary to infuse at least 70 million cells, as demonstrated by the SEED-MSC (SafEty and Efficacy of aDult Mesenchymal Stem Cells), C-CURE Cardiopoietic stem Cell therapy in heart failURE), and MSC-HF (Mesenchymal Stem Cells for Heart Failure) trials [96,97,98].

Table 5.
Clinical trials with BM-MSCs for cardiac regeneration
● Adipose-Derived MSCs (ADSCs)
ADSCs are mesenchymal stem cells derived from adipose tissue, are more abundant than BMSCs, and have been demonstrated to possess a higher capacity to form colonies than BM-MSCs. Also, they have shown greater expansion potential and more resistance to senescence following the culture passages [114]. On the other hand, the surgical procedure for the isolation of the adipose tissue presents a certain risk of organ injury, sepsis, and pulmonary embolism [115]. The first experiment of ADSCs cardiac infusion on a rat model of chronic myocardial infarction improved the LVEF, preventing wall thinning [116], reducing fibrosis, and promoting angiogenesis [117]. On the contrary, clinical trials in humans showed only modest beneficial effects on cardiac function and on myocardial perfusion [88,118,119,120,121] (Table 6). In particular, a reduction of the infarct size was observed in the APOLLO trial [118] and improvement of the exercise tolerance was described in the ATHENA I/II trial [121]. However, no one revealed a significant improvement in the left ventricle function. Thus, it is still unclear as to what mechanisms are responsible for the effects mediated by ADSCs, also because there is contradictory evidence on their ability to differentiate into cardiomyocytes [122,123]. Most likely, ADSCs exert paracrine effects promoting angiogenesis [124] and secrete various cytokines important for tissue regeneration [125,126].

Table 6.
Clinical trials with ADSCs for cardiac regeneration.
● Umbilical Cord-Derived MSCs (UC-MSCs)
In the last few years, great interest has been directed to stem cells isolated from a different part of the umbilical cord because of their high capacity of self-renewal and their reduced potential of forming teratomas [127]. Moreover, their isolation is a simple enzymatic digestion protocol performed on medical waste, thus avoiding invasive biopsy or ethical concerns. Among them, cells isolated from the Wharton’s jelly have properties of both ESCs and adult stem cells [128]. Preclinical studies with human cells showed an increase in cardiac vascularization and attenuated remodeling in rat models of myocardial infarction [129,130,131]. Moreover, an increase in cardiac function was also observed in a swine model [132]. The observed improvements were likely the results of a paracrine effect, rather than the differentiation in new cardiomyocytes, as demonstrated by the increase of angiogenesis, the recruitment of endogenous cardiogenic cells, and by the decrease of apoptosis and fibrosis [129,130,131]. Based on these encouraging results, the use of umbilical cord-derived stem cells was also tested in humans to develop a possible new clinical application for cardiac regeneration. The main scope of these trials was first the safety of the allogeneic transplant of the UC-MSCs: interestingly, no severe adverse effects were observed [133,134,135]. Then, four clinical trials included a control group in order to assess a potential therapeutic effect of the UC-MSCs (Table 7). All these trials reported positive results in terms of LVEF in treated patients as compared to controls at different time points (from 6 to 18 months). Moreover, increases in both exercise tolerance and quality of life were observed [136,137,138]. These preliminary results, although promising, need to be confirmed in larger and more organized clinical trials.

Table 7.
Clinical trials with UC-MSCs for cardiac regeneration.
● Skeletal Muscle-Derived Stem Cells (Satellite Cells)
Satellite cells are the resident stem cells of the skeletal muscle. These mononucleated myogenic cells proliferate during postnatal growth and their number declines during aging. In the adult skeletal muscle, satellite cells remain quiescent under the basal lamina of the muscle fiber, but they result separated from the fiber itself, and they activate after injury in order to repair muscle damage [140]. In the early 1990s, satellite cells were the first adult stem cell population that were tested for cardiac regeneration in animal models of myocardial injury [141,142]. In particular, the effects of the cardiac injection of canine satellite cells have been evaluated in terms of cells engraftment and differentiation into cardiac-like muscle cells. Histological results confirmed that the cardiac environment was sufficient to induce satellite cells differentiation. However, no functional evaluation has been performed in order to corroborate the actual cardiac recovery after satellite cells transplantation.
Ten years later, a phase I clinical trial has been conducted to examine the feasibility and safety of the intramyocardial transplantation of autologous skeletal muscle-derived satellite cells in patients with non-acute myocardial infarction. Treatment was performed on twelve patients by injecting (100–400) × 106 cells previously expanded in vitro. Results confirmed that the procedure was safe and feasible. Moreover, the satellite cells injection was able to improve the LVEF and the viability of the damaged cardiac tissue (Table 8) [143].

Table 8.
Clinical trial with satellite cells for cardiac regeneration.
Despite these encouraging results, several critical issues, such as the in vitro expansion procedure and the disability of the differentiated cells to contract simultaneously with the resident cardiac tissue, limited the use of satellite cells for cardiac regeneration strategies [144].
● Cardiac Stem Cells (CSCs)
The ability of regenerating the heart of some animals, including zebrafish [143] and postnatal mice [144], together with some reports of a cell turnover of cardiac cellular components [7], suggested the idea that a population of resident stem cells could be present also in the heart. Indeed, a series of different stem cell populations have been described in mammals, including humans: c-kit positive cells (c-kit+) [145], sca-1 positive cells (sca-1+) [146], cardiosphere-derived cells (CDCs) [147], side population cells [148], isl1 positive cells (isl-1+) [149], and cardiac atrial appendage stem cells (CASCs) [150]. Actually, c-kit+ cells were the first putative CSCs population identified in the heart, which showed promising results after transplantation, attenuating ventricular remodeling and improving cardiac function in mouse and rat models [151,152]. For these reasons, c-kit+ cells were used in the randomized clinical trial SCIPIO (Stem Cell Infusion in Patients with Ischemic cardiomyopathy), demonstrating a significant increase in left ventricular ejection fraction after one year of follow up in patients who received c-kit+ cells injection [153]. However, many independent groups failed to reproduce these positive results, using c-kit+ cells, raising several still-unanswered questions on the real contribution of c-kit+ cells in cardiac regeneration and cardiac function recovery [154,155,156]. Despite this aspect, another clinical trial with c-kit+ cells is ongoing: indeed, the phase II CONCERT-HF (Combination of Mesenchymal and c-kit+ Cardiac Stem Cells as Regenerative Therapy for Heart Failure) trial aims to investigate the potential therapeutic effects of the infusion of c-kit+ cells, BM-MSCs or the combination of the two populations on patients with myocardial injury [157]. Another cardiac progenitor cell population has been identified using the stem cell antigen-1 (Sca-1), which is a surface marker of somatic and hematopoietic stem cells. Sca-1+ cells were able to differentiate in beating cardiomyocytes when treated with 5-azacytidine [146] and were shown to reduce ventricular remodeling after transplantation, improving cardiac function by the induction of angiogenesis [146,158].
A third type of cardiac progenitors are the cardiosphere-derived cells (CDCs), which have been shown to be clonogenic and have multilineage potential, and that can be safely delivered via intracoronary injections. They have been shown to mediate scar reduction after myocardial infarction, to increase the viable myocardium, and to contribute to the improvement of cardiac function in preclinical models [147]. Recently, CDCs have been used in a clinical trial, CADUCEUS (CArdiosphere-Derived aUtologous stem Cells to reverse ventricular dysfunction), confirming their ability to promote cardiac regeneration, reducing the scar size and thickening the wall of the infarcted zone [159,160,161] (Table 9).

Table 9.
Clinical trials with CSCs for cardiac regeneration.
Finally, cardiac atrial appendage stem cells (CASCs) are the most recent cardiac stem cell population isolated. These cells are characterized by high activity of the aldehyde dehydrogenase, and they have shown better cardiomyogenic potential than other CSCs [150]. Preliminary results on a myocardial infarction model in minipig indicated that CASCs could preserve the cardiac function through the cardiomyogenic differentiation of implanted cells [162].
Overall, while all these results with CSCs seem quite promising, the real regenerative potential and, truly, even the existence of some types of CSCs remains controversial, as the reproducibility of some results reported in the literature is under investigation [156]. Moreover, it seems very unlikely that so many different stem cell populations reside inside the cardiac tissue, as compared to other organs, especially considering the poor intrinsic regenerative capacity of the heart itself. At this stage, further unbiased comparative studies are mandatory to fully understand the role of CSCs in ameliorating cardiac function after acute or chronic injuries, analyzing the mechanisms responsible for the reported, yet somehow difficult to be reproduced, beneficial effects.
2.2.3. Induced-Pluripotent Stem Cells (iPSCs)
In 2006, Yamanaka and colleagues developed a method for the generation of ESC-like cells from mouse fibroblasts by the transduction of defined transcription factors (i.e., Oct-3/4, Sox2, Klf4 and c-Myc) through lentiviral vectors [149]. The next year, the same method was used on human fibroblasts to generate human induced pluripotent stem cells (iPSCs) [150], placing a milestone in the field of stem cell biology. These cells, similarly to ESCs, can differentiate into derivatives of all three germ layers both in vitro and in vivo and can form teratomas when implanted in a nude mouse. The differentiation potential of iPSCs has been extensively employed to generate CMs, although the efficiency of the differentiation process was lower than with ESCs [151,152,153]. However, CMs generated from iPSCs showed a pattern of cardiac gene expressions similar to that of hESC-derived CMs, including the expression of Nkx2.5, troponin T, α-myosin heavy chain, α-actinin, ANF, myosin light chain 2 ventricular isoform (MLC2v), and myosin light chain 2 atrial isoform (MLC2a). Moreover, they exhibit spontaneous contractions, fetal-like ion channel patterns [154], and electrophysiological signals [155]. In the past decade, a series of alternative protocols have been developed to improve CMs generation, including the use of bioreactors [156], the application of a two-medium combination culture protocol [157], the implementation of chemical compounds [158,159,160], and the reproduction of the embryonic heart development by modulating the Wnt/β-catenin pathway [161]. At the same time, iPSCs also showed a great potential for clinical use, as demonstrated in several animal models. For example, human iPSCs-derived CMs, cultured on collagen I patches, were able to form grafts with contractile function in adult rat heart [162]. Moreover, iPSCs restored cardiac function and improved left ventricular remodeling in porcine and mice models of myocardial infarction [163], promoting angiogenesis and interstitial networking [164]. To the best of our knowledge, to date, no iPSCs or iPSC-derived CMs transplantation have been performed on humans, as their use in the clinical practice is still premature and unsafe. Although there is little doubt about the regenerative potential of iPSCs, there are still several important limitations that need to be addressed. For example, the efficiency of the reprogramming process is still quite low, and it greatly (and negatively) influences the time necessary to generate a sufficient number of CMs for any possible clinical application (generation of 100–1000 CMs takes at least six months [165]). Moreover, the delivery of the transcription factors used to reprogram the cells was obtained by retroviruses or lentiviruses infections, and this is known to possibly generate genetic mutations or the de novo copy number variations inside the cells genome [166,167]. Finally, iPSCs could have immunogenic properties that could be responsible for adverse immunorejection after cell transplantation [168]. It is clear that a more accurate evaluation of the genetic and epigenetic modifications, as well as the determination of the actual immunogenicity of iPSCs are all necessary before any possible use of these cells in therapeutic strategies.
3. Considerations on Cell Therapy
More than 20 years have passed since the first experiment on cell transplantation for cardiac regeneration [142] was performed (Table 1). Since then, various clinical trials have been conceived and started. However, it is difficult to draw conclusions, mainly because most trials are still in the early phases (I and II), and they can only offer limited, yet essential, information about safety, but only little insight about the efficacy of the approaches. In fact, they still lack the crucial phase III, which is the one step that can tell us about the efficacy of the therapy. Indeed, most of the endpoints selected for the ongoing clinical trials evaluation often have a low therapeutic impact and are not validated and accepted surrogates for the clinical outcome by the major regulatory agencies, such as the U.S. Food and Drug Administration [169]. Indeed, left ventricular ejection fraction, maximum oxygen consumption, brain natriuretic peptide, or myocardial perfusion are not considered hard endpoints, whereas, on the contrary, primary endpoint of phase III clinical trials should reflect clinically relevant effects, such as mortality, readmission, reintervention, defibrillator event, left ventricular assist device placement, or recurrent heart infarct symptoms [170]. Moreover, the majority of cardiac cell-based trials enrolled patients with acute or convalescent MI, who received prompt and optimal percutaneous reperfusion therapies to preserve cardiac function, leaving little space for any consistent improvement. This patient population has low mortality and morbidity, even without any adjunctive cell therapy [169]. A more detailed and accurate process for patients’ selection based on risk stratification would be helpful to determine the responsiveness to the therapy. Therefore, it seems clear that more appropriately targeted trials, in patients with more severe cardiac dysfunction, are needed to establish the real efficacy of cardiac cell therapy as an effective approach for cardiac regeneration and functional repair. In particular, there is a need for their standardization in order to allow a useful comparison of the results and to increase their reproducibility [171].
Beyond these concerns about the selection of the endpoints and patient population, there are also technical limitations for the procedures that must be faced [172]. For instance, it is important to define the maturation status of the engrafted cells, because while it has been demonstrated that adult mature CMs do not survive transplantation [39], partially differentiated cells, such as those derived from ESCs or MSCs, can cause arrhythmias [59] or tumor formation [173], negatively influencing the outcome of the intervention. Moreover, and possibly most importantly, there is increasing evidence that most of the injected cells do not survive after transplantation, because they are either immunologically rejected, they do not find a proper microenvironment, they are trapped in pulmonary vasculature, or they diffuse throughout the entire body with the circulatory system [174]. To partially circumvent these difficulties, different routes of cell administration have been tested [175], including surgical intramyocardial injection, catheter-based intramyocardial administration, trans-endocardial injection, trans-coronary venous injection, intravenous infusion, intracoronary artery administration, retrograde coronary venous delivery system, and engineered monolayer tissue transplantation. Overall, each different protocol showed both advantages and disadvantages, and there are no real unbiased and comprehensive comparative studies, suggesting that it is almost impossible to define a standard protocol for cell administration [176]. For example, intramyocardial delivery resulted in better cardiac cell retention and allowed the delivery of a high number of cells. However, this technique is very invasive, and the cell distribution might be even too localized. In contrast, intracoronary delivery is simpler than intramyocardial injection, and it enables a homogeneous distribution inside large myocardial regions. However, cell retention is low and the number of cells that can be delivered by each infusion is limited [177,178,179]. Moreover, it is unclear whether the delivered cells can couple and contract with existing cardiac tissue. In fact, the improvement of cardiac function observed in the clinical trials could be related to other aspects, including paracrine effects due to the secretion of soluble factors by transplanted cells that eventually induce different processes, such as myocardial protection, the activation or amplification of endogenous repair processes, neovascularization, and cardiac remodeling [180,181]. This hypothesis was confirmed by a series of studies, both in vitro and in vivo, on the effects of stem cell exosomes’ treatment on cell protection and cardiac regeneration. The results showed that exosomes increased the apoptosis resistance of cultured cardiomyocytes (Xiao et al., 2016), and, moreover, it augmented the cardiac function of infarcted hearts in animal models [182,183,184,185]. In particular, they induced angiogenesis and cardiomyocyte survival, reducing fibrosis and the left ventricle remodeling. The promising results suggest that exosomes could represent the first step for a cell-free therapy for cardiovascular disease, which would be more advantageous than cell therapy, considering different aspects, such as the costs, the standardization of the therapy, the scale-up of the production process and the possibility to engineer ad hoc.
In conclusion, cell therapies for cardiac regeneration could be valid approaches that still need more accurate and extensive studies to determine and improve their feasibility and efficacy. The long-term therapeutic effects have yet to be monitored in order to determine whether cell therapy has the potential to increase lifespan, decrease mortality, and to improve patients’ quality of life. Moreover, as there are some safety issues, especially with the use of more undifferentiated progenitors, a careful evaluation of the potential side effects of cardiac cell therapy has to be conducted before any clinical application can be foreseen.
4. Conclusions
Identifying new therapies for heart diseases, which still represent the primary cause of death in the Western world, is a difficult challenge for modern medicine. As described in this review, in the last two decades, an impressive number of cell-based clinical trials have been conducted with the same overall objective of replacing dead (or damaged) cardiomyocytes with functional ones and restoring the cardiac functionality (Figure 1). However, while the pre-clinical results on animal models generated high expectations, human trials gave very controversial results. In fact, the extremely heterogeneous experimental conditions of the trials, including differences in the cell types used, in the doses and the timings of intervention, in the delivery strategies, in the patients’ selection, and in the time points evaluated, gave rise to often contradictory results. Furthermore, to date, even the most favorable approaches showed only modest long-term outcomes. However, these studies and clinical trials enabled the discovery of critical signaling pathways and transcription-factor networks involved in heart development and regeneration, including cardiac stem cell differentiation. Moreover, it is now unequivocally demonstrated that: (a) cell therapy for cardiac regeneration is feasible and generally safe; (b) the exogenous delivery of adult stem cells suffers from poor engraftment and retention in the heart; and (c) the positive effects of stem cell approaches for cardiac regeneration are mainly mediated by paracrine effects. Therefore, while new cell-based approaches are still under development, many argue that the delivery of regenerating factors, including small molecules and recombinant proteins, might be the winning strategy. Clearly, future trials will need to be closely monitored using more standardized and reliable analytical tools to evaluate the outcomes in order to avoid the previous chaotic generation of unreproducible data, keeping in mind that the number of cardiac diseases are increasingly growing, and patients are in urgent need of novel therapies.
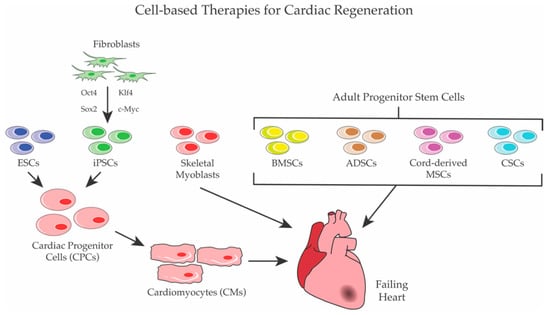
Figure 1.
Cell-based Therapies for cardiac regeneration. Several populations of stem cells were used for cardiac regeneration. ESCs and iPSCs were first differentiated to CPCs and/or to CMs before transplantation, whereas skeletal myoblasts and adult stem cells—such as BMSCs, ADSCs, cord-derived MSCs, and CSCs—were directly employed to regenerate heart.
Author Contributions
All authors made substantial contributions to this work. Acquisition and interpretation of data by online search, writing–original draft and preparing figures/tables: A.G., M.P., and F.C. Revision of the work and English language editing (native speaker): M.M.M. and G.C. Final version approval and supervision of the work: C.P. and L.A.
Funding
This study was partially supported by Ricerca Corrente funding from Italian Ministry of Health to IRCCS Policlinico San Donato.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization: Annual Report on Cardiovascular Diseases. 2017. Available online: http://www.webcitation.org/72fSeoOIs (accessed on 4 September 2018).
- Reimer, K.A.; Ideker, R.E. Myocardial ischemia and infarction: Anatomic and biochemical substrates for ischemic cell death and ventricular arrhythmias. Hum. Pathol. 1987, 18, 462–475. [Google Scholar] [CrossRef]
- Writing Group Member; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; et al. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef] [PubMed]
- Buja, L.M. Myocardial ischemia and reperfusion injury. Cardiovasc. Pathol. 2005, 14, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef]
- Lesyuk, W.; Kriza, C.; Kolominsky-Rabas, P. Cost-of-illness studies in heart failure: A systematic review 2004–2016. BMC Cardiovasc. Disord. 2018, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabe-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.J. Long-term outcome following heart transplantation: Current perspective. J. Thorac. Dis. 2015, 7, 549–551. [Google Scholar] [PubMed]
- Stehlik, J.; Edwards, L.B.; Kucheryavaya, A.Y.; Benden, C.; Christie, J.D.; Dobbels, F.; Kirk, R.; Rahmel, A.O.; Hertz, M.I. The registry of the international society for heart and lung transplantation: Twenty-eighth adult heart transplant report—2011. J. Heart Lung Transplant. 2011, 30, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, M.J.; Boersma, E.; Reimer, W.J.; Balk, A.H.; Komajda, M.; Swedberg, K.; Follath, F.; Jimenez-Navarro, M.; Simoons, M.L.; Cleland, J.G. Under-utilization of evidence-based drug treatment in patients with heart failure is only partially explained by dissimilarity to patients enrolled in landmark trials: A report from the euro heart survey on heart failure. Eur. Heart J. 2005, 26, 2706–2713. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P.; Vanneaux, V. Stem cells for the treatment of heart failure. Curr. Res. Transl. Med. 2016, 64, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Ito, H.; Sano, S. Challenges to success in heart failure: Cardiac cell therapies in patients with heart diseases. J. Cardiol. 2016, 68, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Anastasia, L.; Piccoli, M.; Garatti, A.; Conforti, E.; Scaringi, R.; Bergante, S.; Castelvecchio, S.; Venerando, B.; Menicanti, L.; Tettamanti, G. Cell reprogramming: A new chemical approach to stem cell biology and tissue regeneration. Curr. Pharm. Biotechnol. 2011, 12, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Plonowska, K.; Wu, S.M. Somatic cell reprogramming into cardiovascular lineages. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, M.; Cirillo, F.; Tettamanti, G.; Anastasia, L. A chemical approach to myocardial protection and regeneration. Eur. Heart J. 2016, 18, E1–E7. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Huang, Y.; Spencer, C.I.; Foley, A.; Vedantham, V.; Liu, L.; Conway, S.J.; Fu, J.D.; Srivastava, D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 2012, 485, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Islas, J.F.; Liu, Y.; Weng, K.C.; Robertson, M.J.; Zhang, S.; Prejusa, A.; Harger, J.; Tikhomirova, D.; Chopra, M.; Iyer, D.; et al. Transcription factors ets2 and mesp1 transdifferentiate human dermal fibroblasts into cardiac progenitors. Proc. Natl. Acad. Sci. USA 2012, 109, 13016–13021. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, N.; Yamakawa, H.; Miyamoto, K.; Sadahiro, T.; Umei, T.; Isomi, M.; Nakashima, H.; Akiyama, M.; Wada, R.; Inagawa, K.; et al. Mir-133 promotes cardiac reprogramming by directly repressing snai1 and silencing fibroblast signatures. EMBO J. 2014, 33, 1565–1581. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.J.; Lubczyk, C.; Bhakta, M.; Zang, T.; Fernandez-Perez, A.; McAnally, J.; Bassel-Duby, R.; Olson, E.N.; Munshi, N.V. Induction of diverse cardiac cell types by reprogramming fibroblasts with cardiac transcription factors. Development 2014, 141, 4267–4278. [Google Scholar] [CrossRef] [PubMed]
- Mathison, M.; Gersch, R.P.; Nasser, A.; Lilo, S.; Korman, M.; Fourman, M.; Hackett, N.; Shroyer, K.; Yang, J.; Ma, Y.; et al. In vivo cardiac cellular reprogramming efficacy is enhanced by angiogenic preconditioning of the infarcted myocardium with vascular endothelial growth factor. J. Am. Heart Assoc. 2012, 1, e005652. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.M.; Finch, E.A.; Zhang, L.; Zhang, H.; Hodgkinson, C.P.; Pratt, R.E.; Rosenberg, P.B.; Mirotsou, M.; Dzau, V.J. Microrna induced cardiac reprogramming in vivo: Evidence for mature cardiac myocytes and improved cardiac function. Circ. Res. 2015, 116, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Ghiroldi, A.; Piccoli, M.; Ciconte, G.; Pappone, C.; Anastasia, L. Regenerating the human heart: Direct reprogramming strategies and their current limitations. Basic Res. Cardiol. 2017, 112, 68. [Google Scholar] [CrossRef] [PubMed]
- Stem Cell-Based Clinical Trials for Heart Failure 2018. Available online: http://www.webcitation.org/72fVc36rP (accessed on 14 September 2018).
- Gyongyosi, M.; Haller, P.M.; Blake, D.J.; Martin Rendon, E. Meta-analysis of cell therapy studies in heart failure and acute myocardial infarction. Circ. Res. 2018, 123, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P. Cell therapy trials for heart regeneration—Lessons learned and future directions. Nat. Rev. Cardiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Koh, G.Y.; Klug, M.G.; Soonpaa, M.H.; Field, L.J. Differentiation and long-term survival of c2c12 myoblast grafts in heart. J. Clin. Investig. 1993, 92, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, H.; Poppa, V.; Murry, C.E. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J. Mol. Cell. Cardiol. 2002, 34, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; DerSimonian, H.; Brenner, D.A.; Ngoy, S.; Teller, P.; Edge, A.S.; Zawadzka, A.; Wetzel, K.; Sawyer, D.B.; Colucci, W.S.; et al. Cell therapy attenuates deleterious ventricular remodeling and improves cardiac performance after myocardial infarction. Circulation 2001, 103, 1920–1927. [Google Scholar] [CrossRef] [PubMed]
- Scorsin, M.; Hagege, A.; Vilquin, J.T.; Fiszman, M.; Marotte, F.; Samuel, J.L.; Rappaport, L.; Schwartz, K.; Menasche, P. Comparison of the effects of fetal cardiomyocyte and skeletal myoblast transplantation on postinfarction left ventricular function. J. Thorac. Cardiovasc. Surg. 2000, 119, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.A.; Atkins, B.Z.; Hungspreugs, P.; Jones, T.R.; Reedy, M.C.; Hutcheson, K.A.; Glower, D.D.; Kraus, W.E. Regenerating functional myocardium: Improved performance after skeletal myoblast transplantation. Nat. Med. 1998, 4, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P.; Hagege, A.A.; Scorsin, M.; Pouzet, B.; Desnos, M.; Duboc, D.; Schwartz, K.; Vilquin, J.T.; Marolleau, J.P. Myoblast transplantation for heart failure. Lancet 2001, 357, 279–280. [Google Scholar] [CrossRef]
- Pagani, F.D.; DerSimonian, H.; Zawadzka, A.; Wetzel, K.; Edge, A.S.; Jacoby, D.B.; Dinsmore, J.H.; Wright, S.; Aretz, T.H.; Eisen, H.J.; et al. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J. Am. Coll. Cardiol. 2003, 41, 879–888. [Google Scholar] [CrossRef]
- Siminiak, T.; Kalawski, R.; Fiszer, D.; Jerzykowska, O.; Rzezniczak, J.; Rozwadowska, N.; Kurpisz, M. Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: Phase i clinical study with 12 months of follow-up. Am. Heart J. 2004, 148, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Povsic, T.J.; O’Connor, C.M.; Henry, T.; Taussig, A.; Kereiakes, D.J.; Fortuin, F.D.; Niederman, A.; Schatz, R.; Spencer, R.t.; Owens, D.; et al. A double-blind, randomized, controlled, multicenter study to assess the safety and cardiovascular effects of skeletal myoblast implantation by catheter delivery in patients with chronic heart failure after myocardial infarction. Am. Heart J. 2011, 162, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Gavira, J.J.; Herreros, J.; Perez, A.; Garcia-Velloso, M.J.; Barba, J.; Martin-Herrero, F.; Canizo, C.; Martin-Arnau, A.; Marti-Climent, J.M.; Hernandez, M.; et al. Autologous skeletal myoblast transplantation in patients with nonacute myocardial infarction: 1-year follow-up. J. Thorac. Cardiovasc. Surg. 2006, 131, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Hagege, A.A.; Marolleau, J.P.; Vilquin, J.T.; Alheritiere, A.; Peyrard, S.; Duboc, D.; Abergel, E.; Messas, E.; Mousseaux, E.; Schwartz, K.; et al. Skeletal myoblast transplantation in ischemic heart failure: Long-term follow-up of the first phase i cohort of patients. Circulation 2006, 114, I108–I113. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P.; Alfieri, O.; Janssens, S.; McKenna, W.; Reichenspurner, H.; Trinquart, L.; Vilquin, J.T.; Marolleau, J.P.; Seymour, B.; Larghero, J.; et al. The myoblast autologous grafting in ischemic cardiomyopathy (magic) trial: First randomized placebo-controlled study of myoblast transplantation. Circulation 2008, 117, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Li, R.K.; Jia, Z.Q.; Weisel, R.D.; Mickle, D.A.; Zhang, J.; Mohabeer, M.K.; Rao, V.; Ivanov, J. Cardiomyocyte transplantation improves heart function. Ann. Thorac. Surg. 1996, 62, 654–660, discussion 660–651. [Google Scholar] [CrossRef]
- Reinecke, H.; Zhang, M.; Bartosek, T.; Murry, C.E. Survival, integration, and differentiation of cardiomyocyte grafts: A study in normal and injured rat hearts. Circulation 1999, 100, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Huwer, H.; Winning, J.; Vollmar, B.; Welter, C.; Lohbach, C.; Menger, M.D.; Schafers, H.J. Long-term cell survival and hemodynamic improvements after neonatal cardiomyocyte and satellite cell transplantation into healed myocardial cryoinfarcted lesions in rats. Cell Transplant. 2003, 12, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Muller-Ehmsen, J.; Peterson, K.L.; Kedes, L.; Whittaker, P.; Dow, J.S.; Long, T.I.; Laird, P.W.; Kloner, R.A. Rebuilding a damaged heart: Long-term survival of transplanted neonatal rat cardiomyocytes after myocardial infarction and effect on cardiac function. Circulation 2002, 105, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Muller-Ehmsen, J.; Whittaker, P.; Kloner, R.A.; Dow, J.S.; Sakoda, T.; Long, T.I.; Laird, P.W.; Kedes, L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J. Mol. Cell. Cardiol. 2002, 34, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Methot, D.; Poppa, V.; Fujio, Y.; Walsh, K.; Murry, C.E. Cardiomyocyte grafting for cardiac repair: Graft cell death and anti-death strategies. J. Mol. Cell. Cardiol. 2001, 33, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638. [Google Scholar] [CrossRef] [PubMed]
- Graves, K.H.; Moreadith, R.W. Derivation and characterization of putative pluripotential embryonic stem cells from preimplantation rabbit embryos. Mol. Reprod. Dev. 1993, 36, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, P.M.; Taborn, G.U.; Garton, R.L.; Caplice, M.D.; Brenin, D.R. Pluripotent embryonic stem cells from the rat are capable of producing chimeras. Dev. Biol. 1994, 163, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Kalishman, J.; Golos, T.G.; Durning, M.; Harris, C.P.; Becker, R.A.; Hearn, J.P. Isolation of a primate embryonic stem cell line. Proc. Natl. Acad. Sci. USA 1995, 92, 7844–7848. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Kehat, I.; Kenyagin-Karsenti, D.; Snir, M.; Segev, H.; Amit, M.; Gepstein, A.; Livne, E.; Binah, O.; Itskovitz-Eldor, J.; Gepstein, L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Investig. 2001, 108, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Kehat, I.; Khimovich, L.; Caspi, O.; Gepstein, A.; Shofti, R.; Arbel, G.; Huber, I.; Satin, J.; Itskovitz-Eldor, J.; Gepstein, L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat. Biotechnol. 2004, 22, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Mummery, C.; Ward-van Oostwaard, D.; Doevendans, P.; Spijker, R.; van den Brink, S.; Hassink, R.; van der Heyden, M.; Opthof, T.; Pera, M.; de la Riviere, A.B.; et al. Differentiation of human embryonic stem cells to cardiomyocytes: Role of coculture with visceral endoderm-like cells. Circulation 2003, 107, 2733–2740. [Google Scholar] [CrossRef] [PubMed]
- Passier, R.; Oostwaard, D.W.; Snapper, J.; Kloots, J.; Hassink, R.J.; Kuijk, E.; Roelen, B.; de la Riviere, A.B.; Mummery, C. Increased cardiomyocyte differentiation from human embryonic stem cells in serum-free cultures. Stem Cells 2005, 23, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Kattman, S.J.; Adler, E.D.; Keller, G.M. Specification of multipotential cardiovascular progenitor cell during embryonic stem cell differentiation and embryonic development. Trends Cardiovas. Med. 2007, 17, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Kattman, S.J.; Witty, A.D.; Gagliardi, M.; Dubois, N.C.; Niapour, M.; Hotta, A.; Ellis, J.; Keller, G. Stage-specific optimization of activin/nodal and bmp signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 2011, 8, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, M.A.; Gold, J.; Xu, C.H.; Hassanipour, M.; Rosler, E.; Police, S.; Muskheli, V.; Murry, C.E. Formation of human myocardium in the rat heart from human embryonic stem cells. Am. J. Pathol. 2005, 167, 663–671. [Google Scholar] [CrossRef]
- Kolossov, E.; Bostani, T.; Roell, W.; Breitbach, M.; Pillekamp, F.; Nygren, J.M.; Sasse, P.; Rubenchik, O.; Fries, J.W.U.; Wenzel, D.; et al. Engraftment of engineered es cell-derived cardiomyocytes but not bm cells restores contractile function to the infarcted myocardium. J. Exp. Med. 2006, 203, 2315–2327. [Google Scholar] [CrossRef] [PubMed]
- Min, J.Y.; Yang, Y.K.; Converso, K.L.; Liu, L.X.; Huang, Q.; Morgan, J.P.; Xiao, Y.F. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J. Appl. Physiol. 2002, 92, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Singla, D.K.; Hacker, T.A.; Ma, L.N.; Douglas, P.S.; Sullivan, R.; Lyons, G.E.; Kamp, T.J. Transplantation of embryonic stem cells into the infarcted mouse heart: Formation of multiple cell types. J. Mol. Cell. Cardiol. 2006, 40, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.J.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014, 510, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Chen, B.; Yang, X.; Fugate, J.A.; Kalucki, F.A.; Futakuchi-Tsuchida, A.; Couture, L.; Vogel, K.W.; Astley, C.A.; Baldessari, A.; et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018, 36, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P.; Vanneaux, V.; Hagege, A.; Bel, A.; Cholley, B.; Cacciapuoti, I.; Parouchev, A.; Benhamouda, N.; Tachdjian, G.; Tosca, L.; et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: First clinical case report. Eur. Heart J. 2015, 36, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P.; Vanneaux, V.; Hagege, A.; Bel, A.; Cholley, B.; Parouchev, A.; Cacciapuoti, I.; Al-Daccak, R.; Benhamouda, N.; Blons, H.; et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 2018, 71, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, J.; Minami, E.; Laflamme, M.A.; Virag, J.A.I.; Ware, C.B.; Masino, A.; Muskheli, V.; Pabon, L.; Reinecke, H.; Murry, C.E. Transplantation of undifferentiated murine embryonic stem cells in the heart: Teratoma formation and immune response. FASEB J. 2007, 21, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.J.; Tuan, R.S.; Cheung, K.M.; Leung, V.Y. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Assmus, B.; Schachinger, V.; Teupe, C.; Britten, M.; Lehmann, R.; Dobert, N.; Grunwald, F.; Aicher, A.; Urbich, C.; Martin, H.; et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (topcare-ami). Circulation 2002, 106, 3009–3017. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Meyer, G.P.; Lotz, J.; Ringes-Lichtenberg, S.; Lippolt, P.; Breidenbach, C.; Fichtner, S.; Korte, T.; Hornig, B.; Messinger, D.; et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The boost randomised controlled clinical trial. Lancet 2004, 364, 141–148. [Google Scholar] [CrossRef]
- Schachinger, V.; Erbs, S.; Elsasser, A.; Haberbosch, W.; Hambrecht, R.; Holschermann, H.; Yu, J.; Corti, R.; Mathey, D.G.; Hamm, C.W.; et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: Final 1-year results of the repair-ami trial. Eur. Heart J. 2006, 27, 2775–2783. [Google Scholar] [CrossRef] [PubMed]
- Surder, D.; Schwitter, J.; Moccetti, T.; Astori, G.; Rufibach, K.; Plein, S.; Lo Cicero, V.; Soncin, S.; Windecker, S.; Moschovitis, A.; et al. Cell-based therapy for myocardial repair in patients with acute myocardial infarction: Rationale and study design of the swiss multicenter intracoronary stem cells study in acute myocardial infarction (swiss-ami). Am. Heart J. 2010, 160, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Traverse, J.H.; Henry, T.D.; Pepine, C.J.; Willerson, J.T.; Zhao, D.X.; Ellis, S.G.; Forder, J.R.; Anderson, R.D.; Hatzopoulos, A.K.; Penn, M.S.; et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: The time randomized trial. JAMA 2012, 308, 2380–2389. [Google Scholar] [CrossRef] [PubMed]
- Strauer, B.E.; Brehm, M.; Zeus, T.; Kostering, M.; Hernandez, A.; Sorg, R.V.; Kogler, G.; Wernet, P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 2002, 106, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Meyer, G.P.; Muller-Ehmsen, J.; Tschope, C.; Bonarjee, V.; Larsen, A.I.; May, A.E.; Empen, K.; Chorianopoulos, E.; Tebbe, U.; et al. Intracoronary autologous bone marrow cell transfer after myocardial infarction: The boost-2 randomised placebo-controlled clinical trial. Eur. Heart J. 2017, 38, 2936–2943. [Google Scholar] [CrossRef] [PubMed]
- Hamano, K.; Nishida, M.; Hirata, K.; Mikamo, A.; Li, T.S.; Harada, M.; Miura, T.; Matsuzaki, M.; Esato, K. Local implantation of autologous bone marrow cells for therapeutic angiogenesis in patients with ischemic heart disease: Clinical trial and preliminary results. Jpn. Circ. J. 2001, 65, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Leistner, D.M.; Fischer-Rasokat, U.; Honold, J.; Seeger, F.H.; Schachinger, V.; Lehmann, R.; Martin, H.; Burck, I.; Urbich, C.; Dimmeler, S.; et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (topcare-ami): Final 5-year results suggest long-term safety and efficacy. Clin. Res. Cardiol. 2011, 100, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Lunde, K.; Solheim, S.; Aakhus, S.; Arnesen, H.; Abdelnoor, M.; Forfang, K.; investigators, A. Autologous stem cell transplantation in acute myocardial infarction: The astami randomized controlled trial. Intracoronary transplantation of autologous mononuclear bone marrow cells, study design and safety aspects. Scand. Cardiovasc. J. 2005, 39, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Suerder, D.; Manka, R.; Moccetti, T.; Lo Cicero, V.; Emmert, M.Y.; Klersy, C.; Soncin, S.; Turchetto, L.; Radrizzani, M.; Zuber, M.; et al. The effect of bone marrow derived mononuclear cell treatment, early or late after acute myocardial infarction: Twelve months cmr and long-term clinical results. Circ. Res. 2016, 119, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Wohrle, J.; Merkle, N.; Mailander, V.; Nusser, T.; Schauwecker, P.; von Scheidt, F.; Schwarz, K.; Bommer, M.; Wiesneth, M.; Schrezenmeier, H.; et al. Results of intracoronary stem cell therapy after acute myocardial infarction. Am. J. Cardiol. 2010, 105, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Strauer, B.E.; Yousef, M.; Schannwell, C.M. The acute and long-term effects of intracoronary stem cell transplantation in 191 patients with chronic heart failure: The star-heart study. Eur. J. Heart Fail. 2010, 12, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Stamm, C.; Kleine, H.D.; Choi, Y.H.; Dunkelmann, S.; Lauffs, J.A.; Lorenzen, B.; David, A.; Liebold, A.; Nienaber, C.; Zurakowski, D.; et al. Intramyocardial delivery of cd133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: Safety and efficacy studies. J. Thorac. Cardiovasc. Surg. 2007, 133, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, B.A.; Ebell, W.; Dandel, M.; Kukucka, M.; Gebker, R.; Doltra, A.; Knosalla, C.; Choi, Y.H.; Hetzer, R.; Stamm, C. Autologous cd133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: The cardio133 trial. Eur. Heart J. 2014, 35, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Soonpaa, M.H.; Reinecke, H.; Nakajima, H.; Nakajima, H.O.; Rubart, M.; Pasumarthi, K.B.; Virag, J.I.; Bartelmez, S.H.; Poppa, V.; et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 2004, 428, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Duong Van Huyen, J.P.; Smadja, D.M.; Bruneval, P.; Gaussem, P.; Dal-Cortivo, L.; Julia, P.; Fiessinger, J.N.; Cavazzana-Calvo, M.; Aiach, M.; Emmerich, J. Bone marrow-derived mononuclear cell therapy induces distal angiogenesis after local injection in critical leg ischemia. Mod. Pathol. 2008, 21, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, J.; Luo, R.; Jiang, J.; Wang, J.A. Bone marrow mesenchymal stem cells induce angiogenesis and attenuate the remodeling of diabetic cardiomyopathy. Exp. Clin. Endocrinol. Diabetes 2008, 116, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhou, S.; Liu, Y.; Hu, H. Efficacy and safety of bone marrow cell transplantation for chronic ischemic heart disease: A meta-analysis. Med. Sci. Monit. 2014, 20, 1768–1777. [Google Scholar] [PubMed]
- Seeger, F.H.; Tonn, T.; Krzossok, N.; Zeiher, A.M.; Dimmeler, S. Cell isolation procedures matter: A comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur. Heart J. 2007, 28, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Tendera, M.; Wojakowski, W.; Ruzyllo, W.; Chojnowska, L.; Kepka, C.; Tracz, W.; Musialek, P.; Piwowarska, W.; Nessler, J.; Buszman, P.; et al. Intracoronary infusion of bone marrow-derived selected cd34+cxcr4+ cells and non-selected mononuclear cells in patients with acute stemi and reduced left ventricular ejection fraction: Results of randomized, multicentre myocardial regeneration by intracoronary infusion of selected population of stem cells in acute myocardial infarction (regent) trial. Eur. Heart J. 2009, 30, 1313–1321. [Google Scholar] [PubMed]
- Povsic, T.J.; Henry, T.D.; Traverse, J.H.; Fortuin, F.D.; Schaer, G.L.; Kereiakes, D.J.; Schatz, R.A.; Zeiher, A.M.; White, C.J.; Stewart, D.J.; et al. The renew trial: Efficacy and safety of intramyocardial autologous cd34(+) cell administration in patients with refractory angina. JACC Cardiovasc. Interv. 2016, 9, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Noiseux, N.; Mansour, S.; Weisel, R.; Stevens, L.M.; Der Sarkissian, S.; Tsang, K.; Crean, A.M.; Larose, E.; Li, S.H.; Wintersperger, B.; et al. The impact-cabg trial: A multicenter, randomized clinical trial of cd133(+) stem cell therapy during coronary artery bypass grafting for ischemic cardiomyopathy. J. Thorac. Cardiovasc. Surg. 2016, 152, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A.A.; Mathiasen, A.B.; Mygind, N.D.; Kuhl, J.T.; Jorgensen, E.; Helqvist, S.; Elberg, J.J.; Kofoed, K.F.; Vejlstrup, N.G.; Fischer-Nielsen, A.; et al. Adipose-derived stromal cells for treatment of patients with chronic ischemic heart disease (mystromalcell trial): A randomized placebo-controlled study. Stem Cells Int. 2017, 2017, 5237063. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Suzuki, K. Bone marrow-derived mesenchymal stem cells for the treatment of heart failure. Heart Fail. Rev. 2015, 20, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Davani, S.; Marandin, A.; Mersin, N.; Royer, B.; Kantelip, B.; Herve, P.; Etievent, J.P.; Kantelip, J.P. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation 2003, 108 (Suppl. 1), II253–II258. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Peng, P.; Cheng, L.; Chen, S.; Li, K.; Li, Z.Y.; Mo, Y.H.; Zhou, Z.; Li, M. A natural compound induced cardiogenic differentiation of endogenous mscs for repair of infarcted heart. Differ. Res. Biol. Divers. 2012, 83, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Liu, X.C.; Kong, F.; Qi, T.G.; Cheng, G.H.; Wang, J.; Sun, C.; Luan, Y. Bone marrow mesenchymal stem cells improve myocardial function in a swine model of acute myocardial infarction. Mol. Med. Rep. 2014, 10, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.M.; Fishman, J.E.; Gerstenblith, G.; DiFede Velazquez, D.L.; Zambrano, J.P.; Suncion, V.Y.; Tracy, M.; Ghersin, E.; Johnston, P.V.; Brinker, J.A.; et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The poseidon randomized trial. JAMA 2012, 308, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Heldman, A.W.; DiFede, D.L.; Fishman, J.E.; Zambrano, J.P.; Trachtenberg, B.H.; Karantalis, V.; Mushtaq, M.; Williams, A.R.; Suncion, V.Y.; McNiece, I.K.; et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The tac-hft randomized trial. JAMA 2014, 311, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Tano, N.; Narita, T.; Kaneko, M.; Ikebe, C.; Coppen, S.R.; Campbell, N.G.; Shiraishi, M.; Shintani, Y.; Suzuki, K. Epicardial placement of mesenchymal stromal cell-sheets for the treatment of ischemic cardiomyopathy; in vivo proof-of-concept study. Mol. Ther. 2014, 22, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Bartunek, J.; Behfar, A.; Dolatabadi, D.; Vanderheyden, M.; Ostojic, M.; Dens, J.; El Nakadi, B.; Banovic, M.; Beleslin, B.; Vrolix, M.; et al. Cardiopoietic stem cell therapy in heart failure: The c-cure (cardiopoietic stem cell therapy in heart failure) multicenter randomized trial with lineage-specified biologics. J. Am. Coll. Cardiol. 2013, 61, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, S.H.; Youn, Y.J.; Ahn, M.S.; Kim, J.Y.; Yoo, B.S.; Yoon, J.; Kwon, W.; Hong, I.S.; Lee, K.; et al. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J. Korean Med. Sci. 2014, 29, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Mathiasen, A.B.; Qayyum, A.A.; Jorgensen, E.; Helqvist, S.; Fischer-Nielsen, A.; Kofoed, K.F.; Haack-Sorensen, M.; Ekblond, A.; Kastrup, J. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: A randomized placebo-controlled trial (msc-hf trial). Eur. Heart J. 2015, 36, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Fang, W.W.; Ye, F.; Liu, Y.H.; Qian, J.; Shan, S.J.; Zhang, J.J.; Chunhua, R.Z.; Liao, L.M.; Lin, S.; et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am. J. Cardiol. 2004, 94, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Z.; Tian, N.; Zhang, J.; Yei, F.; Duan, B.; Zhu, Z.; Lin, S.; Kwan, T.W. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J. Invasive Cardiol. 2006, 18, 552–556. [Google Scholar] [PubMed]
- Hare, J.M.; Traverse, J.H.; Henry, T.D.; Dib, N.; Strumpf, R.K.; Schulman, S.P.; Gerstenblith, G.; DeMaria, A.N.; Denktas, A.E.; Gammon, R.S.; et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 2009, 54, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, F.; Ma, W.; Chen, B.; Zhou, F.; Xu, Z.; Zhang, Y.; Zhang, D.; Zhu, T.; Wang, L.; et al. A novel approach to transplanting bone marrow stem cells to repair human myocardial infarction: Delivery via a noninfarct-relative artery. Cardiovasc. Ther. 2010, 28, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.R.; Pei, X.T.; Ding, Q.A.; Chen, Y.; Zhang, N.K.; Chen, H.Y.; Wang, Z.G.; Wang, Y.F.; Zhu, Z.M.; Li, T.C.; et al. A critical challenge: Dosage-related efficacy and acute complication intracoronary injection of autologous bone marrow mesenchymal stem cells in acute myocardial infarction. Int. J. Cardiol. 2013, 168, 3191–3199. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, S.F.; van Ramshorst, J.; Hoogslag, G.E.; Boden, H.; Velders, M.A.; Cannegieter, S.C.; Roelofs, H.; Al Younis, I.; Dibbets-Schneider, P.; Fibbe, W.E.; et al. Intramyocardial injection of autologous bone marrow-derived ex vivo expanded mesenchymal stem cells in acute myocardial infarction patients is feasible and safe up to 5 years of follow-up. J. Cardiovasc. Transl. Res. 2013, 6, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Karantalis, V.; DiFede, D.L.; Gerstenblith, G.; Pham, S.; Symes, J.; Zambrano, J.P.; Fishman, J.; Pattany, P.; McNiece, I.; Conte, J.; et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The prospective randomized study of mesenchymal stem cell therapy in patients undergoing cardiac surgery (prometheus) trial. Circ. Res. 2014, 114, 1302–1310. [Google Scholar] [PubMed]
- Ascheim, D.D.; Gelijns, A.C.; Goldstein, D.; Moye, L.A.; Smedira, N.; Lee, S.; Klodell, C.T.; Szady, A.; Parides, M.K.; Jeffries, N.O.; et al. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation 2014, 129, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Chullikana, A.; Majumdar, A.S.; Gottipamula, S.; Krishnamurthy, S.; Kumar, A.S.; Prakash, V.S.; Gupta, P.K. Randomized, double-blind, phase i/ii study of intravenous allogeneic mesenchymal stromal cells in acute myocardial infarction. Cytotherapy 2015, 17, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.C.; Borow, K.M.; Silva, G.V.; DeMaria, A.N.; Marroquin, O.C.; Huang, P.P.; Traverse, J.H.; Krum, H.; Skerrett, D.; Zheng, Y.; et al. A phase ii dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ. Res. 2015, 117, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Guijarro, D.; Lebrin, M.; Lairez, O.; Bourin, P.; Piriou, N.; Pozzo, J.; Lande, G.; Berry, M.; Le Tourneau, T.; Cussac, D.; et al. Intramyocardial transplantation of mesenchymal stromal cells for chronic myocardial ischemia and impaired left ventricular function: Results of the mesami 1 pilot trial. Int. J. Cardiol. 2016, 209, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Guo, S.; Gao, C.; Dai, G.; Gao, Y.; Li, M.; Wang, X.; Hu, D. A randomized comparative study on the efficacy of intracoronary infusion of autologous bone marrow mononuclear cells and mesenchymal stem cells in patients with dilated cardiomyopathy. Int. Heart J. 2017, 58, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Florea, V.; Rieger, A.C.; DiFede, D.L.; El-Khorazaty, J.; Natsumeda, M.; Banerjee, M.N.; Tompkins, B.A.; Khan, A.; Schulman, I.H.; Landin, A.M.; et al. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (the trident study). Circ. Res. 2017, 121, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Epstein, S.E.; Greene, S.J.; Quyyumi, A.A.; Sikora, S.; Kim, R.J.; Anderson, A.S.; Wilcox, J.E.; Tankovich, N.I.; Lipinski, M.J.; et al. Intravenous allogeneic mesenchymal stem cells for nonischemic cardiomyopathy: Safety and efficacy results of a phase ii-a randomized trial. Circ. Res. 2017, 120, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Bartunek, J.; Terzic, A.; Davison, B.A.; Filippatos, G.S.; Radovanovic, S.; Beleslin, B.; Merkely, B.; Musialek, P.; Wojakowski, W.; Andreka, P.; et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: Results at 39 weeks of the prospective, randomized, double blind, sham-controlled chart-1 clinical trial. Eur. Heart J. 2017, 38, 648–660. [Google Scholar] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Rigol, M.; Solanes, N.; Roura, S.; Roque, M.; Novensa, L.; Dantas, A.P.; Martorell, J.; Sitges, M.; Ramirez, J.; Bayes-Genis, A.; et al. Allogeneic adipose stem cell therapy in acute myocardial infarction. Eur. J. Clin. Investig. 2014, 44, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, Y.; Nagaya, N.; Kataoka, M.; Yanagawa, B.; Tanaka, K.; Hao, H.; Ishino, K.; Ishida, H.; Shimizu, T.; Kangawa, K.; et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat. Med. 2006, 12, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Mazo, M.; Planat-Benard, V.; Abizanda, G.; Pelacho, B.; Leobon, B.; Gavira, J.J.; Penuelas, I.; Cemborain, A.; Penicaud, L.; Laharrague, P.; et al. Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. Eur. J. Heart Fail. 2008, 10, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Houtgraaf, J.H.; den Dekker, W.K.; van Dalen, B.M.; Springeling, T.; de Jong, R.; van Geuns, R.J.; Geleijnse, M.L.; Fernandez-Aviles, F.; Zijlsta, F.; Serruys, P.W.; et al. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with st-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2012, 59, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.C.; Sanz-Ruiz, R.; Sanchez, P.L.; Lasso, J.; Perez-Cano, R.; Alonso-Farto, J.C.; Perez-David, E.; Fernandez-Santos, M.E.; Serruys, P.W.; Duckers, H.J.; et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The precise trial. Am. Heart J. 2014, 168, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Kastrup, J.; Schou, M.; Gustafsson, I.; Nielsen, O.W.; Mogelvang, R.; Kofoed, K.F.; Kragelund, C.; Hove, J.D.; Fabricius-Bjerre, A.; Heitman, M.; et al. Rationale and design of the first double-blind, placebo-controlled trial with allogeneic adipose tissue-derived stromal cell therapy in patients with ischemic heart failure: A phase ii danish multicentre study. Stem Cells Int. 2017, 2017, 8506370. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.D.; Pepine, C.J.; Lambert, C.R.; Traverse, J.H.; Schatz, R.; Costa, M.; Povsic, T.J.; David Anderson, R.; Willerson, J.T.; Kesten, S.; et al. The athena trials: Autologous adipose-derived regenerative cells for refractory chronic myocardial ischemia with left ventricular dysfunction. Catheter. Cardiovasc. Interv. 2017, 89, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Okura, H.; Matsuyama, A.; Lee, C.M.; Saga, A.; Kakuta-Yamamoto, A.; Nagao, A.; Sougawa, N.; Sekiya, N.; Takekita, K.; Shudo, Y.; et al. Cardiomyoblast-like cells differentiated from human adipose tissue-derived mesenchymal stem cells improve left ventricular dysfunction and survival in a rat myocardial infarction model. Tissue Eng. Part C Methods 2010, 16, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.E.; Yang, D.; Li, L.; Wang, W.; Peng, Y.; Chen, C.; Chen, P.; Xia, X.; Wang, H.; Jiang, J.; et al. Prolyl hydroxylase domain protein 2 silencing enhances the survival and paracrine function of transplanted adipose-derived stem cells in infarcted myocardium. Circ. Res. 2013, 113, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Lee, H.C.; Park, J.H.; Kim, B.W.; Ahn, J.; Kim, J.H.; Park, J.S.; Oh, J.H.; Choi, J.H.; Cha, K.S.; et al. Effects of intracoronary administration of autologous adipose tissue-derived stem cells on acute myocardial infarction in a porcine model. Yonsei Med. J. 2015, 56, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, S.; Wenisch, S. Adipose tissue derived mesenchymal stem cells for musculoskeletal repair in veterinary medicine. Am. J. Stem Cells 2015, 4, 1–12. [Google Scholar] [PubMed]
- Timmers, L.; Lim, S.K.; Hoefer, I.E.; Arslan, F.; Lai, R.C.; van Oorschot, A.A.; Goumans, M.J.; Strijder, C.; Sze, S.K.; Choo, A.; et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011, 6, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.Y.; Chak, L.L.; Biswas, A.; Tan, J.H.; Gauthaman, K.; Chan, W.K.; Bongso, A. Human wharton’s jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev. 2011, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.R.; Zhang, N.K.; Ding, Q.A.; Chen, H.Y.; Hu, X.; Jiang, S.; Li, T.C.; Chen, Y.; Wang, Z.G.; Ye, Y.; et al. Common expression of stemness molecular markers and early cardiac transcription factors in human wharton’s jelly-derived mesenchymal stem cells and embryonic stem cells. Cell Transplant. 2013, 22, 1883–1900. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.J.; Kim, H.; Lee, S.K.; Kim, J.; Shen, Y.; Jung, S.; Kang, K.S.; Im, S.G.; Lee, S.Y.; Choi, M.; et al. Umbilical-cord-blood-derived mesenchymal stem cells seeded onto fibronectin-immobilized polycaprolactone nanofiber improve cardiac function. Acta Biomater. 2014, 10, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Choi, E.K.; Kang, S.K.; Kim, G.H.; Park, J.Y.; Kang, H.J.; Lee, S.W.; Kim, K.H.; Kwon, J.S.; Lee, K.H.; et al. N-cadherin determines individual variations in the therapeutic efficacy of human umbilical cord blood-derived mesenchymal stem cells in a rat model of myocardial infarction. Mol. Ther. 2012, 20, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Lilyanna, S.; Martinez, E.C.; Vu, T.D.; Ling, L.H.; Gan, S.U.; Tan, A.L.; Phan, T.T.; Kofidis, T. Cord lining-mesenchymal stem cells graft supplemented with an omental flap induces myocardial revascularization and ameliorates cardiac dysfunction in a rat model of chronic ischemic heart failure. Tissue Eng. Part A 2013, 19, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, X.C.; Yang, L.; Zhu, D.L.; Zhang, Y.D.; Chen, Y.; Zhang, H.Y. Wharton’s jelly-derived mesenchymal stem cells promote myocardial regeneration and cardiac repair after miniswine acute myocardial infarction. Coronary Artery Dis. 2013, 24, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Yin, X.; Wang, J.; Tian, N.; Ao, Q.; Gu, Y.; Liu, Y. Functional characterization of human umbilical cord-derived mesenchymal stem cells for treatment of systolic heart failure. Exp. Ther. Med. 2016, 12, 3328–3332. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, Y.D.; Guo, Y.; Chen, Y.; Guo, D.X.; Zhou, H.L.; Zhang, F.L.; Zhao, Q.N. Safety and efficacy of intracoronary human umbilical cord-derived mesenchymal stem cell treatment for very old patients with coronary chronic total occlusion. Curr. Pharm. Des. 2015, 21, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Musialek, P.; Mazurek, A.; Jarocha, D.; Tekieli, L.; Szot, W.; Kostkiewicz, M.; Banys, R.P.; Urbanczyk, M.; Kadzielski, A.; Trystula, M.; et al. Myocardial regeneration strategy using wharton’s jelly mesenchymal stem cells as an off-the-shelf ‘unlimited’ therapeutic agent: Results from the acute myocardial infarction first-in-man study. Adv. Int. Cardiol. 2015, 11, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, J.; Verdugo, F.J.; Gonzalez, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A.; et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: A phase 1/2 randomized controlled trial (rimecard trial [randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]). Circ. Res. 2017, 121, 1192–1204. [Google Scholar] [PubMed]
- Gao, L.R.; Chen, Y.; Zhang, N.K.; Yang, X.L.; Liu, H.L.; Wang, Z.G.; Yan, X.Y.; Wang, Y.; Zhu, Z.M.; Li, T.C.; et al. Intracoronary infusion of wharton’s jelly-derived mesenchymal stem cells in acute myocardial infarction: Double-blind, randomized controlled trial. BMC Med. 2015, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.F.; Xu, Y.; Zhu, Z.Y.; Gao, C.Y.; Shi, Y.N. Clinical observation of umbilical cord mesenchymal stem cell treatment of severe systolic heart failure. Genet. Mol. Res. 2015, 14, 3010–3017. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Celikkan, F.T.; Cinar, O. Umbilical cord mesenchymal stromal cell transplantations: A systemic analysis of clinical trials. Cytotherapy 2017, 19, 1351–1382. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.E.; Partridge, T.A. Muscle satellite cells. Int. J. Biochem. Cell. Biol. 2003, 35, 1151–1156. [Google Scholar] [CrossRef]
- Chiu, R.C.; Zibaitis, A.; Kao, R.L. Cellular cardiomyoplasty: Myocardial regeneration with satellite cell implantation. Ann. Thorac. Surg. 1995, 60, 12–18. [Google Scholar] [CrossRef]
- Marelli, D.; Desrosiers, C.; el-Alfy, M.; Kao, R.L.; Chiu, R.C. Cell transplantation for myocardial repair: An experimental approach. Cell Transplant. 1992, 1, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Herreros, J.; Prosper, F.; Perez, A.; Gavira, J.J.; Garcia-Velloso, M.J.; Barba, J.; Sanchez, P.L.; Canizo, C.; Rabago, G.; Marti-Climent, J.M.; et al. Autologous intramyocardial injection of cultured skeletal muscle-derived stem cells in patients with non-acute myocardial infarction. Eur. Heart J. 2003, 24, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P. Stem cell therapy for heart failure: Are arrhythmias a real safety concern? Circulation 2009, 119, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Bolli, R.; Chugh, A.R.; D’Amario, D.; Loughran, J.H.; Stoddard, M.F.; Ikram, S.; Beache, G.M.; Wagner, S.G.; Leri, A.; Hosoda, T.; et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet 2011, 378, 1847–1857. [Google Scholar] [CrossRef]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.; Berman, D.; Czer, L.S.; Marban, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (caduceus): A prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef]
- Malliaras, K.; Makkar, R.R.; Smith, R.R.; Cheng, K.; Wu, E.; Bonow, R.O.; Marban, L.; Mendizabal, A.; Cingolani, E.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells after myocardial infarction: Evidence of therapeutic regeneration in the final 1-year results of the caduceus trial (cardiosphere-derived autologous stem cells to reverse ventricular dysfunction). J. Am. Coll. Cardiol. 2014, 63, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Bolli, R.; Hare, J.M.; March, K.L.; Pepine, C.J.; Willerson, J.T.; Perin, E.C.; Yang, P.C.; Henry, T.D.; Traverse, J.H.; Mitrani, R.D.; et al. Rationale and design of the concert-hf trial (combination of mesenchymal and c-kit(+) cardiac stem cells as regenerative therapy for heart failure). Circ. Res. 2018, 122, 1703–1715. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Mauritz, C.; Schwanke, K.; Reppel, M.; Neef, S.; Katsirntaki, K.; Maier, L.S.; Nguemo, F.; Menke, S.; Haustein, M.; Hescheler, J.; et al. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation 2008, 118, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Montserrat, N.; Bahima, E.G.; Batlle, L.; Hafner, S.; Rodrigues, A.M.; Gonzalez, F.; Izpisua Belmonte, J.C. Generation of pig ips cells: A model for cell therapy. J. Cardiovasc. Transl. Res. 2011, 4, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wilson, G.F.; Soerens, A.G.; Koonce, C.H.; Yu, J.; Palecek, S.P.; Thomson, J.A.; Kamp, T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009, 104, e30–41. [Google Scholar] [CrossRef] [PubMed]
- Beqqali, A.; Kloots, J.; Ward-van Oostwaard, D.; Mummery, C.; Passier, R. Genome-wide transcriptional profiling of human embryonic stem cells differentiating to cardiomyocytes. Stem Cells 2006, 24, 1956–1967. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.P.; van den Berg, C.W.; Casini, S.; Braam, S.R.; Mummery, C.L. Pluripotent stem cell models of cardiac disease and their implication for drug discovery and development. Trends Mol. Med. 2011, 17, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Wada, M.; Konishi, K.; Sato, M.; Iwamoto, U.; Sato, Y.; Tachibana, A.; Kikuchi, T.; Iwamiya, T.; Shimizu, T.; et al. Fabrication of mouse embryonic stem cell-derived layered cardiac cell sheets using a bioreactor culture system. PLoS ONE 2012, 7, e52176. [Google Scholar] [CrossRef] [PubMed]
- So, K.H.; Han, Y.J.; Park, H.Y.; Kim, J.G.; Sung, D.J.; Bae, Y.M.; Yang, B.C.; Park, S.B.; Chang, S.K.; Kim, E.Y.; et al. Generation of functional cardiomyocytes from mouse induced pluripotent stem cells. Int. J. Cardiol. 2011, 153, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Gai, H.; Leung, E.L.; Costantino, P.D.; Aguila, J.R.; Nguyen, D.M.; Fink, L.M.; Ward, D.C.; Ma, Y. Generation and characterization of functional cardiomyocytes using induced pluripotent stem cells derived from human fibroblasts. Cell Biol. Int. 2009, 33, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Quattrocelli, M.; Palazzolo, G.; Agnolin, I.; Martino, S.; Bouche, M.; Anastasia, L.; Sampaolesi, M. Synthetic sulfonyl-hydrazone-1 positively regulates cardiomyogenic microrna expression and cardiomyocyte differentiation of induced pluripotent stem cells. J. Cell. Biochem. 2011, 112, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Tan, G.; Manasi; Qiu, S.; Kong, G.; Yong, P.; Koh, C.; Ooi, T.H.; Lim, S.Y.; Wong, P.; et al. One-step derivation of cardiomyocytes and mesenchymal stem cells from human pluripotent stem cells. Stem Cell Res. 2012, 9, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating wnt/beta-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Tulloch, N.L.; Muskheli, V.; Razumova, M.V.; Korte, F.S.; Regnier, M.; Hauch, K.D.; Pabon, L.; Reinecke, H.; Murry, C.E. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ. Res. 2011, 109, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Miyagawa, S.; Miki, K.; Saito, A.; Fukushima, S.; Higuchi, T.; Kawamura, T.; Kuratani, T.; Daimon, T.; Shimizu, T.; et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 2012, 126, S29–S37. [Google Scholar] [CrossRef] [PubMed]
- Ja, K.P.; Miao, Q.; Zhen Tee, N.G.; Lim, S.Y.; Nandihalli, M.; Ramachandra, J.A.; Mehta, A.; Shim, W. Ipsc-derived human cardiac progenitor cells improve ventricular remodelling via angiogenesis and interstitial networking of infarcted myocardium. J. Cell. Mol. Med. 2016, 20, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Robinton, D.A.; Daley, G.Q. The promise of induced pluripotent stem cells in research and therapy. Nature 2012, 481, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Abyzov, A.; Mariani, J.; Palejev, D.; Zhang, Y.; Haney, M.S.; Tomasini, L.; Ferrandino, A.F.; Rosenberg Belmaker, L.A.; Szekely, A.; Wilson, M.; et al. Somatic copy number mosaicism in human skin revealed by induced pluripotent stem cells. Nature 2012, 492, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Young, M.A.; Larson, D.E.; Sun, C.W.; George, D.R.; Ding, L.; Miller, C.A.; Lin, L.; Pawlik, K.M.; Chen, K.; Fan, X.; et al. Background mutations in parental cells account for most of the genetic heterogeneity of induced pluripotent stem cells. Cell Stem Cell 2012, 10, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, Z.N.; Rong, Z.; Xu, Y. Immunogenicity of induced pluripotent stem cells. Nature 2011, 474, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Malliaras, K.; Marban, E. Moving beyond surrogate endpoints in cell therapy trials for heart disease. Stem Cells Transl. Med. 2014, 3, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Garcia, A.A.; Anker, S.D.; Armstrong, P.W.; Calvo, G.; Cleland, J.G.; Cohn, J.N.; Dickstein, K.; Domanski, M.J.; Ekman, I.; et al. Clinical outcome endpoints in heart failure trials: A european society of cardiology heart failure association consensus document. Eur. J. Heart Fail. 2013, 15, 1082–1094. [Google Scholar] [CrossRef] [PubMed]
- Gyongyosi, M.; Wojakowski, W.; Lemarchand, P.; Lunde, K.; Tendera, M.; Bartunek, J.; Marban, E.; Assmus, B.; Henry, T.D.; Traverse, J.H.; et al. Meta-analysis of cell-based cardiac studies (accrue) in patients with acute myocardial infarction based on individual patient data. Circ. Res. 2015, 116, 1346–1360. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, Z.; Sun, Z. The potential and challenges of using stem cells for cardiovascular repair and regeneration. Genes Dis. 2014, 1, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.O.; Han, J.W.; Kim, J.M.; Cho, H.J.; Park, C.; Lee, N.; Kim, D.W.; Yoon, Y.S. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ. Res. 2011, 108, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Ptaszek, L.M.; Mansour, M.; Ruskin, J.N.; Chien, K.R. Towards regenerative therapy for cardiac disease. Lancet 2012, 379, 933–942. [Google Scholar] [CrossRef]
- Sheng, C.C.; Zhou, L.; Hao, J. Current stem cell delivery methods for myocardial repair. BioMed Res. Int. 2013, 2013, 547902. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Youssef, E.A.; Brinton, T.J.; Zhang, P.; Rogers, P.; Price, E.T.; Yeung, A.C.; Johnstone, B.H.; Yock, P.G.; March, K.L. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: Implications for current clinical trials. Circulation 2005, 112, I150–156. [Google Scholar] [PubMed]
- Freyman, T.; Polin, G.; Osman, H.; Crary, J.; Lu, M.M.; Cheng, L.; Palasis, M.; Wilensky, R.L. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur. Heart J. 2006, 27, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.H.; Guo, Y.; Ou, Q.H.; Chen, N.; Wu, W.J.; Yuan, F.P.; O’Brien, E.; Wang, T.; Luo, L.; Hunt, G.N.; et al. Intracoronary administration of cardiac stem cells in mice: A new, improved technique for cell therapy in murine models. Basic Res. Cardiol. 2011, 106, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.V.; Sasano, T.; Mills, K.; Evers, R.; Lee, S.T.; Smith, R.R.; Lardo, A.C.; Lai, S.H.; Steenbergen, C.; Gerstenblith, G.; et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation 2009, 120, U1075–U1095. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, F.S.; Steinhauser, M.L.; Gannon, J.; Lee, R.T. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell 2011, 8, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Mirotsou, M.; Jayawardena, T.M.; Schmeckpeper, J.; Gnecchi, M.; Dzau, V.J. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J. Mol. Cell. Cardiol. 2011, 50, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Pan, Y.; Li, X.H.; Yang, X.Y.; Feng, Y.L.; Tan, H.H.; Jiang, L.; Feng, J.; Yu, X.Y. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal mir-21 by targeting pdcd4. Cell Death Dis. 2016, 7, e2277. [Google Scholar] [CrossRef] [PubMed]
- Cambier, L.; de Couto, G.; Ibrahim, A.; Echavez, A.K.; Valle, J.; Liu, W.; Kreke, M.; Smith, R.R.; Marban, L.; Marban, E. Y rna fragment in extracellular vesicles confers cardioprotection via modulation of il-10 expression and secretion. EMBO Mol. Med. 2017, 9, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Gallet, R.; Dawkins, J.; Valle, J.; Simsolo, E.; de Couto, G.; Middleton, R.; Tseliou, E.; Luthringer, D.; Kreke, M.; Smith, R.R.; et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 2017, 38, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Tseliou, E.; Fouad, J.; Reich, H.; Slipczuk, L.; de Couto, G.; Aminzadeh, M.; Middleton, R.; Valle, J.; Weixin, L.; Marban, E. Fibroblasts rendered antifibrotic, antiapoptotic, and angiogenic by priming with cardiosphere-derived extracellular membrane vesicles. J. Am. Coll. Cardiol. 2015, 66, 599–611. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).