Zinc Chloride Exposure Inhibits Brain Acetylcholine Levels, Produces Neurotoxic Signatures, and Diminishes Memory and Motor Activities in Adult Zebrafish
Abstract
:1. Introduction
2. Results
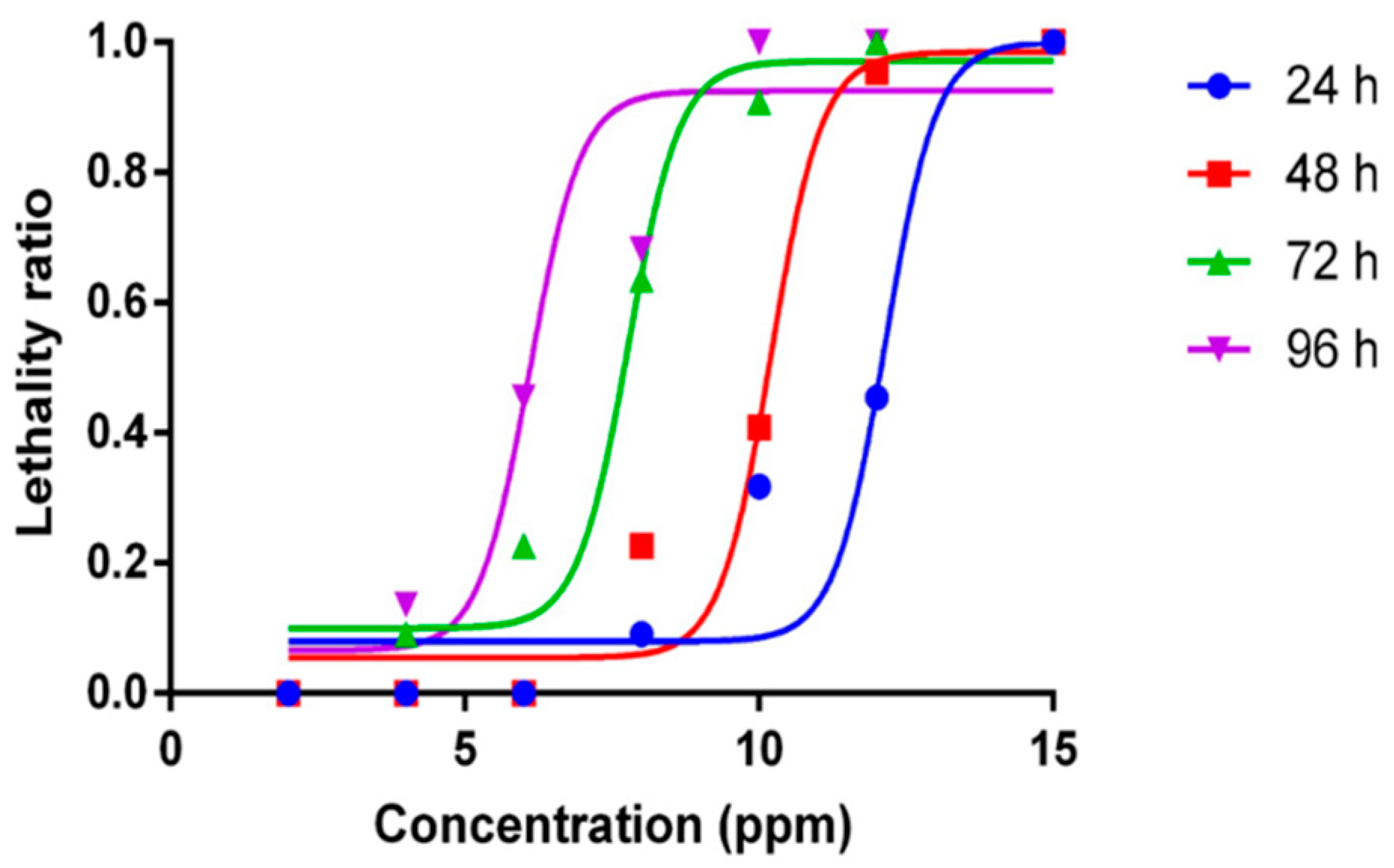
2.1. Mortality Curve and Median Lethal Concentration for ZnCl2 Exposure
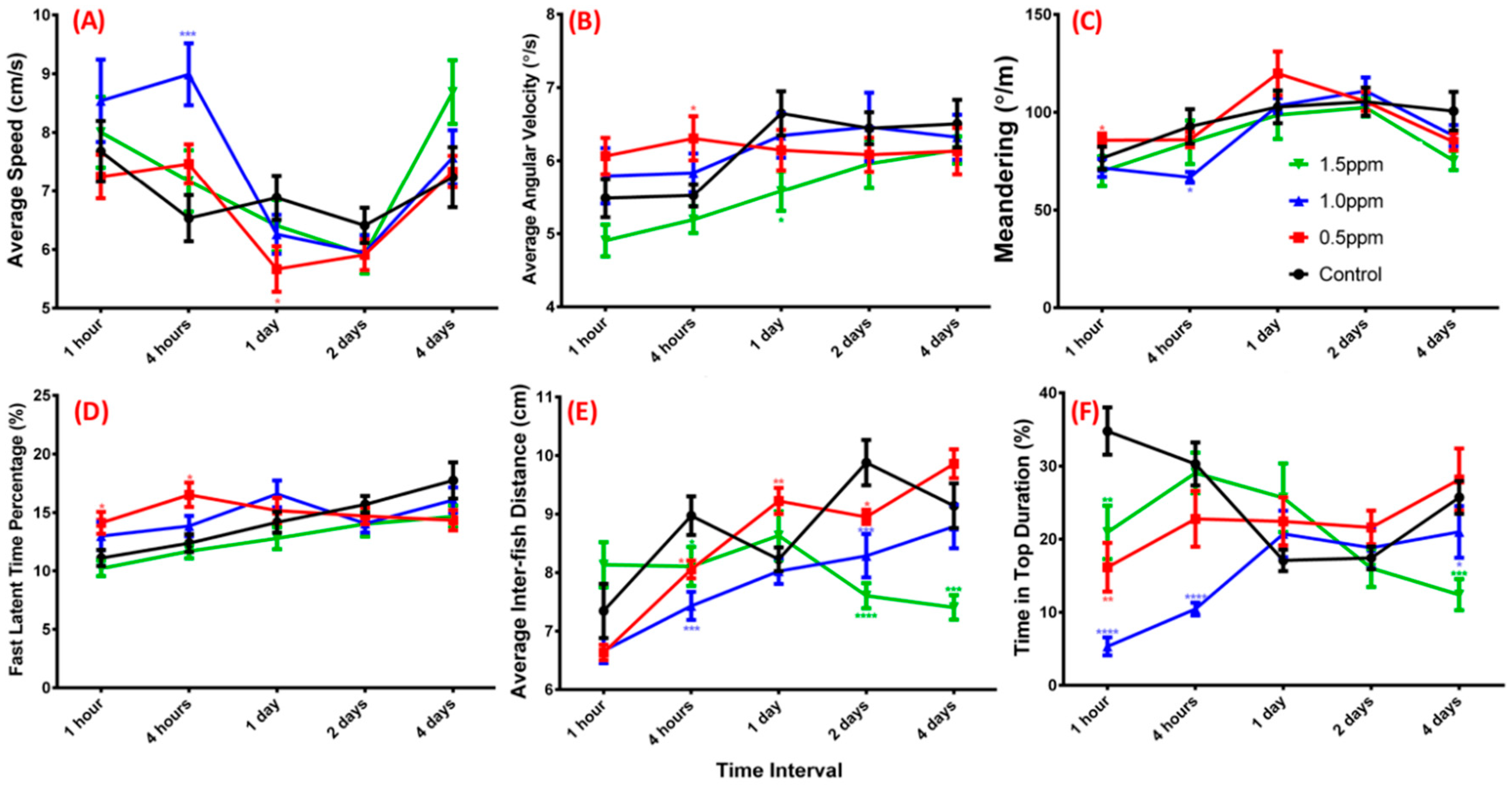
2.2. ZnCl2 Exposure Impaired Zebrafish Three-Dimensional Locomotor Activity
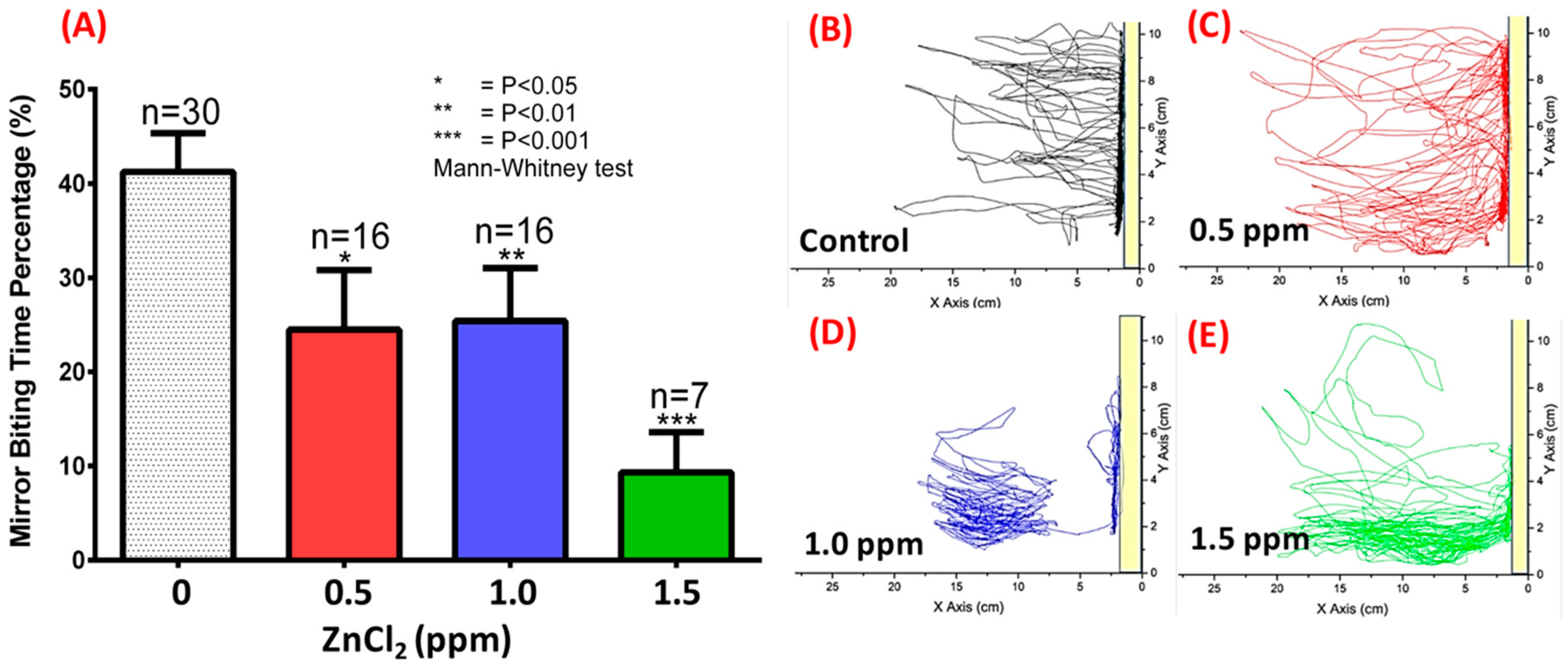
2.3. ZnCl2 Exposure Reduced Zebrafish Aggression
2.4. ZnCl2 Exposure Reduced Memory in the Passive Avoidance Test
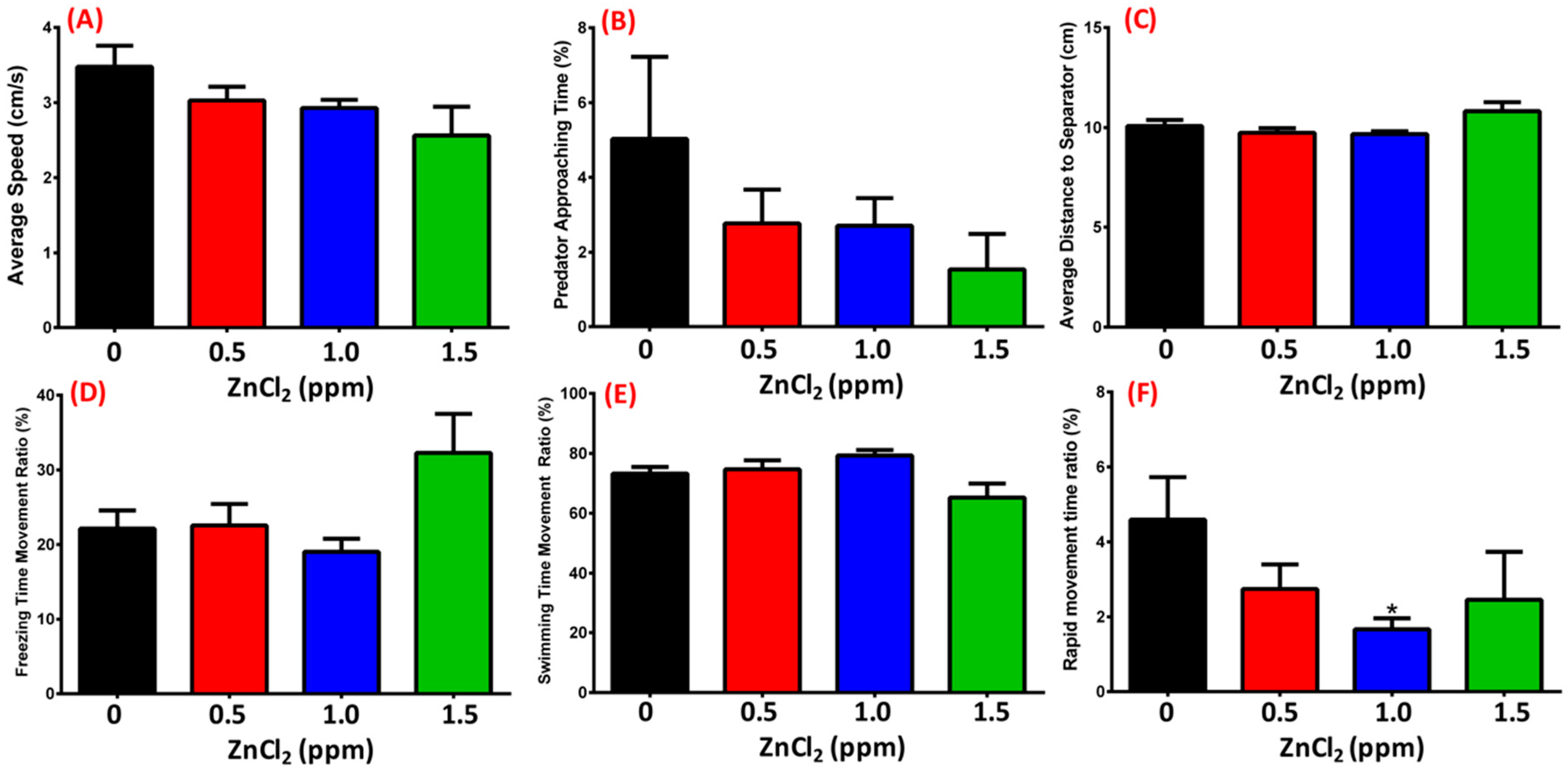
2.5. ZnCl2 Exposure Does Not Change Predator Avoidance Behavior
2.6. ZnCl2 Exposure Deregulated the Circadian Rhythm
2.7. ZnCl2 Exposure Increased Oxidative Stress in the Brain
2.8. ZnCl2 Causes the Downregulation of Neurotransmitter and AD-Related Marker Expression
3. Discussion
4. Materials and Methods
4.1. Animal Ethics
4.2. Zebrafish Rearing
4.3. Acute Toxicity Test
4.4. Chronic Toxicity Test
4.5. Adult Neurobehavioral Analysis
4.6. Zebrafish 3D Locomotor Activity
4.7. Passive Avoidance Test (Short-Term Memory Test)
4.8. Aggressiveness Test
4.9. Predator Avoidance Test
4.10. Circadian Rhythm Test
4.11. Biochemical Parameters
4.11.1. Tissue Preparation and Total Protein Determination
4.11.2. Analysis of Zinc and Metallothionein (MT) Content in Tissues
4.11.3. Determination of Acetylcholine and Acetylcholinesterase in Brain Samples
4.11.4. Quantification of Hydrogen Peroxide and Reactive Oxygen Species Levels in Brain Samples
4.11.5. Determination of MDA, TBARS, 4-HNE, 8-OH-dG, Catecholamine, and Cortisol Levels in Brain Samples
4.11.6. Determination of Antioxidant Capacity in Brain Samples
4.11.7. Evaluation of Neurotransmitter Levels in Brain Samples
4.11.8. Estimation of Amyloid Beta (Aβ-42) and p-Tau Levels in the Brain
4.11.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A

| Toxicant | Model Organism | Concentration | Study | Behavior Endpoints | Toxic Effects | LC50 | References |
|---|---|---|---|---|---|---|---|
| ZnCl2 | Mice | 7.5, 10, 15 mg/kg | In vivo | No | Cytotoxicity, head abnormalities | NA | [77] |
| ZnCl2 | Neuronal cells | 0.15 mmol/L | In vitro | No | Loss of viability | NA | [78] |
| ZnCl2 + ZnO | MDKC cells | 100 μg/mL | In vitro | No | Cytotoxicity, genotoxicity | NA | [79] |
| ZnCl2 | Mice | 10 ppm | In vivo | Yes | Depressive Behavior | NA | [80] |
| Zinc | Mice | equimolar | In vitro | No | Stimulate hippocampal microglia activation | NA | [20] |
| ZnCl2 | D. magna | 0.16 ppm | In vivo | No | Toxic effects on growth and reproduction | NA | [81] |
| ZnCl2 + ZnO | C. elegans larvae | 614 μM | In vivo | No | Genotoxicity, Apoptosis | NA | [82] |
| Zinc | Red sea bream | 2.5 ppm | In vivo | No | Toxic effects on development and growth | 4.3 ppm | [18] |
| ZnCl2 | Zebrafish embryo | 0.5 ppm | In vivo | No | Skeletal anomalies | NA | [83] |
| ZnCl2 | Zebrafish embryo | 0.1–0.5 ppm | In vivo | No | Developmental deformities | NA | [84] |
| This study Low concentration of ZnCl2 | Adult zebrafish | 0.1–1.5 ppm | In vivo | Yes, we used 5 behavioralparameters | Memory impairment, locomotor deficit, and high AChE content in brain | 6 ppm | This study |
References
- Martins, R.N.; Villemagne, V.; Sohrabi, H.R.; Chatterjee, P.; Shah, T.M.; Verdile, G.; Fraser, P.; Taddei, K.; Gupta, V.B.; Rainey-Smith, S.R. Alzheimer’s Disease: A Journey from Amyloid Peptides and Oxidative Stress, to Biomarker Technologies and Disease Prevention Strategies—Gains from AIBL and DIAN Cohort Studies. J. Alzheimers Dis. 2018, 62, 965–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markesbery, W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef]
- Nitsch, R.M.; Blusztajn, J.K.; Pittas, A.G.; Slack, B.E.; Growdon, J.H.; Wurtman, R.J. Evidence for a membrane defect in Alzheimer disease brain. Proc. Natl. Acad. Sci. USA 1992, 89, 1671–1675. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A. Zinc and Zinc Transport and Sequestration Proteins in the Brain in the Progression of Alzheimer’s Disease. In Neurochemical Mechanisms in Disease; Springer: Berlin, Germany, 2011; pp. 669–693. [Google Scholar]
- Divakaruni, A.S.; Wiley, S.E.; Rogers, G.W.; Andreyev, A.Y.; Petrosyan, S.; Loviscach, M.; Wall, E.A.; Yadava, N.; Heuck, A.P.; Ferrick, D.A. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc. Natl. Acad. Sci. USA 2013, 110, 5422–5427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandon, L.; Ni, B.-F.; Ding, X.; Ehmann, W.; Kasarskis, E.; Markesbery, W. RNAA for arsenic, cadmium, copper, and molybdenum in CNS tissues from subjects with age-related neurodegenerative diseases. J. Radioanal. Nucl. Chem. 1994, 179, 331–339. [Google Scholar] [CrossRef]
- Thompson, C.; Markesbery, W.; Ehmann, W.; Mao, Y.; Vance, D. Regional brain trace-element studies in Alzheimer’s disease. Neurotoxicology 1988, 9, 1–7. [Google Scholar] [PubMed]
- Lovell, M.; Robertson, J.; Teesdale, W.; Campbell, J.; Markesbery, W. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- DeGrado, T.R.; Kemp, B.J.; Pandey, M.K.; Jiang, H.; Gunderson, T.M.; Linscheid, L.R.; Woodwick, A.R.; McConnell, D.M.; Fletcher, J.G.; Johnson, G.B. First PET imaging studies with 63Zn-zinc citrate in healthy human participants and patients with Alzheimer disease. Mol. Imaging 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid β-peptide (1–42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Murgia, C.; Lang, C.; Truong-Tran, A.; Grosser, D.; Jayaram, L.; Ruffin, R.; Perozzi, G.; Zalewski, P. Zinc and its specific transporters as potential targets in airway disease. Curr. Drug Targets 2006, 7, 607–627. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, P.D. Zinc metabolism in the airway: Basic mechanisms and drug targets. Curr. Opin. Pharmacol. 2006, 6, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Zastrow, M.L.; Pecoraro, V.L. Designing hydrolytic zinc metalloenzymes. Biochemistry 2014, 53, 957–978. [Google Scholar] [CrossRef] [PubMed]
- Fay, M. Exposure to contaminant mixtures at US hazardous waste sites. WIT Trans. Ecol. Environ. 2005, 85. [Google Scholar] [CrossRef]
- Stankevičiūtė, M.; Sauliutė, G.; Svecevičius, G.; Kazlauskienė, N.; Baršienė, J. Genotoxicity and cytotoxicity response to environmentally relevant complex metal mixture (Zn, Cu, Ni, Cr, Pb, Cd) accumulated in Atlantic salmon (Salmo salar). Part I: Importance of exposure time and tissue dependence. Ecotoxicology 2017, 26, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, M.S.; Stevens, D.; Kamunde, C. Zinc and calcium alter the relationship between mitochondrial respiration, ROS and membrane potential in rainbow trout (Oncorhynchus mykiss) liver mitochondria. Aquat. Toxicol. 2017, 189, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xiao, Y.-Y.; Ji, Y.-H.; Liu, M.-Z.; Chen, Y.; Zeng, Y.-L.; Zhang, Y.-G.; Jin, L. CuInS2/ZnS QD exposure induces developmental toxicity, oxidative stress and DNA damage in rare minnow (Gobiocypris rarus) embryos and larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 198, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Cao, L.; Shan, X.; Xiao, Z.; Wang, Q.; Dou, S. Toxic effects of zinc on the development, growth, and survival of red sea bream Pagrus major embryos and larvae. Arch. Environ. Contam. Toxicol. 2010, 58, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, M.; Antunes, C.; Guilhermino, L. Enzymatic biomarkers in the crab Carcinus maenas from the Minho River estuary (NW Portugal) exposed to zinc and mercury. Chemosphere 2007, 66, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-C.; Yu, W.-C.; Shih, Y.-H.; Chen, C.-Y.; Guo, Z.-H.; Huang, S.-J.; Chan, J.C.; Chen, Y.-R. Zinc ion rapidly induces toxic, off-pathway amyloid-β oligomers distinct from amyloid-β derived diffusible ligands in Alzheimer’s disease. Sci. Rep. 2018, 8, 4772. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.; Wu, P.c.; Chung, I.f.; Jiang, J.H.; Fann, M.J.; Kao, L.S. Cell death caused by the synergistic effects of zinc and dopamine is mediated by a stress sensor gene Gadd45b–implication in the pathogenesis of Parkinson’s disease. J. Neurochem. 2016, 139, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.L.; Zipfel, G.J.; Sheline, C.T. Zinc neurotoxicity is dependent on intracellular NAD+ levels and the sirtuin pathway. Eur. J. Neurosci. 2006, 24, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W.; Koh, J.Y. Zinc and brain injury. Annu. Rev. Neurosci. 1998, 21, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Kimura, K.; Morioka, S.; Tamaki, N. Inhibitory effects of Zn2+ on muscle glycolysis and their reversal by histidine. J. Nutr. Sci. Vitaminol. 1980, 26, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Krotkiewska, B.; Banas, T. Interaction of Zn2+, and Cu2+, Ions with glyceraldehyde-3-phosphate dehydrogenase from bovine heart and rabbit muscle. Int. J. Biochem. 1992, 24, 1501–1505. [Google Scholar] [CrossRef]
- Dineley, K.; Brocard, J.; Reynolds, I. Elevated intracellular zinc and altered proton homeostasis in forebrain neurons. Neuroscience 2002, 114, 439–449. [Google Scholar] [CrossRef]
- Conte-Daban, A.; Day, A.; Faller, P.; Hureau, C. How Zn can impede Cu detoxification by chelating agents in Alzheimer’s disease: A proof-of-concept study. Dalton Trans. 2016, 45, 15671–15678. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.-I.; Kawahara, M. Copper enhances zinc-induced neurotoxicity and the endoplasmic reticulum stress response in a neuronal model of vascular dementia. Front. Neurosci. 2017, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhu, L.; Dong, M.; Wang, J.; Wang, J.; Xie, H.; Liu, T.; Guo, Y. Oxidative stress and genotoxicity of the ionic liquid 1-octyl-3-methylimidazolium bromide in zebrafish (Danio rerio). Arch. Environ. Contam. Toxicol. 2014, 67, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, D.; Nunes, M.M.; Wallau, G.; Martins, I.; Zemolin, A.P.; Cruz, L.; Rodrigues, N.; Lopes, A.; Posser, T.; Franco, J. Oxidative stress markers in fish (Astyanax sp. and Danio rerio) exposed to urban and agricultural effluents in the Brazilian Pampa biome. Environ. Sci. Pollut. Res. 2015, 22, 15526–15535. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, R.; Das, S.K.; Patri, M. Modulation of benzo [a]pyrene induced anxiolytic-like behavior by retinoic acid in zebrafish: Involvement of oxidative stress and antioxidant defense system. Neurotox. Res. 2017, 31, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.J. Cholinergic control of GABA release: Emerging parallels between neocortex and hippocampus. Trends Neurosci. 2008, 31, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, C.; Chatonnet, A.; Takke, C.; Yan, Y.; Postlethwait, J.; Toutant, J.-P.; Cousin, X. Zebrafish acetylcholinesterase is encoded by a single gene localized on linkage group 7 gene structure and polymorphism; molecular forms and expression pattern during development. J. Biol. Chem. 2001, 276, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Senger, M.R.; Rico, E.P.; de Bem Arizi, M.; Frazzon, A.P.G.; Dias, R.D.; Bogo, M.R.; Bonan, C.D. Exposure to Hg2+ and Pb2+ changes NTPDase and ecto-5′-nucleotidase activities in central nervous system of zebrafish (Danio rerio). Toxicology 2006, 226, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Rico, E.P.; Rosemberg, D.B.; Senger, M.R.; de Bem Arizi, M.; Bernardi, G.F.; Dias, R.D.; Bogo, M.R.; Bonan, C.D. Methanol alters ecto-nucleotidases and acetylcholinesterase in zebrafish brain. Neurotoxicol. Teratol. 2006, 28, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Dix, D.J.; Houck, K.A.; Martin, M.T.; Richard, A.M.; Setzer, R.W.; Kavlock, R.J. The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol. Sci. 2006, 95, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Boran, H.; Ulutas, G. Genotoxic effects and gene expression changes in larval zebrafish after exposure to ZnCl2 and ZnO nanoparticles. Dis. Aquat. Organ. 2016, 117, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Audira, G.; Sampurna, B.P.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Hsiao, C.-D. A simple setup to perform 3D locomotion tracking in zebrafish by using a single camera. Inventions 2018, 3, 11. [Google Scholar] [CrossRef]
- Levin, E.D.; Bencan, Z.; Cerutti, D.T. Anxiolytic effects of nicotine in zebrafish. Physiol. Behav. 2007, 90, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.; Raymond, J.; Hester, J.; Kyzar, E.; Gaikwad, S.; Bruce, I.; Fryar, C.; Chanin, S.; Enriquez, J.; Bagawandoss, S. Assessing social behavior phenotypes in adult zebrafish: Shoaling, social preference, and mirror biting tests. In Zebrafish Protocols for Neurobehavioral Research; Springer: Berlin, Germany, 2012; pp. 231–246. [Google Scholar]
- Maximino, C.; da Silva, A.W.B.; Gouveia, A., Jr.; Herculano, A.M. Pharmacological analysis of zebrafish (Danio rerio) scototaxis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Serra, E.; Medalha, C.; Mattioli, R. Natural preference of zebrafish (Danio rerio) for a dark environment. Braz. J. Med. Biol. Res. 1999, 32, 1551–1553. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.P.; Chen, H.-C. Effects of cadmium and zinc on oxygen consumption, ammonium excretion, and osmoregulation of white shrimp (Litopenaeus vannamei). Chemosphere 2004, 57, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Ciji, P.; Nandan, S.B. Toxicity of copper and zinc to Puntius parrah (Day, 1865). Mar. Environ. Res. 2014, 93, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Loro, V.L.; Nogueira, L.; Nadella, S.R.; Wood, C.M. Zinc bioaccumulation and ionoregulatory impacts in Fundulus heteroclitus exposed to sublethal waterborne zinc at different salinities. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 166, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Oshima, Y.; Yamaguchi, T.; Tsuruda, Y.; Kang, I.J.; Kobayashi, M.; Imada, N.; Honjo, T. Fertilization success and sexual behavior in male medaka, Oryzias latipes, exposed to tributyltin. Chemosphere 2004, 55, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-S.; Chung, N.-I.; Choi, K.-H.; Cha, E.Y.; Lee, S.-K.; Chon, T.-S. Computational characterization of behavioral response of medaka (Oryzias latipes) treated with diazinon. Aquat. Toxicol. 2005, 71, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Azizullah, A.; Richter, P.; Häder, D.-P. Comparative toxicity of the pesticides carbofuran and malathion to the freshwater flagellate Euglena gracilis. Ecotoxicology 2011, 20, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Garaventa, F.; Gambardella, C.; Di Fino, A.; Pittore, M.; Faimali, M. Swimming speed alteration of Artemia sp. and Brachionus plicatilis as a sub-lethal behavioural end-point for ecotoxicological surveys. Ecotoxicology 2010, 19, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Makaras, T.; Svecevičius, G.; Kazlauskienė, N.; Montvydienė, D. Rapid Detection of Sublethal Toxicity Using Locomotor Activity of Rainbow Trout Juveniles. Bull. Environ. Contam. Toxicol. 2018, 100, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Fan, C.; Chen, X.; Liu, J.; Wang, B.; Huang, Z.; Zhang, Y.; Meng, X.; Zou, F. Effects of cadmium, manganese, and lead on locomotor activity and neurexin 2a expression in zebrafish. Environ. Toxicol. Chem. 2017, 36, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.S.; Giacomini, A.C.; Rodriguez, R.; Kalueff, A.V.; Barcellos, L.J. Effects of ZnSO4-induced peripheral anosmia on zebrafish behavior and physiology. Behav. Brain Res. 2017, 320, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Mocelin, R.; Herrmann, A.P.; Marcon, M.; Rambo, C.L.; Rohden, A.; Bevilaqua, F.; de Abreu, M.S.; Zanatta, L.; Elisabetsky, E.; Barcellos, L.J. N-acetylcysteine prevents stress-induced anxiety behavior in zebrafish. Pharmacol. Biochem. Behav. 2015, 139, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Deibel, M.; Ehmann, W.; Markesbery, W. Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer’s disease: Possible relation to oxidative stress. J. Neurol. Sci. 1996, 143, 137–142. [Google Scholar] [CrossRef]
- Tekkök, S.B.; Ye, Z.; Ransom, B.R. Excitotoxic mechanisms of ischemic injury in myelinated white matter. J. Cereb. Blood Flow Metab. 2007, 27, 1540–1552. [Google Scholar] [CrossRef] [PubMed]
- De Matos Mansur, B.; Cavalcante, C.N.S.; dos Santos, B.R.; Gouveia, A. Effects of mercury chloride (HgCl2) on Betta splendens aggressive display. Span. J. Psychol. 2012, 15, 442–450. [Google Scholar] [CrossRef]
- Weber, D.N.; Ghorai, J.K. Experimental design affects social behavior outcomes in adult zebrafish developmentally exposed to lead. Zebrafish 2013, 10, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.R.; Sloman, K.A. The effects of environmental pollutants on complex fish behaviour: Integrating behavioural and physiological indicators of toxicity. Aquat. Toxicol. 2004, 68, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Bégay, V.R.; Falcón, J.; Cahill, G.M.; Klein, D.C.; Coon, S.L. Transcripts encoding two melatonin synthesis enzymes in the teleost pineal organ: Circadian regulation in pike and zebrafish, but not in trout. Endocrinology 1998, 139, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Noche, R.R.; Lu, P.-N.; Goldstein-Kral, L.; Glasgow, E.; Liang, J.O. Circadian rhythms in the pineal organ persist in zebrafish larvae that lack ventral brain. BMC Neurosci. 2011, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Aton, S.J.; Huettner, J.E.; Straume, M.; Herzog, E.D. GABA and Gi/o differentially control circadian rhythms and synchrony in clock neurons. Proc. Natl. Acad. Sci. USA 2006, 103, 19188–19193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardinali, D.P.; Furio, A.M.; Brusco, L.I. Clinical aspects of melatonin intervention in Alzheimer’s disease progression. Curr. Neuropharmacol. 2010, 8, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Morris, S.; Dinman, J.; Pine, R.; Smith, B. Role of metallothionein in detoxification and tolerance to transition metals. In Metallothionein II; Springer: Berlin, Germany, 1987; pp. 439–446. [Google Scholar]
- De Lisle, R.C.; Sarras, M.P., Jr.; Hidalgo, J.; Andrews, G.K. Metallothionein is a component of exocrine pancreas secretion: Implications for zinc homeostasis. Am. J. Physiol. Cell Physiol. 1996, 271, C1103–C1110. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.L.; Wasserloos, K.; Croix, C.M.S.; Gandley, R.; Levitan, E.S.; Pitt, B.R. Metallothionein, nitric oxide and zinc homeostasis in vascular endothelial cells. J. Nutr. 2000, 130, 1467S–1470S. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.A.; Stürzenbaum, S.; Nicolaus, B.; Morgan, A.J.; Harwood, J.L.; Kille, P. Identification and characterisation of metallothioneins from environmental indicator species as potential biomonitors. In Metallothionein IV; Springer: Berlin, Germany, 1999; pp. 621–627. [Google Scholar]
- Knapen, D.; Reynders, H.; Bervoets, L.; Verheyen, E.; Blust, R. Metallothionein gene and protein expression as a biomarker for metal pollution in natural gudgeon populations. Aquat. Toxicol. 2007, 82, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D. Oxidative stress hypothesis in Alzheimer’s disease: A reappraisal. Trends Pharmacol. Sci. 2008, 29, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Condello, C.; Yuan, P.; Schain, A.; Grutzendler, J. Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques. Nat. Commun. 2015, 6, 6176. [Google Scholar] [CrossRef] [PubMed]
- Sliwinska, A.; Kwiatkowski, D.; Czarny, P.; Milczarek, J.; Toma, M.; Korycinska, A.; Szemraj, J.; Sliwinski, T. Genotoxicity and cytotoxicity of ZnO and Al2O3 nanoparticles. Toxicol. Mech. Methods 2015, 25, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Blank, M.; Guerim, L.D.; Cordeiro, R.F.; Vianna, M.R. A one-trial inhibitory avoidance task to zebrafish: Rapid acquisition of an NMDA-dependent long-term memory. Neurobiol. Learn. Mem. 2009, 92, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Gerlai, R.; Lahav, M.; Guo, S.; Rosenthal, A. Drinks like a fish: Zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol. Biochem. Behav. 2000, 67, 773–782. [Google Scholar] [CrossRef]
- Moretz, J.A.; Martins, E.P.; Robison, B.D. The effects of early and adult social environment on zebrafish (Danio rerio) behavior. Environ. Biol. Fishes 2007, 80, 91–101. [Google Scholar] [CrossRef]
- Bass, S.L.; Gerlai, R. Zebrafish (Danio rerio) responds differentially to stimulus fish: The effects of sympatric and allopatric predators and harmless fish. Behav. Brain Res. 2008, 186, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Patterns of avoidance behaviours in the light/dark preference test in young juvenile zebrafish: A pharmacological study. Behav. Brain Res. 2011, 222, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Talukder, G.; Sharma, A. Cytotoxicity of zinc chloride in mice in vivo. Biol. Trace Elem. Res. 1991, 30, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Zyśk, M.; Bielarczyk, H.; Gul-Hinc, S.; Dyś, A.; Gapys, B.; Ronowska, A.; Sakowicz-Burkiewicz, M.; Szutowicz, A. Phenotype-dependent interactions between N-acetyl-l-aspartate and acetyl-coa in septal sn56 cholinergic cells exposed to an excess of zinc. J. Alzheimer’s Dis. 2017, 56, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Kononenko, V.; Repar, N.; Marušič, N.; Drašler, B.; Romih, T.; Hočevar, S.; Drobne, D. Comparative in vitro genotoxicity study of zno nanoparticles, zno macroparticles and ZnCl2 to mdck kidney cells: Size matters. Toxicol. In Vitro 2017, 40, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Manosso, L.M.; Moretti, M.; Colla, A.R.; Ribeiro, C.M.; Dal-Cim, T.; Tasca, C.I.; Rodrigues, A.L.S. Involvement of glutamatergic neurotransmission in the antidepressant-like effect of zinc in the chronic unpredictable stress model of depression. J. Neural Transm. 2016, 123, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Adam, N.; Schmitt, C.; Galceran, J.; Companys, E.; Vakurov, A.; Wallace, R.; Knapen, D.; Blust, R. The chronic toxicity of zno nanoparticles and ZnCl2 to daphnia magna and the use of different methods to assess nanoparticle aggregation and dissolution. Nanotoxicology 2014, 8, 709–717. [Google Scholar] [PubMed]
- O’donnell, B.; Huo, L.; Polli, J.R.; Qiu, L.; Collier, D.N.; Zhang, B.; Pan, X. From the cover: Zno nanoparticles enhanced germ cell apoptosis in Caenorhabditis elegans, in comparison with ZnCl2. Toxicol. Sci. 2016, 156, 336–343. [Google Scholar]
- Salvaggio, A.; Marino, F.; Albano, M.; Pecoraro, R.; Camiolo, G.; Tibullo, D.; Bramanti, V.; Lombardo, B.M.; Saccone, S.; Mazzei, V. Toxic effects of zinc chloride on the bone development in Danio rerio (hamilton, 1822). Front. Physiol. 2016, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Küçükoğlu, M.; Binokay, U.S.; Pekmezekmek, A.B. The effects of zinc chloride during early embryonic development in zebrafish (Brachydanio rerio). Turkish J. Biol. 2013, 37, 158–164. [Google Scholar]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarasamma, S.; Audira, G.; Juniardi, S.; Sampurna, B.P.; Liang, S.-T.; Hao, E.; Lai, Y.-H.; Hsiao, C.-D. Zinc Chloride Exposure Inhibits Brain Acetylcholine Levels, Produces Neurotoxic Signatures, and Diminishes Memory and Motor Activities in Adult Zebrafish. Int. J. Mol. Sci. 2018, 19, 3195. https://doi.org/10.3390/ijms19103195
Sarasamma S, Audira G, Juniardi S, Sampurna BP, Liang S-T, Hao E, Lai Y-H, Hsiao C-D. Zinc Chloride Exposure Inhibits Brain Acetylcholine Levels, Produces Neurotoxic Signatures, and Diminishes Memory and Motor Activities in Adult Zebrafish. International Journal of Molecular Sciences. 2018; 19(10):3195. https://doi.org/10.3390/ijms19103195
Chicago/Turabian StyleSarasamma, Sreeja, Gilbert Audira, Stevhen Juniardi, Bonifasius Putera Sampurna, Sung-Tzu Liang, Erwei Hao, Yu-Heng Lai, and Chung-Der Hsiao. 2018. "Zinc Chloride Exposure Inhibits Brain Acetylcholine Levels, Produces Neurotoxic Signatures, and Diminishes Memory and Motor Activities in Adult Zebrafish" International Journal of Molecular Sciences 19, no. 10: 3195. https://doi.org/10.3390/ijms19103195
APA StyleSarasamma, S., Audira, G., Juniardi, S., Sampurna, B. P., Liang, S.-T., Hao, E., Lai, Y.-H., & Hsiao, C.-D. (2018). Zinc Chloride Exposure Inhibits Brain Acetylcholine Levels, Produces Neurotoxic Signatures, and Diminishes Memory and Motor Activities in Adult Zebrafish. International Journal of Molecular Sciences, 19(10), 3195. https://doi.org/10.3390/ijms19103195






