The Emerging Role of Cyclin-Dependent Kinases (CDKs) in Pancreatic Ductal Adenocarcinoma
Abstract
:1. Background
2. Family of CDKs
3. Cell Cycle Regulation by CDKs
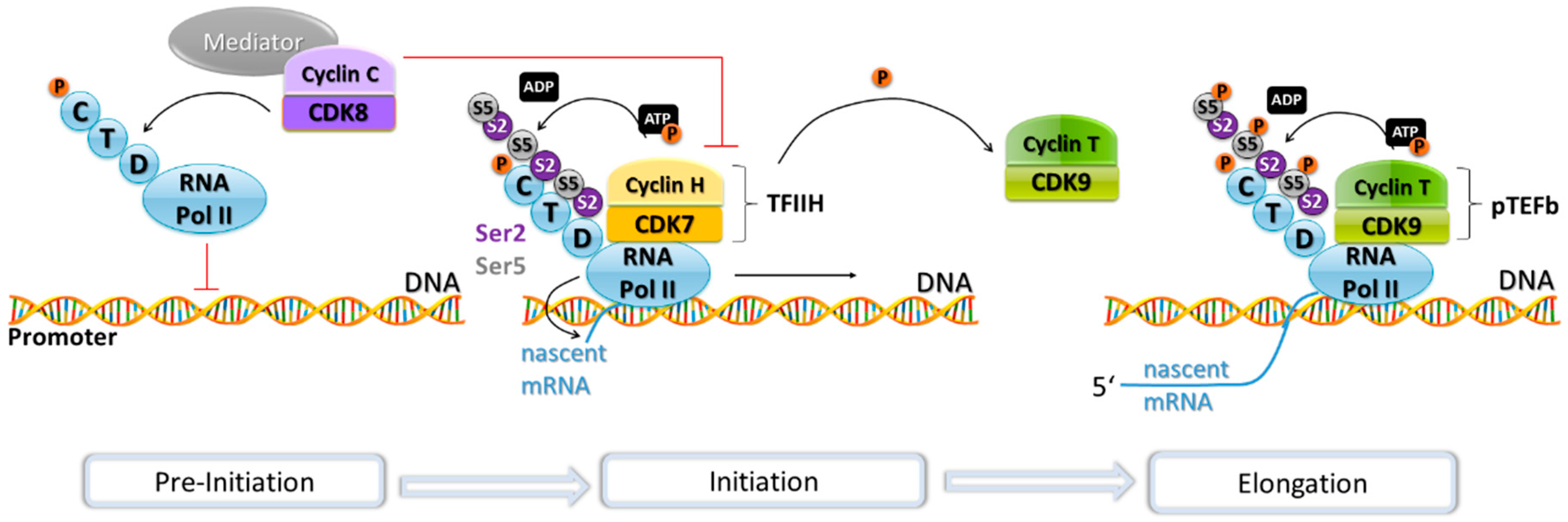
4. Transcriptional Regulation by CDKs
5. The Role of CDKs in Pancreatic Cancer
6. CDK Inhibition in PDAC
7. Clinical Trials of CDK-Inhibitors
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CAK | CDK-activating kinase |
| CDK | Cyclin-dependent kinase |
| CDKN2A | Cyclin-dependent kinase Inhibitor 2A |
| CLL | Chronic lymphocytic leukemia |
| CMGC | CDKs, MAPKs, Gsk3β, and CDK-like kinases |
| CTD | C-Terminal Domain |
| DYRK | Dual-specificity tyrosine-regulated kinase |
| ELL2 | Elongation Factor for RNA Polymerase II |
| FACT protein | Facilitates chromatin transcription |
| FDA | US Food and Drug Administration |
| FOLFIRINOX | FOL = Folinic acid, L = Leucovorin, F = 5-FU = 5-Fluorouracil, IRIN = Irinotecan, OX = Oxaliplatin |
| FoxM1 | Forkhead box protein M1 |
| GADD45 | Growth Arrest and DNA Damage-inducible protein |
| GSK3β | Glycogen synthase kinase-3 β |
| hESC | Human embryonic stem cells |
| hiPSC | Human induced pluripotent stem cells |
| INK4 | Inhibitor of Cyclin-Dependent Kinase 4 |
| MAPK | Mitogen-activated protein kinase |
| MAT1 | Menage á trois 1 |
| Mcl-1 | Myeloid leukaemia cell differentiation protein 1 |
| MED12 | Mediator complex subunit 12 |
| MED13 | Mediator complex subunit 13 |
| MPK7 | Mitogen-activated protein kinase 7 |
| mTOR | Mechanistic target of rapamycin |
| NSCLC | Non-small cell lung cancer |
| PanIN | Pancreatic intraepithelial neoplasia |
| PDAC | Pancreatic ductal adenocarcinoma |
| pTEFb | Positive transcription elongation factor b |
| RalA | RAS-Like Protein A |
| Rb | Retinoblastoma protein |
| RNA Pol II | RNA polymerase II |
| ROS | Reactive oxygen species |
| TAK | Tat-associated kinase |
| TCF/LEF | T-cell factor/lymphoid enhancer factor |
| TF | Transcription factor |
| TFIIH | Transcription factor II H |
| TP53 | Tumour protein p53 |
| TPKII | Tau protein kinase II |
| TRAIL-R1/2 | Tumour necrosis factor-related, apoptosis-inducing ligand receptors 1/2 |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M. Pancreatic Cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Simoes, P.K.; Olson, S.H.; Saldia, A.; Kurtz, R.C. Epidemiology of pancreatic adenocarcinoma. Chin. Clin. Oncol. 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Kupfer, S.S.; Brim, H.; Carethers, J.M. Accepted Manuscript Racial Disparity in Gastrointestinal Cancer Risk. Gastroenterol. Gastrointest. Cancer Risk Gastroenterol. 2017. [Google Scholar] [CrossRef]
- Sanabria Mateos, R.; Conlon, K.C. Pancreatic cancer. Surgery 2016, 34, 282–291. [Google Scholar] [CrossRef]
- Mancuso, A.; Calabrò, F.; Sternberg, C.N. Current therapies and advances in the treatment of pancreatic cancer. Crit. Rev. Oncol. Hematol. 2006, 58, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Chand, S.; O’Hayer, K.; Blanco, F.F.; Winter, J.M.; Brody, J.R. The landscape of pancreatic cancer therapeutic resistance mechanisms. Int. J. Biol. Sci. 2016, 12, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Westphal, S.; Kalthoff, H. Apoptosis: Targets in Pancreatic Cancer. Mol. Cancer 2003, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.W.; Ruefli, A.A.; Lowe, S.W. Apoptosis: A link between cancer genetics and chemotherapy. Cell 2002, 108, 153–164. [Google Scholar] [CrossRef]
- Conroy, T.; Bachet, J.-B.; Ayav, A.; Huguet, F.; Lambert, A.; Caramella, C.; Maréchal, R.; Van Laethem, J.-L.; Ducreux, M. Current standards and new innovative approaches for treatment of pancreatic cancer. Eur. J. Cancer 2016, 57, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Kretz, A.-L.; von Karstedt, S.; Hillenbrand, A.; Henne-Bruns, D.; Knippschild, U.; Trauzold, A.; Lemke, J. Should We Keep Walking along the Trail for Pancreatic Cancer Treatment? Revisiting TNF-Related Apoptosis-Inducing Ligand for Anticancer Therapy. Cancers 2018, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Unicancer GI PRODIGE 24/CCTG PA.6 Trial: A Multicenter International Randomized Phase III Trial of Adjuvant mFOLFIRINOX versus Gemcitabine (gem) in Patients with Resected Pancreatic Ductal Adenocarcinomas. 2018 ASCO Annual Meeting Abstracts. Available online: http://abstracts.asco.org/214/AbstView_214_218335.html (accessed on 2 August 2018).
- Rossi, M.L.; Rehman, A.A.; Gondi, C.S. Therapeutic options for the management of pancreatic cancer. World J. Gastroenterol. 2014, 20, 11142–11159. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Van Buren, G.; Fisher, W.E. Pancreatic cancer: Advances in treatment. World J. Gastroenterol. 2014, 20, 9354–9360. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, W.; Huang, P.; Xu, Y.Z.; Heinemann, V.; Grunewald, R.; Gandhi, V. Gemcitabine: Metabolism, mechanisms of action, and self-potentiation. Semin. Oncol. 1995, 22, 3–10. [Google Scholar] [PubMed]
- Burris, H.A.; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.S.; Park, S.W. Systemic Chemotherapy in Advanced Pancreatic Cancer. Gut Liver 2016, 10, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Saung, M.T.; Zheng, L. Current Standards of Chemotherapy for Pancreatic Cancer. Clin. Ther. 2017, 39, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Assaf, E.; Verlinde-Carvalho, M.; Delbaldo, C.; Grenier, J.; Sellam, Z.; Pouessel, D.; Bouaita, L.; Baumgaertner, I.; Sobhani, I.; Tayar, C.; et al. 5-fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncology 2011, 80, 301–306. [Google Scholar] [CrossRef] [PubMed]
- de W Marsh, R.; Talamonti, M.S.; Baker, M.S.; Posner, M.; Roggin, K.; Matthews, J.; Catenacci, D.; Kozloff, M.; Polite, B.; Britto, M.; et al. Primary systemic therapy in resectable pancreatic ductal adenocarcinoma using mFOLFIRINOX: A pilot study. J. Surg. Oncol. 2018, 117, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Gunturu, K.S.; Yao, X.; Cong, X.; Thumar, J.R.; Hochster, H.S.; Stein, S.M.; Lacy, J. FOLFIRINOX for locally advanced and metastatic pancreatic cancer: Single institution retrospective review of efficacy and toxicity. Med. Oncol. 2013, 30, 361. [Google Scholar] [CrossRef] [PubMed]
- Faris, J.E.; Blaszkowsky, L.S.; McDermott, S.; Guimaraes, A.R.; Szymonifka, J.; Huynh, M.A.; Ferrone, C.R.; Wargo, J.A.; Allen, J.N.; Dias, L.E.; et al. FOLFIRINOX in locally advanced pancreatic cancer: The Massachusetts General Hospital Cancer Center experience. Oncologist 2013, 18, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awan, F.T.; Jones, J.A.; Maddocks, K.; Poi, M.; Grever, M.R.; Johnson, A.; Byrd, J.C.; Andritsos, L.A. A phase 1 clinical trial of flavopiridol consolidation in chronic lymphocytic leukemia patients following chemoimmunotherapy. Ann. Hematol. 2016, 95, 1137–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [PubMed]
- Hanks, S.K.; Hunter, T. Protein kinases 6. The eukaryotic protein kinase superfamily: Kinase (catalytic) domain structure and classification. FASEB J. 1995, 9, 576–596. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef] [PubMed]
- Mikolcevic, P.; Rainer, J.; Geley, S. Orphan kinases turn eccentric: A new class of cyclin Y-activated, membrane-targeted CDKs. Cell Cycle 2012, 11, 3758–3768. [Google Scholar] [CrossRef] [PubMed]
- Rane, S.G.; Dubus, P.; Mettus, R.V.; Galbreath, E.J.; Boden, G.; Reddy, E.P.; Barbacid, M. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in β-islet cell hyperplasia. Nat. Genet. 1999, 22, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Berthet, C.; Aleem, E.; Coppola, V.; Tessarollo, L.; Kaldis, P. Cdk2 knockout mice are viable. Curr. Biol. 2003, 13, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Ortega, S.; Prieto, I.; Odajima, J.; Martin, A.; Dubus, P.; Sotillo, R.; Barbero, J.L.; Malumbres, M.; Barbacid, M. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat. Genet. 2003, 35, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Sotillo, R.; Santamaria, D.; Galan, J.; Cerezo, A.; Ortega, S.; Dubus, P.; Barbacid, M. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 2004, 118, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Harlow, E.; Hunt, T.; Hunter, T.; Lahti, J.M.; Manning, G.; Morgan, D.O.; Tsai, L.H.; Wolgemuth, D.J. Cyclin-dependent kinases: A family portrait. Nat. Cell Biol. 2009, 11, 1275–1276. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, L.; Ratnacaram, C.K.; Kaldis, P. Established and novel Cdk/cyclin complexes regulating the cell cycle and development. Results Probl. Cell Differ. 2011, 53, 365–389. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Roberts, J.M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. To cycle or not to cycle: A critical decision in cancer. Nat. Rev. Cancer 2001, 1, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Bendris, N.; Lemmers, B.; Blanchard, J.M. Cell cycle, cytoskeleton dynamics and beyond: The many functions of cyclins and CDK inhibitors. Cell Cycle 2015, 14, 1786–1798. [Google Scholar] [CrossRef] [PubMed]
- Pavletich, N.P. Mechanisms of cyclin-dependent kinase regulation: Structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J. Mol. Biol. 1999, 287, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.O. Principles of CDK regulation. Nature 1995, 374, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.G.; Norbury, C.J.; Spurr, N.K.; Nurse, P. Regulated expression and phosphorylation of a possible mammalian cell-cycle control protein. Nature 1988, 333, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Jing, T.; Liu, B.; Yao, J.; Tan, M.; McDonnell, T.J.; Hung, M.C. Overexpression of ErbB2 blocks Taxol-induced apoptosis by upregulation of p21Cip1, which inhibits p34Cdc2 kinase. Mol. Cell 1998, 2, 581–591. [Google Scholar] [CrossRef]
- Moore, J.D.; Kirk, J.A.; Hunt, T. Unmasking the S-phase-promoting potential of cyclin B1. Science 2003, 300, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, D.; Barriere, C.; Cerqueira, A.; Hunt, S.; Tardy, C.; Newton, K.; Caceres, J.F.; Dubus, P.; Malumbres, M.; Barbacid, M. Cdk1 is sufficient to drive the mammalian cell cycle. Nature 2007, 448, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.H.; Harlow, E.; Meyerson, M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature 1991, 353, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Sheaff, R.J.; Groudine, M.; Gordon, M.; Roberts, J.M.; Clurman, B.E. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997, 11, 1464–1478. [Google Scholar] [CrossRef] [PubMed]
- Hinchcliffe, E.H.; Li, C.; Thompson, E.A.; Maller, J.L.; Sluder, G. Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science 1999, 283, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Maller, J.L. A centrosomal localization signal in cyclin E required for Cdk2-independent S phase entry. Science 2004, 306, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Zariwala, M.; Liu, J.; Xiong, Y. Cyclin E2, a novel human G1 cyclin and activating partner of CDK2 and CDK3, is induced by viral oncoproteins. Oncogene 1998, 17, 2787–2798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Zhu, C.; Harper, J.W. A premature-termination mutation in the Mus musculus cyclin-dependent kinase 3 gene. Proc. Natl. Acad. Sci. USA 2001, 98, 1682–1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, S.; Rollins, B.J. Cyclin C/cdk3 promotes Rb-dependent G0 exit. Cell 2004, 117, 239–251. [Google Scholar] [CrossRef]
- Harbour, J.W.; Luo, R.X.; Dei Santi, A.; Postigo, A.A.; Dean, D.C. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell 1999, 98, 859–869. [Google Scholar] [CrossRef]
- Lazarov, M.; Kubo, Y.; Cai, T.; Dajee, M.; Tarutani, M.; Lin, Q.; Fang, M.; Tao, S.; Green, C.L.; Khavari, P.A. CDK4 coexpression with Ras generates malignant human epidermal tumorigenesis. Nat. Med. 2002, 8, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, I.; Denissova, N.G.; Wang, G.; He, D.; Long, J.; Liu, F. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature 2004, 430, 226–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyerson, M.; Harlow, E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol. Cell Biol. 1994, 14, 2077–2086. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Fernandes, H.; Rocha, B. High expression of active CDK6 in the cytoplasm of CD8 memory cells favors rapid division. Nat. Immunol. 2004, 5, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Patrick, G.N.; Zukerberg, L.; Nikolic, M.; de la Monte, S.; Dikkes, P.; Tsai, L.H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 1999, 402, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Dhavan, R.; Tsai, L.H. A decade of CDK5. Nat. Rev. Mol. Cell Biol. 2001, 2, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Brinkkoetter, P.T.; Olivier, P.; Wu, J.S.; Henderson, S.; Krofft, R.D.; Pippin, J.W.; Hockenbery, D.; Roberts, J.M.; Shankland, S.J. Cyclin I activates Cdk5 and regulates expression of Bcl-2 and Bcl-XL in postmitotic mouse cells. J. Clin. Investig. 2009, 119, 3089–3101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagano, T.; Hashimoto, T.; Nakashima, A.; Hisanaga, S.; Kikkawa, U.; Kamada, S. Cyclin I is involved in the regulation of cell cycle progression. Cell Cycle 2013, 12, 2617–2624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, R.P.; Morgan, D.O. A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell 1994, 78, 713–724. [Google Scholar] [CrossRef]
- Shiekhattar, R.; Mermelstein, F.; Fisher, R.P.; Drapkin, R.; Dynlacht, B.; Wessling, H.C.; Morgan, D.O.; Reinberg, D. Cdk-activating kinase complex is a component of human transcription factor TFIIH. Nature 1995, 374, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Akoulitchev, S.; Chuikov, S.; Reinberg, D. TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature 2000, 407, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Tassan, J.P.; Jaquenoud, M.; Leopold, P.; Schultz, S.J.; Nigg, E.A. Identification of human cyclin-dependent kinase 8, a putative protein kinase partner for cyclin C. Proc. Natl. Acad. Sci. USA 1995, 92, 8871–8875. [Google Scholar] [CrossRef] [PubMed]
- Firestein, R.; Bass, A.J.; Kim, S.Y.; Dunn, I.F.; Silver, S.J.; Guney, I.; Freed, E.; Ligon, A.H.; Vena, N.; Ogino, S.; et al. CDK8 is a colorectal cancer oncogene that regulates β-catenin activity. Nature 2008, 455, 547–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsutsui, T.; Fukasawa, R.; Tanaka, A.; Hirose, Y.; Ohkuma, Y. Identification of target genes for the CDK subunits of the Mediator complex. Genes Cells 2011, 16, 1208–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shore, S.M.; Byers, S.A.; Maury, W.; Price, D.H. Identification of a novel isoform of Cdk9. Gene 2003, 307, 175–182. [Google Scholar] [CrossRef]
- Garriga, J.; Grana, X. Cellular control of gene expression by T-type cyclin/CDK9 complexes. Gene 2004, 337, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Guen, V.J.; Gamble, C.; Flajolet, M.; Unger, S.; Thollet, A.; Ferandin, Y.; Superti-Furga, A.; Cohen, P.A.; Meijer, L.; Colas, P. CDK10/cyclin M is a protein kinase that controls ETS2 degradation and is deficient in STAR syndrome. Proc. Natl. Acad. Sci. USA 2013, 110, 19525–19530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.H.; Wang, Y.C.; Fann, M.J. Identification and characterization of the CDK12/cyclin L1 complex involved in alternative splicing regulation. Mol. Cell. Biol. 2006, 26, 2736–2745. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yang, W.; Ni, T.; Tang, Z.; Nakadai, T.; Zhu, J.; Roeder, R.G. RNA polymerase II-associated factor 1 regulates the release and phosphorylation of paused RNA polymerase II. Science 2015, 350, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Kohoutek, J.; Blazek, D. Cyclin K goes with Cdk12 and Cdk13. Cell Div. 2012, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, D.; Mayeda, A.; Trembley, J.H.; Lahti, J.M.; Kidd, V.J. CDK11 complexes promote pre-mRNA splicing. J. Biol. Chem. 2003, 278, 8623–8629. [Google Scholar] [CrossRef] [PubMed]
- Loyer, P.; Trembley, J.H.; Grenet, J.A.; Busson, A.; Corlu, A.; Zhao, W.; Kocak, M.; Kidd, V.J.; Lahti, J.M. Characterization of cyclin L1 and L2 interactions with CDK11 and splicing factors: Influence of cyclin L isoforms on splice site selection. J. Biol. Chem. 2008, 283, 7721–7732. [Google Scholar] [CrossRef] [PubMed]
- Davidson, G.; Shen, J.; Huang, Y.L.; Su, Y.; Karaulanov, E.; Bartscherer, K.; Hassler, C.; Stannek, P.; Boutros, M.; Niehrs, C. Cell cycle control of wnt receptor activation. Dev. Cell 2009, 17, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Romano, G. Deregulations in the cyclin-dependent kinase-9-related pathway in cancer: Implications for drug discovery and development. ISRN Oncol. 2013, 2013, 305371. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Kim, S.Y.; Kim, Y.J.; Chung, Y.H. ALS2CR7 (CDK15) attenuates TRAIL induced apoptosis by inducing phosphorylation of survivin Thr34. Biochem. Biophys. Res. Commun. 2014, 450, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Dixon-Clarke, S.E.; Shehata, S.N.; Krojer, T.; Sharpe, T.D.; von Delft, F.; Sakamoto, K.; Bullock, A.N. Structure and inhibitor specificity of the PCTAIRE-family kinase CDK16. Biochem. J. 2017, 474, 699–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirose, T.; Tamaru, T.; Okumura, N.; Nagai, K.; Okada, M. PCTAIRE 2, a Cdc2-related serine/threonine kinase, is predominantly expressed in terminally differentiated neurons. Eur. J. Biochem. 1997, 249, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Besset, V.; Rhee, K.; Wolgemuth, D.J. The cellular distribution and kinase activity of the Cdk family member Pctaire1 in the adult mouse brain and testis suggest functions in differentiation. Cell Growth Differ. 1999, 10, 173–181. [Google Scholar] [PubMed]
- Okuda, T.; Cleveland, J.L.; Downing, J.R. PCTAIRE-1 and PCTAIRE-3, two members of a novel cdc2/CDC28-related protein kinase gene family. Oncogene 1992, 7, 2249–2258. [Google Scholar] [PubMed]
- Barone, G.; Staples, C.J.; Ganesh, A.; Patterson, K.W.; Bryne, D.P.; Myers, K.N.; Patil, A.A.; Eyers, C.E.; Maslen, S.; Skehel, J.M.; et al. Human CDK18 promotes replication stress signaling and genome stability. Nucleic Acids Res. 2016, 44, 8772–8785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, S.; Kawamoto, K.; Miyamoto, K.; Tsuji, A.; Yuasa, K. PCTK3/CDK18 regulates cell migration and adhesion by negatively modulating FAK activity. Sci. Rep. 2017, 7, 45545. [Google Scholar] [CrossRef] [PubMed]
- Wohlbold, L.; Larochelle, S.; Liao, J.C.; Livshits, G.; Singer, J.; Shokat, K.M.; Fisher, R.P. The cyclin-dependent kinase (CDK) family member PNQALRE/CCRK supports cell proliferation but has no intrinsic CDK-activating kinase (CAK) activity. Cell Cycle 2006, 5, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, J.; Lu, Y.; Zhang, S.; Huang, J.; Chen, J.; Bei, J.X.; Yang, K.; Wu, G.; Huang, K.; et al. CDK20 interacts with KEAP1 to activate NRF2 and promotes radiochemoresistance in lung cancer cells. Oncogene 2017, 36, 5321–5330. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Fabbro, D.; Kelly, E.; Marrion, N.V.; Peters, J.A.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; Sharman, J.L.; Southan, C.; et al. The Concise Guide to Pharmacology 2017/18: Enzymes. Br. J. Pharmacol. 2017, 174 (Suppl. 1), S272–S359. [Google Scholar] [CrossRef]
- Tang, D.; Chun, A.C.; Zhang, M.; Wang, J.H. Cyclin-dependent kinase 5 (Cdk5) activation domain of neuronal Cdk5 activator. Evidence of the existence of cyclin fold in neuronal Cdk5a activator. J. Biol. Chem. 1997, 272, 12318–12327. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.C.; Watenpaugh, K.D.; Heinrikson, R.L. A model of the complex between cyclin-dependent kinase 5 and the activation domain of neuronal Cdk5 activator. Biochem. Biophys. Res. Commun. 1999, 259, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Woo, R.A.; Poon, R.Y. Cyclin-dependent kinases and S phase control in mammalian cells. Cell Cycle 2003, 2, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 2005, 30, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Sanso, M.; Fisher, R.P. Modelling the CDK-dependent transcription cycle in fission yeast. Biochem. Soc. Trans. 2013, 41, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Roberts, J.M. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004, 18, 2699–2711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Di Liberto, M.; Jayabalan, D.; Liang, J.; Ely, S.; Bretz, J.; Shaffer, A.L., 3rd; Louie, T.; Chen, I.; Randolph, S.; et al. Prolonged early G(1) arrest by selective CDK4/CDK6 inhibition sensitizes myeloma cells to cytotoxic killing through cell cycle-coupled loss of IRF4. Blood 2012, 120, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Winston, J.; Dong, F.; Pledger, W.J. Differential modulation of G1 cyclins and the Cdk inhibitor p27kip1 by platelet-derived growth factor and plasma factors in density-arrested fibroblasts. J. Biol. Chem. 1996, 271, 11253–11260. [Google Scholar] [CrossRef] [PubMed]
- Aktas, H.; Cai, H.; Cooper, G.M. Ras links growth factor signaling to the cell cycle machinery via regulation of cyclin D1 and the Cdk inhibitor p27KIP1. Mol. Cell. Biol. 1997, 17, 3850–3857. [Google Scholar] [CrossRef] [PubMed]
- Diehl, J.A.; Cheng, M.; Roussel, M.F.; Sherr, C.J. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998, 12, 3499–3511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobrinik, D. Pocket proteins and cell cycle control. Oncogene 2005, 24, 2796–2809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyson, N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998, 12, 2245–2262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friend, S.H.; Bernards, R.; Rogelj, S.; Weinberg, R.A.; Rapaport, J.M.; Albert, D.M.; Dryja, T.P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature 1986, 323, 643–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lees, J.A.; Buchkovich, K.J.; Marshak, D.R.; Anderson, C.W.; Harlow, E. The retinoblastoma protein is phosphorylated on multiple sites by human cdc2. EMBO J. 1991, 10, 4279–4290. [Google Scholar] [CrossRef] [PubMed]
- Harbour, J.W.; Dean, D.C. Rb function in cell-cycle regulation and apoptosis. Nat. Cell Biol. 2000, 2, E65–E67. [Google Scholar] [CrossRef] [PubMed]
- Narasimha, A.M.; Kaulich, M.; Shapiro, G.S.; Choi, Y.J.; Sicinski, P.; Dowdy, S.F. Cyclin D activates the Rb tumor suppressor by mono-phosphorylation. eLife 2014, 3, e02872. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, C.; Klier, S.; McGowan, C.; Wittenberg, C.; de Bruin, R.A. Chk1 inhibits E2F6 repressor function in response to replication stress to maintain cell-cycle transcription. Curr. Biol. 2013, 23, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.K.; Wang, X.; Li, Y.; Ekholm-Reed, S.; Wu, X.; Wang, P.; Reed, S.I. Cyclin E deregulation promotes loss of specific genomic regions. Curr. Biol. 2015, 25, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.; Murphy, M.; Kubelka, M.; Ravnik, S.E.; Hawkins, C.F.; Wolgemuth, D.J.; Carrington, M. A distinct cyclin A is expressed in germ cells in the mouse. Development 1996, 122, 53–64. [Google Scholar] [PubMed]
- Coverley, D.; Laman, H.; Laskey, R.A. Distinct roles for cyclins E and A during DNA replication complex assembly and activation. Nat. Cell Biol. 2002, 4, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Wang, C.; Li, Z.; Sakamaki, T.; Pestell, R.G. Minireview: Cyclin D1: Normal and abnormal functions. Endocrinology 2004, 145, 5439–5447. [Google Scholar] [CrossRef] [PubMed]
- Crasta, K.; Lim, H.H.; Zhang, T.; Nirantar, S.; Surana, U. Consorting kinases, end of destruction and birth of a spindle. Cell Cycle 2008, 7, 2960–2966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, H.; Drozdowska, A.; Dobrynin, G. A role for Cdc48/p97 and Aurora B in controlling chromatin condensation during exit from mitosis. Biochem. Cell Biol. 2010, 88, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Gavet, O.; Pines, J. Activation of cyclin B1-Cdk1 synchronizes events in the nucleus and the cytoplasm at mitosis. J. Cell Biol. 2010, 189, 247–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draviam, V.M.; Orrechia, S.; Lowe, M.; Pardi, R.; Pines, J. The localization of human cyclins B1 and B2 determines CDK1 substrate specificity and neither enzyme requires MEK to disassemble the Golgi apparatus. J. Cell Biol. 2001, 152, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Mailand, N.; Podtelejnikov, A.V.; Groth, A.; Mann, M.; Bartek, J.; Lukas, J. Regulation of G(2)/M events by Cdc25A through phosphorylation-dependent modulation of its stability. EMBO J. 2002, 21, 5911–5920. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, K.A.; Michelson, R.J.; Putnam, C.W.; Weinert, T.A. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 2002, 36, 617–656. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, J.M.; Lees, J.A. Sibling rivalry in the E2F family. Nat. Rev. Mol. Cell Biol. 2002, 3, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Dominguez-Brauer, C.; Wang, Z.; Asara, J.M.; Costa, R.H.; Tyner, A.L.; Lau, L.F.; Raychaudhuri, P. A conserved phosphorylation site within the forkhead domain of FoxM1B is required for its activation by cyclin-CDK1. J. Biol. Chem. 2009, 284, 30695–30707. [Google Scholar] [CrossRef] [PubMed]
- Major, M.L.; Lepe, R.; Costa, R.H. Forkhead box M1B transcriptional activity requires binding of Cdk-cyclin complexes for phosphorylation-dependent recruitment of p300/CBP coactivators. Mol. Cell. Biol. 2004, 24, 2649–2661. [Google Scholar] [CrossRef] [PubMed]
- Laoukili, J.; Alvarez, M.; Meijer, L.A.; Stahl, M.; Mohammed, S.; Kleij, L.; Heck, A.J.; Medema, R.H. Activation of FoxM1 during G2 requires cyclin A/Cdk-dependent relief of autorepression by the FoxM1 N-terminal domain. Mol. Cell. Biol. 2008, 28, 3076–3087. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Wang, Z.; Costa, R.H.; Tyner, A.; Lau, L.F.; Raychaudhuri, P. An N-terminal inhibitory domain modulates activity of FoxM1 during cell cycle. Oncogene 2008, 27, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Rickert, P.; Corden, J.L.; Lees, E. Cyclin C/CDK8 and cyclin H/CDK7/p36 are biochemically distinct CTD kinases. Oncogene 1999, 18, 1093–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsten, J.O.; Zhu, X.; Gustafsson, C.M. The multitalented Mediator complex. Trends Biochem. Sci. 2013, 38, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Nemet, J.; Jelicic, B.; Rubelj, I.; Sopta, M. The two faces of Cdk8, a positive/negative regulator of transcription. Biochimie 2014, 97, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Serizawa, H.; Makela, T.P.; Conaway, J.W.; Conaway, R.C.; Weinberg, R.A.; Young, R.A. Association of Cdk-activating kinase subunits with transcription factor TFIIH. Nature 1995, 374, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liu, M.; Marion, J.; Zhu, Y.; Price, D.H. RNA polymerase II elongation control. Cold Spring Harb. Symp. Quant. Biol. 1998, 63, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.J.; Peng, J.; Lee, G.; Price, D.H.; Flores, O. Cyclin K functions as a CDK9 regulatory subunit and participates in RNA polymerase II transcription. J. Biol. Chem. 1999, 274, 34527–34530. [Google Scholar] [CrossRef] [PubMed]
- Oqani, R.K.; Kim, H.R.; Diao, Y.F.; Park, C.S.; Jin, D.I. The CDK9/cyclin T1 subunits of P-TEFb in mouse oocytes and preimplantation embryos: A possible role in embryonic genome activation. BMC Dev. Biol. 2011, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yik, J.H. The Yin and Yang of P-TEFb regulation: Implications for human immunodeficiency virus gene expression and global control of cell growth and differentiation. Microbiol. Mol. Biol. Rev. 2006, 70, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, L.A.; Edgar, A.J.; Ehley, J.; Gottesfeld, J.M. Cyclin L is an RS domain protein involved in pre-mRNA splicing. J. Biol. Chem. 2002, 277, 25465–25473. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak, B.; Liu, P.; Phatnani, H.P.; Fuda, N.J.; Cooper, J.J.; Price, D.H.; Adelman, K.; Lis, J.T.; Greenleaf, A.L. CDK12 is a transcription elongation-associated CTD kinase, the metazoan ortholog of yeast Ctk1. Genes Dev. 2010, 24, 2303–2316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.W.; Kuzyk, M.A.; Moradian, A.; Ichu, T.A.; Chang, V.C.; Tien, J.F.; Vollett, S.E.; Griffith, M.; Marra, M.A.; Morin, G.B. Interaction of cyclin-dependent kinase 12/CrkRS with cyclin K1 is required for the phosphorylation of the C-terminal domain of RNA polymerase II. Mol. Cell. Biol. 2012, 32, 4691–4704. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Bukholm, I.R.; Bukholm, G.; Nesland, J.M. Over-expression of cyclin A is highly associated with early relapse and reduced survival in patients with primary breast carcinomas. Int. J. Cancer 2001, 93, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Yam, C.H.; Fung, T.K.; Poon, R.Y. Cyclin A in cell cycle control and cancer. Cell. Mol. Life Sci. 2002, 59, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Kretz, A.L.; Schaum, M.; Richter, J.; Kitzig, E.F.; Engler, C.C.; Leithauser, F.; Henne-Bruns, D.; Knippschild, U.; Lemke, J. CDK9 is a prognostic marker and therapeutic target in pancreatic cancer. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, H.Q.; Ying, H.; Tian, T.; Ling, J.; Fu, J.; Lu, Y.; Wu, M.; Yang, L.; Achreja, A.; Chen, G.; et al. Mutant Kras- and p16-regulated NOX4 activation overcomes metabolic checkpoints in development of pancreatic ductal adenocarcinoma. Nat. Commun. 2017, 8, 14437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldas, C.; Hahn, S.A.; da Costa, L.T.; Redston, M.S.; Schutte, M.; Seymour, A.B.; Weinstein, C.L.; Hruban, R.H.; Yeo, C.J.; Kern, S.E. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat. Genet. 1994, 8, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.K.; Grimmond, S.M.; Biankin, A.V. Pancreatic cancer genomics. Curr. Opin. Genet. Dev. 2014, 24, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Hruban, R.H.; Wilentz, R.E.; Kern, S.E. Genetic progression in the pancreatic ducts. Am. J. Pathol. 2000, 156, 1821–1825. [Google Scholar] [CrossRef]
- LaPak, K.M.; Burd, C.E. The molecular balancing act of p16(INK4a) in cancer and aging. Mol. Cancer Res. 2014, 12, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J. Cancer cell cycles. Science 1996, 274, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, D.L.; Sage, J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat. Rev. Cancer 2008, 8, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.; Infante, J.R. Targeting CDK4/6 in patients with cancer. Cancer Treat. Rev. 2016, 45, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Zinczuk, J.; Zareba, K.; Guzinska-Ustymowicz, K.; Kedra, B.; Kemona, A.; Pryczynicz, A. p16, p21, and p53 proteins play an important role in development of pancreatic intraepithelial neoplastic. Ir. J. Med. Sci. 2018, 187, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.M.; Fraser, M.C.; Struewing, J.P.; Hussussian, C.J.; Ranade, K.; Zametkin, D.P.; Fontaine, L.S.; Organic, S.M.; Dracopoli, N.C.; Clark, W.H., Jr.; et al. Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N. Engl. J. Med. 1995, 333, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef]
- Bartkova, J.; Rezaei, N.; Liontos, M.; Karakaidos, P.; Kletsas, D.; Issaeva, N.; Vassiliou, L.V.; Kolettas, E.; Niforou, K.; Zoumpourlis, V.C.; et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 2006, 444, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.; Serrano, M. Senescence in tumours: Evidence from mice and humans. Nat. Rev. Cancer 2010, 10, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, H.; Beach, D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell 1992, 71, 505–514. [Google Scholar] [CrossRef]
- Serrano, M.; Hannon, G.J.; Beach, D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 1993, 366, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J. Mammalian G1 cyclins. Cell 1993, 73, 1059–1065. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, H.; Beach, D. Subunit rearrangement of the cyclin-dependent kinases is associated with cellular transformation. Genes Dev. 1993, 7, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Witkiewicz, A.K.; Borja, N.A.; Franco, J.; Brody, J.R.; Yeo, C.J.; Mansour, J.; Choti, M.A.; McCue, P.; Knudsen, E.S. Selective impact of CDK4/6 suppression on patient-derived models of pancreatic cancer. Oncotarget 2015, 6, 15788–15801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chicas, A.; Wang, X.; Zhang, C.; McCurrach, M.; Zhao, Z.; Mert, O.; Dickins, R.A.; Narita, M.; Zhang, M.; Lowe, S.W. Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer Cell 2010, 17, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Whelan, A.J.; Bartsch, D.; Goodfellow, P.J. Brief report: A familial syndrome of pancreatic cancer and melanoma with a mutation in the CDKN2 tumor-suppressor gene. N. Engl. J. Med. 1995, 333, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Eggers, J.P.; Grandgenett, P.M.; Collisson, E.C.; Lewallen, M.E.; Tremayne, J.; Singh, P.K.; Swanson, B.J.; Andersen, J.M.; Caffrey, T.C.; High, R.R.; et al. Cyclin-dependent kinase 5 is amplified and overexpressed in pancreatic cancer and activated by mutant K-Ras. Clin. Cancer Res. 2011, 17, 6140–6150. [Google Scholar] [CrossRef] [PubMed]
- Dhariwala, F.A.; Rajadhyaksha, M.S. An unusual member of the Cdk family: Cdk5. Cell. Mol. Neurobiol. 2008, 28, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, Z.; Zhang, W.; Qian, K.; Li, H.; Kong, D.; Li, Y.; Tang, Y. Mutated K-ras activates CDK8 to stimulate the epithelial-to-mesenchymal transition in pancreatic cancer in part via the Wnt/β-catenin signaling pathway. Cancer Lett. 2015, 356, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Allaway, R.J.; Fischer, D.A.; de Abreu, F.B.; Gardner, T.B.; Gordon, S.R.; Barth, R.J.; Colacchio, T.A.; Wood, M.; Kacsoh, B.Z.; Bouley, S.J.; et al. Genomic characterization of patient-derived xenograft models established from fine needle aspirate biopsies of a primary pancreatic ductal adenocarcinoma and from patient-matched metastatic sites. Oncotarget 2016, 7, 17087–17102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Dadon, T.; Chenna, V.; Yabuuchi, S.; Bannerji, R.; Booher, R.; Strack, P.; Azad, N.; Nelkin, B.D.; Maitra, A. Combined Inhibition of Cyclin-Dependent Kinases (Dinaciclib) and AKT (MK-2206) Blocks Pancreatic Tumor Growth and Metastases in Patient-Derived Xenograft Models. Mol. Cancer Ther. 2015, 14, 1532–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puyol, M.; Martin, A.; Dubus, P.; Mulero, F.; Pizcueta, P.; Khan, G.; Guerra, C.; Santamaria, D.; Barbacid, M. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell 2010, 18, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Guerra, C.; Collado, M.; Navas, C.; Schuhmacher, A.J.; Hernandez-Porras, I.; Canamero, M.; Rodriguez-Justo, M.; Serrano, M.; Barbacid, M. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell 2011, 19, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, T.P.; Peltier, J.; Hartlova, A.; Gierlinski, M.; Jansen, V.M.; Trost, M.; Bjorklund, M. Thermal proteome profiling of breast cancer cells reveals proteasomal activation by CDK4/6 inhibitor palbociclib. EMBO J. 2018, 37. [Google Scholar] [CrossRef] [PubMed]
- Dulic, V.; Kaufmann, W.K.; Wilson, S.J.; Tlsty, T.D.; Lees, E.; Harper, J.W.; Elledge, S.J.; Reed, S.I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell 1994, 76, 1013–1023. [Google Scholar] [CrossRef]
- Elledge, S.J. Cell cycle checkpoints: Preventing an identity crisis. Science 1996, 274, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.M. Mammalian G1 and G2 phase checkpoints. Cancer Surv. 1997, 29, 151–182. [Google Scholar] [PubMed]
- Bartek, J.; Lukas, J. Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr. Opin. Cell Biol. 2001, 13, 738–747. [Google Scholar] [CrossRef]
- Attardi, L.D.; de Vries, A.; Jacks, T. Activation of the p53-dependent G1 checkpoint response in mouse embryo fibroblasts depends on the specific DNA damage inducer. Oncogene 2004, 23, 973–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wierod, L.; Rosseland, C.M.; Lindeman, B.; Oksvold, M.P.; Grosvik, H.; Skarpen, E.; Huitfeldt, H.S. Activation of the p53-p21(Cip1) pathway is required for CDK2 activation and S-phase entry in primary rat hepatocytes. Oncogene 2008, 27, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Siddik, Z.H.; Huang, Z.; Wang, R.; Koomen, J.; Kobayashi, R.; Khokhar, A.R.; Kuang, J. Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene 2005, 24, 2929–2943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calbo, J.; Serna, C.; Garriga, J.; Grana, X.; Mazo, A. The fate of pancreatic tumor cell lines following p16 overexpression depends on the modulation of CDK2 activity. Cell Death Differ. 2004, 11, 1055–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Heuvel, S.; Harlow, E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 1993, 262, 2050–2054. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, K.; Kataoka, M.; Uno, F.; Murakami, T.; Kondo, T.; Roth, J.A.; Tanaka, N.; Fujiwara, T. Activation of caspase-3 and cleavage of Rb are associated with p16-mediated apoptosis in human non-small cell lung cancer cells. Oncogene 2002, 21, 2108–2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Katayose, Y.; Rojanala, L.; Shah, S.; Sgagias, M.; Jang, L.; Jung, Y.J.; Lee, S.H.; Hwang, S.G.; Cowan, K.H. Induction of apoptosis in p16INK4A mutant cell lines by adenovirus-mediated overexpression of p16INK4A protein. Cell Death Differ. 2000, 7, 706–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeannot, P.; Callot, C.; Baer, R.; Duquesnes, N.; Guerra, C.; Guillermet-Guibert, J.; Bachs, O.; Besson, A. Loss of p27Kip(1) promotes metaplasia in the pancreas via the regulation of Sox9 expression. Oncotarget 2015, 6, 35880–35892. [Google Scholar] [CrossRef] [PubMed]
- Lukas, J.; Herzinger, T.; Hansen, K.; Moroni, M.C.; Resnitzky, D.; Helin, K.; Reed, S.I.; Bartek, J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997, 11, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Alevizopoulos, K.; Vlach, J.; Hennecke, S.; Amati, B. Cyclin E and c-Myc promote cell proliferation in the presence of p16INK4a and of hypophosphorylated retinoblastoma family proteins. EMBO J. 1997, 16, 5322–5333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neganova, I.; Tilgner, K.; Buskin, A.; Paraskevopoulou, I.; Atkinson, S.P.; Peberdy, D.; Passos, J.F.; Lako, M. CDK1 plays an important role in the maintenance of pluripotency and genomic stability in human pluripotent stem cells. Cell Death Dis. 2014, 5, e1508. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, L.T.; Tovar, C.; Chen, S.; Knezevic, D.; Zhao, X.; Sun, H.; Heimbrook, D.C.; Chen, L. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc. Natl. Acad. Sci. USA 2006, 103, 10660–10665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diril, M.K.; Ratnacaram, C.K.; Padmakumar, V.C.; Du, T.; Wasser, M.; Coppola, V.; Tessarollo, L.; Kaldis, P. Cyclin-dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re-replication but not for liver regeneration. Proc. Natl. Acad. Sci. USA 2012, 109, 3826–3831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berthet, C.; Kaldis, P. Cell-specific responses to loss of cyclin-dependent kinases. Oncogene 2007, 26, 4469–4477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Kleeff, J.; Li, J.; Ding, J.; Hammer, J.; Zhao, Y.; Giese, T.; Korc, M.; Buchler, M.W.; Friess, H. Expression and functional significance of CDC25B in human pancreatic ductal adenocarcinoma. Oncogene 2004, 23, 71–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, R.P. Secrets of a double agent: CDK7 in cell-cycle control and transcription. J. Cell Sci. 2005, 118, 5171–5180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Fischer, P.M. Cyclin-dependent kinase 9: A key transcriptional regulator and potential drug target in oncology, virology and cardiology. Trends Pharmacol. Sci. 2008, 29, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Pevarello, P.; Barbacid, M.; Bischoff, J.R. CDK inhibitors in cancer therapy: What is next? Trends Pharmacol. Sci. 2008, 29, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.A.; Schwartz, G.K. Development of cell-cycle inhibitors for cancer therapy. Curr. Oncol. 2009, 16, 36–43. [Google Scholar] [PubMed]
- Lemke, J.; Schäfer, D.; Sander, S.; Henne-Bruns, D.; Kornmann, M. Survival and prognostic factors in pancreatic and ampullary cancer. Anticancer Res. 2014, 34, 3011–3020. [Google Scholar] [PubMed]
- Fry, D.W.; Harvey, P.J.; Keller, P.R.; Elliott, W.L.; Meade, M.; Trachet, E.; Albassam, M.; Zheng, X.; Leopold, W.R.; Pryer, N.K.; et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol. Cancer Ther. 2004, 3, 1427–1438. [Google Scholar] [PubMed]
- Rader, J.; Russell, M.R.; Hart, L.S.; Nakazawa, M.S.; Belcastro, L.T.; Martinez, D.; Li, Y.; Carpenter, E.L.; Attiyeh, E.F.; Diskin, S.J.; et al. Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin. Cancer Res. 2013, 19, 6173–6182. [Google Scholar] [CrossRef] [PubMed]
- Gelbert, L.M.; Cai, S.; Lin, X.; Sanchez-Martinez, C.; Del Prado, M.; Lallena, M.J.; Torres, R.; Ajamie, R.T.; Wishart, G.N.; Flack, R.S.; et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: In-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Investig. New Drugs 2014, 32, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, A.M.; Perera, R.M.; Ecker, V.; Nicolay, B.N.; Bardeesy, N.; Benes, C.H.; Dyson, N.J. CDK4/6 and IGF1 receptor inhibitors synergize to suppress the growth of p16INK4A-deficient pancreatic cancers. Cancer Res. 2014, 74, 3947–3958. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.; Froio, D.; Nagrial, A.M.; Parkin, A.; Murphy, K.J.; Chin, V.T.; Wohl, D.; Steinmann, A.; Stark, R.; Drury, A.; et al. Tailored first-line and second-line CDK4-targeting treatment combinations in mouse models of pancreatic cancer. Gut 2017. [Google Scholar] [CrossRef] [PubMed]
- Fajas, L. Re-thinking cell cycle regulators: The cross-talk with metabolism. Front. Oncol. 2013, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Tu, B.P. Driving the cell cycle through metabolism. Annu. Rev. Cell Dev. Biol. 2012, 28, 59–87. [Google Scholar] [CrossRef] [PubMed]
- Bryant, K.L.; Mancias, J.D.; Kimmelman, A.C.; Der, C.J. KRAS: Feeding pancreatic cancer proliferation. Trends Biochem. Sci. 2014, 39, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.M.; Kimmelman, A.C. The complex landscape of pancreatic cancer metabolism. Carcinogenesis 2014, 35, 1441–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, J.; Balaji, U.; Freinkman, E.; Witkiewicz, A.K.; Knudsen, E.S. Metabolic re-programming of pancreatic cancer mediated by CDK4/6 inhibition elicits unique vulnerabilities. Cell Rep. 2016, 14, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.; Witkiewicz, A.K.; Knudsen, E.S. CDK4/6 inhibitors have potent activity in combination with pathway selective therapeutic agents in models of pancreatic cancer. Oncotarget 2014, 5, 6512–6525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Retzer-Lidl, M.; Schmid, R.M.; Schneider, G. Inhibition of CDK4 impairs proliferation of pancreatic cancer cells and sensitizes towards TRAIL-induced apoptosis via downregulation of survivin. Int. J. Cancer 2007, 121, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, P.J.; Bisi, J.E.; Strum, J.C.; Combest, A.J.; Darr, D.B.; Usary, J.E.; Zamboni, W.C.; Wong, K.K.; Perou, C.M.; Sharpless, N.E. Multiple Roles of Cyclin-Dependent Kinase 4/6 Inhibitors in Cancer Therapy. J. Natl. Cancer Inst. 2012, 104, 476–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, J.L.; McClendon, A.K.; Knudsen, E.S. Modification of the DNA damage response by therapeutic CDK4/6 inhibition. J. Biol. Chem. 2012, 287, 29075–29087. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, D.; Periyasamy, G.; Ponnurangam, S.; Chakrabarti, D.; Sugumar, A.; Padigaru, M.; Weir, S.J.; Balakrishnan, A.; Sharma, S.; Anant, S. CDK-4 inhibitor P276 sensitizes pancreatic cancer cells to gemcitabine-induced apoptosis. Mol. Cancer Ther. 2012, 11, 1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Kong, L.; Xiao, Y.; Yuan, H.; Song, Y.; Wang, J.; Yu, H.; Mao, S.; Xu, W. CDK8 regulates the angiogenesis of pancreatic cancer cells in part via the CDK8-β-catenin-KLF2 signal axis. Exp. Cell Res. 2018, 369, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Cros, J.; Raffenne, J.; Couvelard, A.; Pote, N. Tumor Heterogeneity in Pancreatic Adenocarcinoma. Pathobiology 2018, 85, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, V.L.; Garcia-Garijo, A.; van Laarhoven, H.W.; Bijlsma, M.F. Extracellular Influences: Molecular Subclasses and the Microenvironment in Pancreatic Cancer. Cancers 2018, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.; Guzi, T.; Shanahan, F.; Davis, N.; Prabhavalkar, D.; Wiswell, D.; Seghezzi, W.; Paruch, K.; Dwyer, M.P.; Doll, R.; et al. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol. Cancer Ther. 2010, 9, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Nemunaitis, J.J.; Small, K.A.; Kirschmeier, P.; Zhang, D.; Zhu, Y.; Jou, Y.M.; Statkevich, P.; Yao, S.L.; Bannerji, R. A first-in-human, phase 1, dose-escalation study of dinaciclib, a novel cyclin-dependent kinase inhibitor, administered weekly in subjects with advanced malignancies. J. Transl. Med. 2013, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Ghia, P.; Scarfò, L.; Perez, S.; Pathiraja, K.; Derosier, M.; Small, K.; McCrary Sisk, C.; Patton, N. Efficacy and safety of dinaciclib vs ofatumumab in patients with relapsed/refractory chronic lymphocytic leukemia. Blood 2017, 129, 1876–1878. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, G.; Mishra, A.; Bisht, S.; Karikari, C.; Garrido-Laguna, I.; Rasheed, Z.; Ottenhof, N.A.; Dadon, T.; Alvarez, H.; Fendrich, V.; et al. Cyclin-dependent kinase inhibitor Dinaciclib (SCH727965) inhibits pancreatic cancer growth and progression in murine xenograft models. Cancer Biol. Ther. 2011, 12, 598–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamad, N.M.; Elconin, J.H.; Karnoub, A.E.; Bai, W.; Rich, J.N.; Abraham, R.T.; Der, C.J.; Counter, C.M. Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev. 2002, 16, 2045–2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, K.H.; Baines, A.T.; Fiordalisi, J.J.; Shipitsin, M.; Feig, L.A.; Cox, A.D.; Der, C.J.; Counter, C.M. Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell 2005, 7, 533–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, K.H.; O’Hayer, K.; Adam, S.J.; Kendall, S.D.; Campbell, P.M.; Der, C.J.; Counter, C.M. Divergent roles for RalA and RalB in malignant growth of human pancreatic carcinoma cells. Curr. Biol. 2006, 16, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wierda, W.G.; Chubb, S.; Hawtin, R.E.; Fox, J.A.; Keating, M.J.; Gandhi, V.; Plunkett, W. Mechanism of action of SNS-032, a novel cyclin-dependent kinase inhibitor, in chronic lymphocytic leukemia. Blood 2009, 113, 4637–4645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conroy, A.; Stockett, D.E.; Walker, D.; Arkin, M.R.; Hoch, U.; Fox, J.A.; Hawtin, R.E. SNS-032 is a potent and selective CDK 2, 7 and 9 inhibitor that drives target modulation in patient samples. Cancer Chemother. Pharmacol. 2009, 64, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Heath, E.I.; Bible, K.; Martell, R.E.; Adelman, D.C.; Lorusso, P.M. A phase 1 study of SNS-032 (formerly BMS-387032), a potent inhibitor of cyclin-dependent kinases 2, 7 and 9 administered as a single oral dose and weekly infusion in patients with metastatic refractory solid tumors. Investig. New Drugs 2008, 26, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.G.; Chen, R.; Plunkett, W.; Siegel, D.; Sinha, R.; Harvey, R.D.; Badros, A.Z.; Popplewell, L.; Coutre, S.; Fox, J.A.; et al. Phase I and Pharmacologic Study of SNS-032, a Potent and Selective Cdk2, 7, and 9 Inhibitor, in Patients with Advanced Chronic Lymphocytic Leukemia and Multiple Myeloma. J. Clin. Oncol. 2010, 28, 3015–3022. [Google Scholar] [CrossRef] [PubMed]
- Senderowicz, A.M. Flavopiridol: The first cyclin-dependent kinase inhibitor in human clinical trials. Investig. New Drugs 1999, 17, 313–320. [Google Scholar] [CrossRef]
- Cassaday, R.D.; Goy, A.; Advani, S.; Chawla, P.; Nachankar, R.; Gandhi, M.; Gopal, A.K. A phase II, single-arm, open-label, multicenter study to evaluate the efficacy and safety of P276-00, a cyclin-dependent kinase inhibitor, in patients with relapsed or refractory mantle cell lymphoma. Clin. Lymphoma Myeloma Leuk. 2015, 15, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Stetler-Stevenson, M.; Sebers, S.; Worland, P.; Sedlacek, H.; Myers, C.; Czech, J.; Naik, R.; Sausville, E. Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone L86-8275. J. Natl. Cancer Inst. 1992, 84, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.S.; Rathos, M.J.; Mahajan, P.; Wagh, V.; Shenoy, S.; Bhatia, D.; Chile, S.; Sivakumar, M.; Maier, A.; Fiebig, H.H.; et al. P276-00, a novel cyclin-dependent inhibitor induces G1-G2 arrest, shows antitumor activity on cisplatin-resistant cells and significant in vivo efficacy in tumor models. Mol. Cancer Ther. 2007, 6, 926–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Guru, S.K.; Jain, S.K.; Joshi, P.; Gandhi, S.G.; Bharate, S.B.; Bhushan, S.; Bharate, S.S.; Vishwakarma, R.A. A chromatography-free isolation of rohitukine from leaves of Dysoxylum binectariferum: Evaluation for in vitro cytotoxicity, CDK inhibition and physicochemical properties. Bioorg. Med. Chem. Lett. 2016, 26, 3457–3463. [Google Scholar] [CrossRef] [PubMed]
- Rathkopf, D.; Dickson, M.A.; Feldman, D.R.; Carvajal, R.D.; Shah, M.A.; Wu, N.; Lefkowitz, R.; Gonen, M.; Cane, L.M.; Dials, H.J.; et al. Phase I study of flavopiridol with oxaliplatin and fluorouracil/leucovorin in advanced solid tumors. Clin. Cancer Res. 2009, 15, 7405–7411. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.A.; Shah, M.A.; Rathkopf, D.; Tse, A.; Carvajal, R.D.; Wu, N.; Lefkowitz, R.A.; Gonen, M.; Cane, L.M.; Dials, H.J.; et al. A phase I clinical trial of FOLFIRI in combination with the pan-cyclin-dependent kinase (CDK) inhibitor flavopiridol. Cancer Chemother. Pharmacol. 2010, 66, 1113–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fornier, M.N.; Rathkopf, D.; Shah, M.; Patil, S.; O’Reilly, E.; Tse, A.N.; Hudis, C.; Lefkowitz, R.; Kelsen, D.P.; Schwartz, G.K. Phase I Dose-Finding Study of Weekly Docetaxel Followed by Flavopiridol for Patients with Advanced Solid Tumors. Clin. Cancer Res. 2007, 13. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; D’Adamo, D.R.; Dickson, M.A.; Keohan, M.L.; Carvajal, R.D.; Maki, R.G.; de Stanchina, E.; Musi, E.; Singer, S.; Schwartz, G.K. The cyclin-dependent kinase inhibitor flavopiridol potentiates doxorubicin efficacy in advanced sarcomas: Preclinical investigations and results of a phase I dose-escalation clinical trial. Clin. Cancer Res. 2012, 18, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Identifier: NCT00047307. Available online: ClinicalTrials.gov (accessed on 20 September 2018).
- Carvajal, R.D.; Tse, A.; Shah, M.A.; Lefkowitz, R.A.; Gonen, M.; Gilman-Rosen, L.; Kortmansky, J.; Kelsen, D.P.; Schwartz, G.K.; O’Reilly, E.M. A phase II study of flavopiridol (Alvocidib) in combination with docetaxel in refractory, metastatic pancreatic cancer. Pancreatology 2009, 9, 404–409. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Identifier: NCT00331682. Available online: ClinicalTrials.gov (accessed on 20 September 2018).
- Flynn, J.; Jones, J.; Johnson, A.J.; Andritsos, L.; Maddocks, K.; Jaglowski, S.; Hessler, J.; Grever, M.R.; Im, E.; Zhou, H.; et al. Dinaciclib is a novel cyclin-dependent kinase inhibitor with significant clinical activity in relapsed and refractory chronic lymphocytic leukemia. Leukemia 2015, 29, 1524–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitri, Z.; Karakas, C.; Wei, C.; Briones, B.; Simmons, H.; Ibrahim, N.; Alvarez, R.; Murray, J.L.; Keyomarsi, K.; Moulder, S. A phase 1 study with dose expansion of the CDK inhibitor dinaciclib (SCH 727965) in combination with epirubicin in patients with metastatic triple negative breast cancer. Investig. New Drugs 2015, 33, 890–894. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Identifier: NCT01783171. Available online: ClinicalTrials.gov (accessed on 20 September 2018).
- Kumar, S.K.; LaPlant, B.; Chng, W.J.; Zonder, J.; Callander, N.; Fonseca, R.; Fruth, B.; Roy, V.; Erlichman, C.; Stewart, A.K.; et al. Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood 2015, 125, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.M.; Joy, A.A.; Mita, A.; Sankhala, K.; Jou, Y.-M.; Zhang, D.; Statkevich, P.; Zhu, Y.; Yao, S.-L.; Small, K.; et al. Randomized phase II trial of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus capecitabine in patients with advanced breast cancer. Clin. Breast Cancer 2014, 14, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.J.; Nemunaitis, J.; Joy, A.A.; Martin, J.C.; Jou, Y.-M.; Zhang, D.; Statkevich, P.; Yao, S.-L.; Zhu, Y.; Zhou, H.; et al. Randomized phase 2 study of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus erlotinib in patients with non-small cell lung cancer. Lung Cancer 2014, 83, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, S.; Liu, Q.; Yuan, X.; Mani, S.; Pestell, R.G.; Wu, K. Recent advances of highly selective CDK4/6 inhibitors in breast cancer. J. Hematol. Oncol. 2017, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Identifier: NCT02981342. Available online: ClinicalTrials.gov (accessed on 20 September 2018).
- Dickler, M.N.; Tolaney, S.M.; Rugo, H.S.; Cortés, J.; Diéras, V.; Patt, D.; Wildiers, H.; Hudis, C.A.; O’Shaughnessy, J.; Zamora, E.; et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2−Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 5218–5224. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination with Fulvestrant in Women With HR+/HER2− Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.W.; Shi, P.; Reck, M.; Paz-Ares, L.; Koustenis, A.; Hurt, K.C. Treatment Rationale and Study Design for the JUNIPER Study: A Randomized Phase III Study of Abemaciclib With Best Supportive Care Versus Erlotinib With Best Supportive Care in Patients with Stage IV Non-Small-Cell Lung Cancer with a Detectable KRAS Mutati. Clin. Lung Cancer 2016, 17, 80–84. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Press Announcements—FDA Approves New Treatment for Certain Advanced or Metastatic Breast Cancers. Available online: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm578071.htm (accessed on 31 July 2018).
- U.S. National Library of Medicine. Identifier: NCT02897375. Available online: ClinicalTrials.gov (accessed on 20 September 2018).
- U.S. National Library of Medicine. Identifier: NCT03454035. Available online: ClinicalTrials.gov (accessed on 20 September 2018).
- U.S. National Library of Medicine. Identifier: NCT03065062. Available online: ClinicalTrials.gov (accessed on 20 September 2018).
- Ma, C.X.; Gao, F.; Luo, J.; Northfelt, D.W.; Goetz, M.; Forero, A.; Hoog, J.; Naughton, M.; Ademuyiwa, F.; Suresh, R.; et al. NeoPalAna: Neoadjuvant Palbociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor, and Anastrozole for Clinical Stage 2 or 3 Estrogen Receptor-Positive Breast Cancer. Clin. Cancer Res. 2017, 23, 4055–4065. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Ro, J.; André, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Huang Bartlett, C.; Zhang, K.; et al. Palbociclib in Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 209–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J. Palbociclib: A first-in-class CDK4/CDK6 inhibitor for the treatment of hormone-receptor positive advanced breast cancer. J. Hematol. Oncol. 2015, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Identifier: NCT02703571. Available online: ClinicalTrials.gov (accessed on 20 September 2018).
- Asiedu, C.; Biggs, J.; Lilly, M.; Kraft, A.S. Inhibition of leukemic cell growth by the protein kinase C activator bryostatin 1 correlates with the dephosphorylation of cyclin-dependent kinase 2. Cancer Res. 1995, 55, 3716–3720. [Google Scholar] [PubMed]
- Lam, A.P.; Sparano, J.A.; Vinciguerra, V.; Ocean, A.J.; Christos, P.; Hochster, H.; Camacho, F.; Goel, S.; Mani, S.; Kaubisch, A. Phase II study of paclitaxel plus the protein kinase C inhibitor bryostatin-1 in advanced pancreatic carcinoma. Am. J. Clin. Oncol. 2010, 33, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Aspeslagh, S.; Shailubhai, K.; Bahleda, R.; Gazzah, A.; Varga, A.; Hollebecque, A.; Massard, C.; Spreafico, A.; Reni, M.; Soria, J.-C. Phase I dose-escalation study of milciclib in combination with gemcitabine in patients with refractory solid tumors. Cancer Chemother. Pharmacol. 2017, 79, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Motwani, M.; Delohery, T.M.; Schwartz, G.K.; Patil, S.; O’Reilly, E.; Tse, A.N.; Hudis, C.; Lefkowitz, R.; Kelsen, D.P.; Schwartz, G.K. Sequential dependent enhancement of caspase activation and apoptosis by flavopiridol on paclitaxel-treated human gastric and breast cancer cells. Clin. Cancer Res. 1999, 5, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B. Ofatumumab. mAbs 2009, 1, 326–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| CDK Subfamily * | Name ** | Other Names * | Cyclins | Main Functions | References |
|---|---|---|---|---|---|
| CDK1 | CDK1 | CDC2, CDC28A, cell division cycle 2 homolog A, p34 protein kinase, p34 | Cyclin A Cyclin B Cyclin D Cyclin E | Cell cycle—S phase Cell cycle—G2/M phase | [45,46,47,48] |
| CDK2 | cell division protein kinase 2 | Cyclin A Cyclin B Cyclin D Cyclin E | Cell cycle—G1/S transition Cell cycle—S phase | [49,50,51,52] | |
| CDK3 | Cdkn3, Cell division protein kinase 3 | Cyclin C Cyclin E | Cell cycle—S phase | [53,54,55] | |
| CDK4 | CDK4 | cell division protein kinase 4, CRK3, p34/cdk4, PSK-J3 | Cyclin D1, D2, D3 | Cell cycle—G1 phase Cell cycle—G1/S transition | [56,57,58] |
| CDK6 | CR2 protein kinase, CRK2, Serine/threonine-protein kinase PLSTIRE | Cyclin D1, D2, D3 | Cell cycle—G1 phase Cell cycle—G1/S transition | [56,59,60] | |
| CDK5 | CDK5 | cell division protein kinase 5, CR6 protein kinase, CRK6, serine/threonine-protein kinase PSSALRE, tau protein kinase II catalytic subunit, TPKII catalytic subunit | p35 *** Cyclin I | Development of the mammalian central nervous system Apoptosis Cell cycle regulation | [61,62,63,64] |
| CDK7 | CDK7 | CAK1, CDKN7, STK1, 39 kDa protein kinase, CDK-activating kinase 1, cell division protein kinase 7, CRK4, protein-tyrosine kinase MPK-7, TFIIH basal transcription factor complex kinase subunit | Cyclin H | Transcription regulation | [65,66,67] |
| CDK8 | CDK8 | Cell division protein kinase 8, Mediator of RNA polymerase II transcription subunit CDK8 | Cyclin C | Transcription regulation Wnt pathway modulation | [68,69,70] |
| CDK19 | CDC2L6, CDC2-related protein kinase 6, CDK11 | Cyclin C | Transcription regulation Cell cycle | [70] | |
| CDK9 | CDK9 | CDC2L4, cell division protein kinase 9, TAK | Cyclin K Cyclin T1, T2a, T2b | Transcription regulation | [71,72] |
| CDK10 | CDK10 | cell division protein kinase 10, PISSLRE | Cyclin M | Transcription regulation | [73] |
| CRK7 | CDK12 | CDC2 related protein kinase 7, cell division cycle 2-related protein kinase 7, CRK7, CRKR | Cyclin K Cyclin L | Transcription regulation Splicing | [74,75] |
| CDK13 | CDC2L, CDC2L5, CDC2-related protein kinase 5, cell division cycle 2-like 5, cell division protein kinase 13 | Cyclin K Cyclin L | Transcription regulation Splicing | [74,76] | |
| PITSLRE | CDK11A | CDC2L2, CDC2L3, CDK11-p110, CDK11-p46, CDK11-p58, cell division cycle 2-like 2 (PITSLRE proteins), Cell division cycle 2-like protein kinase 2, Cell division protein kinase 11A, PITSLRE serine/threonine-protein kinase CDC2L2 | Cyclin L1, L2 | Splicing | [77,78] |
| CDK11B | CDC2L1, CDK11-p110, CDK11-p46, CDK11-p58, cell division cycle 2-like protein kinase 1, cell division protein kinase 11, PITSLRE serine/threonine-protein kinase CDC2L1, PITSLRE serine/threonine-protein kinase CDC2L1CDK11-p110 | Cyclin L1, L2 | Splicing | [77,78] | |
| TAIRE | CDK14 | cell division protein kinase 14, PFTAIRE protein kinase 1, PFTAIRE1, PFTK1 | Cyclin D Cyclin Y | Cell cycle progression Wnt signaling | [79,80] |
| CDK15 | ALS2CR7, PFTAIRE protein kinase 2, PFTAIRE2, Cell division protein kinase 15, frizzled family receptor 7 | Associated with TRAIL resistance in cancer cells | [81] | ||
| CDK16 | cell division protein kinase 16, CRK5, PCTAIRE protein kinase 1, PCTAIRE1 | Cyclin Y | Neurite outgrowth regulation Vesicle trafficking Cancer cell proliferation | [82] | |
| CDK17 | PCTAIRE protein kinase 2, PCTAIRE2 | Expressed in brain | [83,84] | ||
| CDK18 | cell division protein kinase 18, PCTAIRE protein kinase 3, PCTAIRE3 | Cyclin A2 Cyclin E | Actin reorganization regulation Genome integrity regulation | [85,86,87] | |
| CCRK | CDK20 | CCRK, CDK-related protein kinase PNQLARE, cell division protein kinase 20, Cyclin-dependent protein kinase H, Cyclin-kinase-activating kinase p42 | Cell cycle regulation | [88,89] |
| Drug | Targeted CDKs | Clinical Trial Phase | Disease | Observations |
|---|---|---|---|---|
| Flavopiridol | 1, 2, 4, 6, 7, 9 [26] | Phase I | Chronic Lymphocytic Leukemia (CLL) [27] | After chemoimmunotherapy |
| Advanced solid tumours [227] | In combination with oxaliplatin and fluorouracil/leucovorin | |||
| Advanced solid tumours [228] | In combination with FOLFIRI | |||
| Advanced solid tumours [229] | In combination with docetaxel | |||
| Advanced sarcomas [230] | In combination with doxorubicin | |||
| Pancreatic cancer [231] | In combination with radiation and gemcitabine | |||
| Phase II | Metastatic melanoma, endometrial adenocarcinoma, multiple myeloma [190] | |||
| Pancreatic cancer [232,233] | In combination with docetaxel | |||
| Dinaciclib | 1, 2, 5, 9 [211] | Phase I | CLL [234] | |
| Advanced malignancies [212] | ||||
| Triple-negative breast cancer [235] | In combination with epirubicin | |||
| Pancreatic cancer [236] | No results published yet | |||
| Phase II | Multiple myeloma [237] | |||
| Advanced breast cancer [238] | In comparison vs. capecitabine | |||
| Non-small cell lung cancer (NSCLC) [239] | In comparison vs. erlotinib | |||
| Phase III | CLL [213] | In comparison vs. ofatumumab | ||
| SNS-032 | 2, 7, 9 [219] | Phase I | Metastatic refractory solid tumours [220] | |
| CLL, multiple myeloma [221] | ||||
| Abemaciclib | 4, 6 [240] | Phase II | Pancreatic cancer [241] | In combination with gemcitabine, capecitabine |
| Advanced breast cancer [242] (MONARCH 1) | ||||
| Phase III | Advanced breast cancer [243] (MONARCH 2) | In combination with fulvestrant | ||
| Advanced breast cancer [244] (MONARCH 3) | In combination with aromatase inhibitor | |||
| NSCLC (KRAS-mutation) [245] (JUNIPER) | In comparison vs. erlotinib | |||
| FDA-approved for advanced breast cancer [246] | ||||
| Palbociclib | 4, 6 [240] | Phase I | Advanced solid tumours [247,248,249], including pancreatic cancer | In combination with cisplatin, carboplatin, ulixertinib, gedatolisib |
| Phase II | Advanced breast cancer [250,251] | In combination with anastrozole, letrozole | ||
| Phase III | Advanced breast cancer [252] | In combination with fulvestrant | ||
| FDA-approved for advanced breast cancer [253] | ||||
| Ribociclib | 4, 6 [240] | Phase I/II | Advanced solid tumours [254] | |
| Bryostatin-1 | 2 [190,255] | Phase II | Advanced pancreatic carcinoma [256] | In combination to paclitaxel |
| Milciclib | 1, 2, 4, 5 [257] | Phase I | Refractory solid tumours [257] | In combination with gemcitabine |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Reyes, B.; Kretz, A.-L.; Ruff, J.-P.; Von Karstedt, S.; Hillenbrand, A.; Knippschild, U.; Henne-Bruns, D.; Lemke, J. The Emerging Role of Cyclin-Dependent Kinases (CDKs) in Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2018, 19, 3219. https://doi.org/10.3390/ijms19103219
García-Reyes B, Kretz A-L, Ruff J-P, Von Karstedt S, Hillenbrand A, Knippschild U, Henne-Bruns D, Lemke J. The Emerging Role of Cyclin-Dependent Kinases (CDKs) in Pancreatic Ductal Adenocarcinoma. International Journal of Molecular Sciences. 2018; 19(10):3219. https://doi.org/10.3390/ijms19103219
Chicago/Turabian StyleGarcía-Reyes, Balbina, Anna-Laura Kretz, Jan-Philipp Ruff, Silvia Von Karstedt, Andreas Hillenbrand, Uwe Knippschild, Doris Henne-Bruns, and Johannes Lemke. 2018. "The Emerging Role of Cyclin-Dependent Kinases (CDKs) in Pancreatic Ductal Adenocarcinoma" International Journal of Molecular Sciences 19, no. 10: 3219. https://doi.org/10.3390/ijms19103219
APA StyleGarcía-Reyes, B., Kretz, A.-L., Ruff, J.-P., Von Karstedt, S., Hillenbrand, A., Knippschild, U., Henne-Bruns, D., & Lemke, J. (2018). The Emerging Role of Cyclin-Dependent Kinases (CDKs) in Pancreatic Ductal Adenocarcinoma. International Journal of Molecular Sciences, 19(10), 3219. https://doi.org/10.3390/ijms19103219





