Outer Membrane Protein of Gut Commensal Microorganism Induces Autoantibody Production and Extra-Intestinal Gland Inflammation in Mice
Abstract
1. Introduction
2. Results
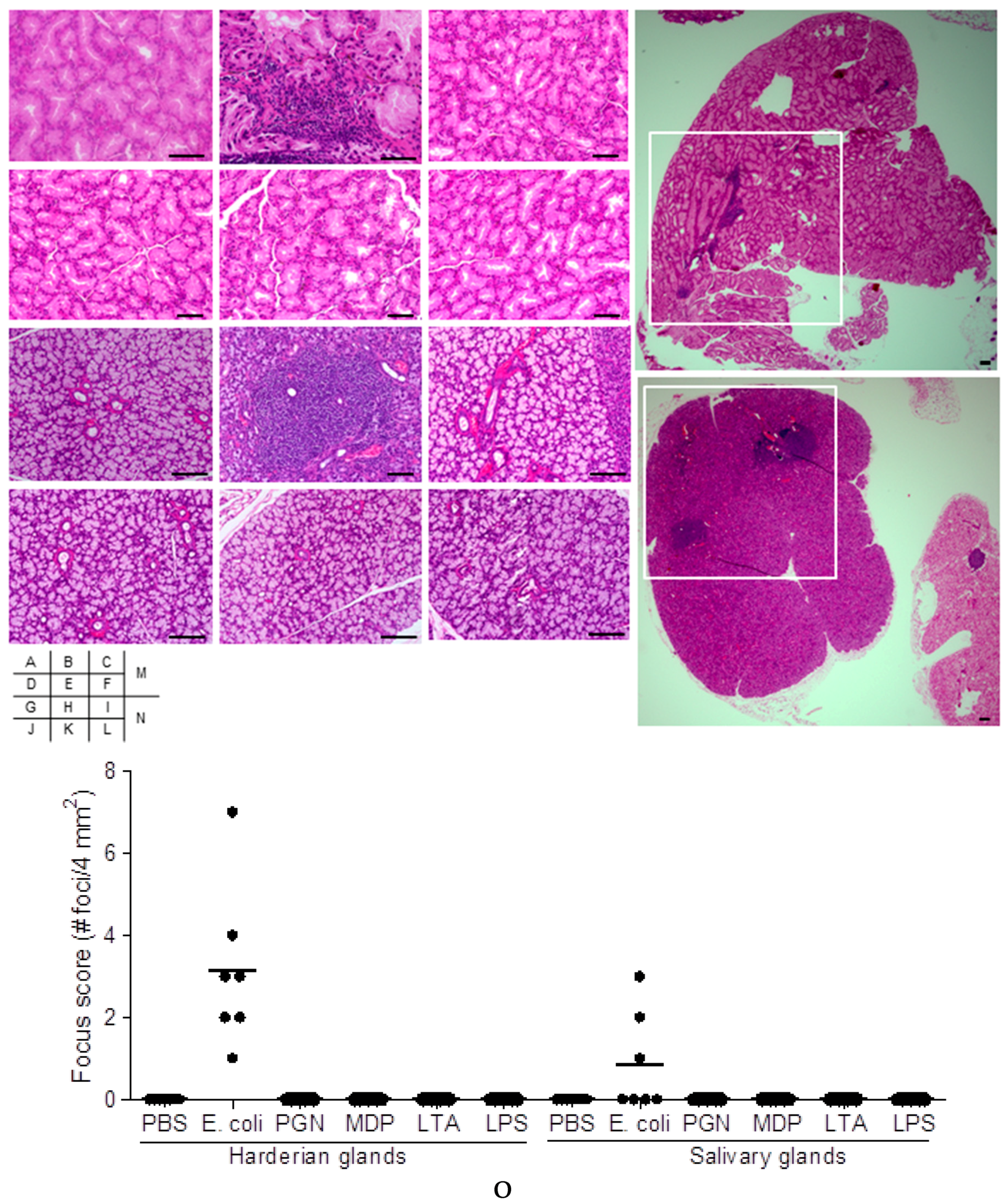
2.1. Effect on Extra-Intestinal Gland Inflammation by Systemic Treatment of Mice with Bacterial Components
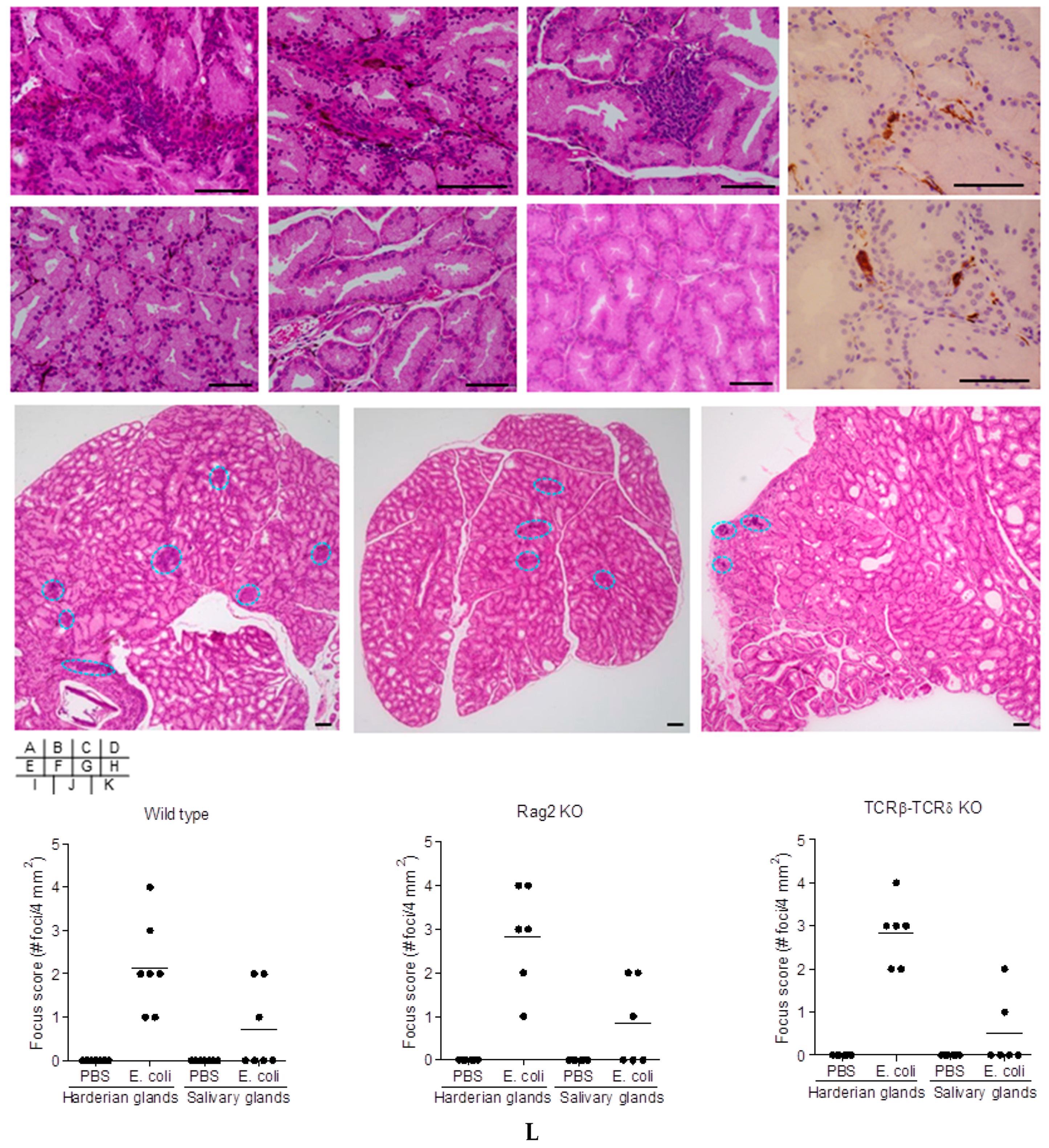
2.2. Cell-Mediated Immunity in Bacteria-Treated Mice
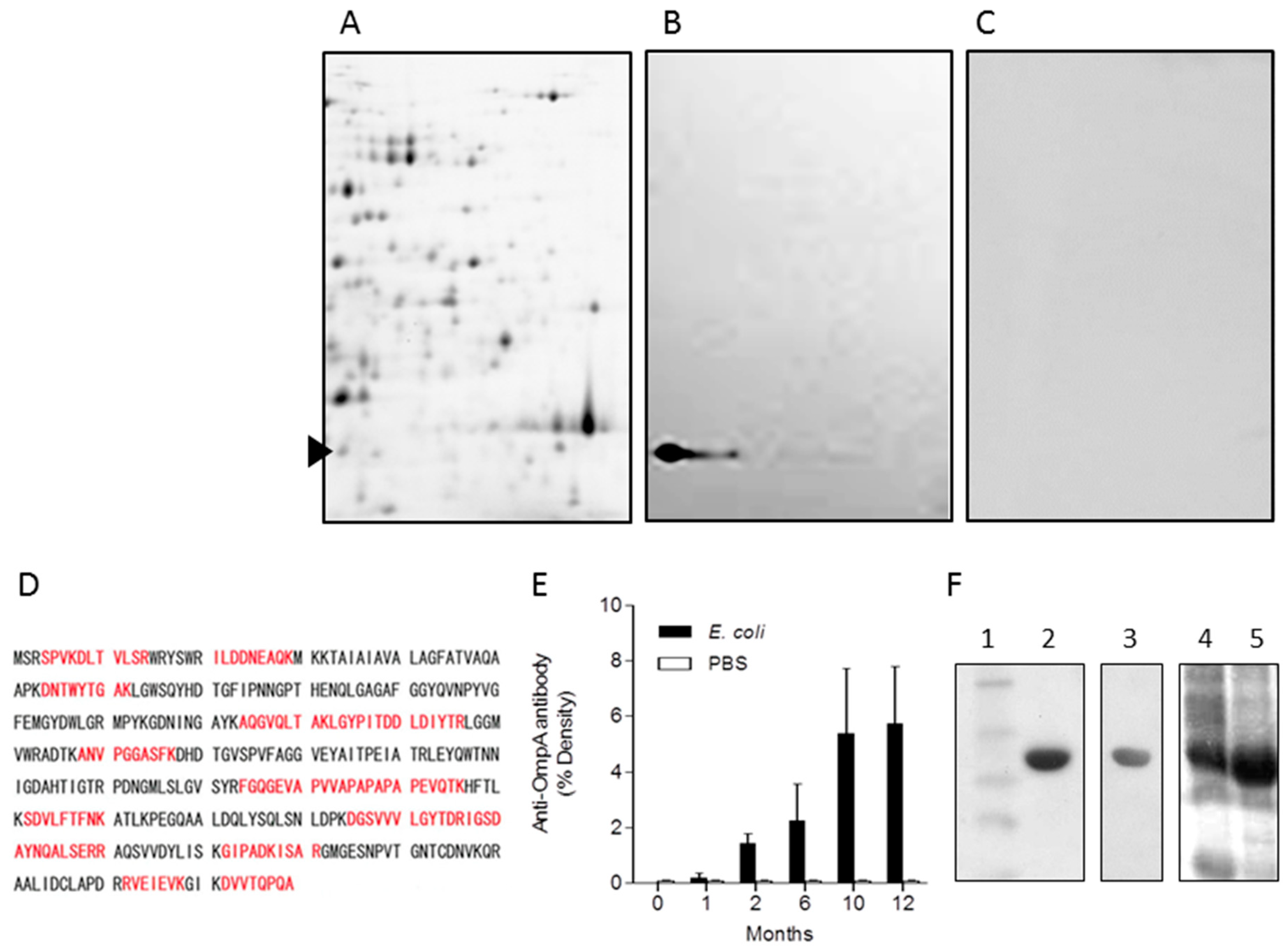
2.3. Identification of OmpA from E. coli as a Representative Immunogenic Protein in Mice
2.4. Analysis of the Amino Acid Sequences of OmpA from Phylum Proteobacteria
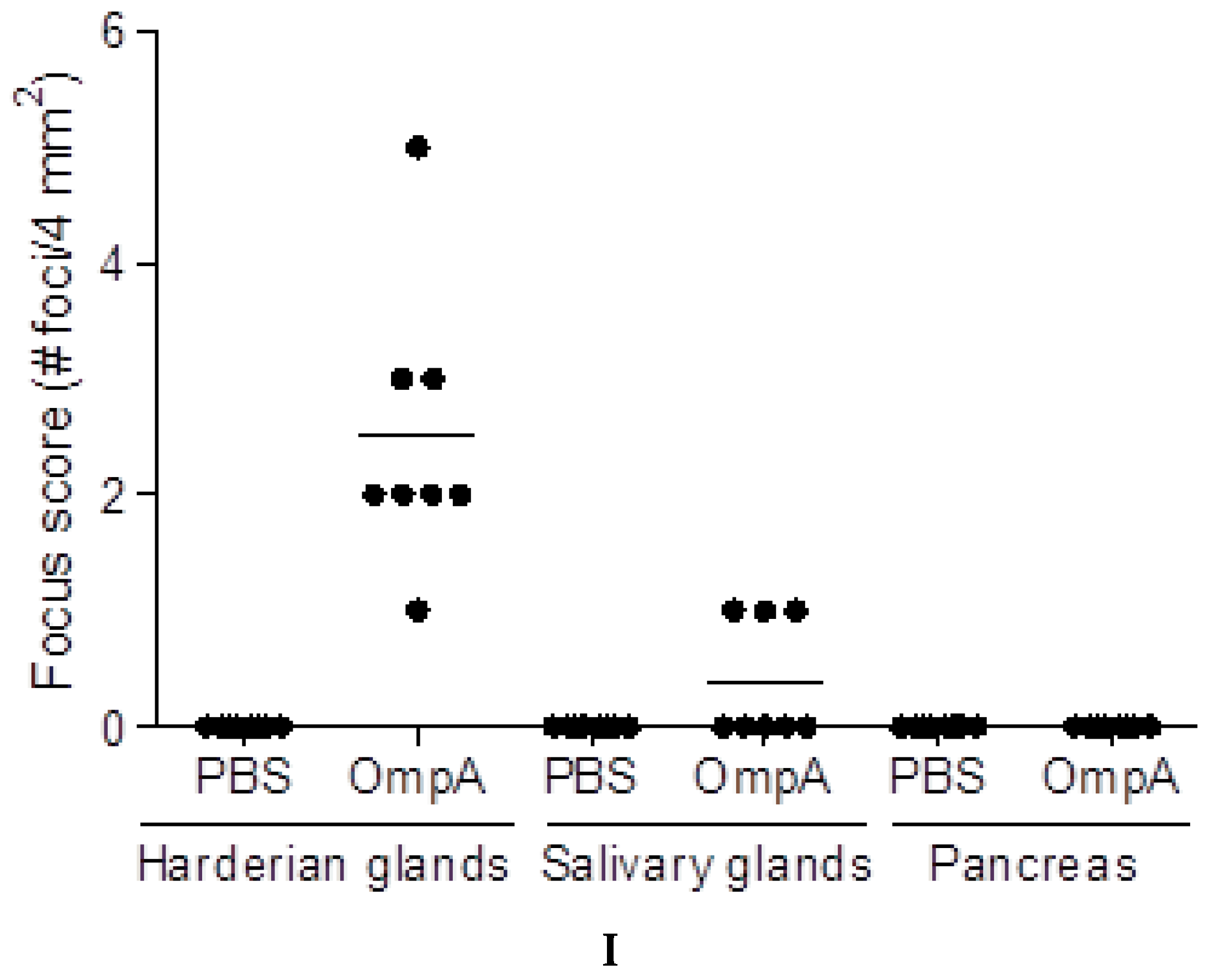
2.5. Induction of Extra-Intestinal Gland Inflammation by OmpA
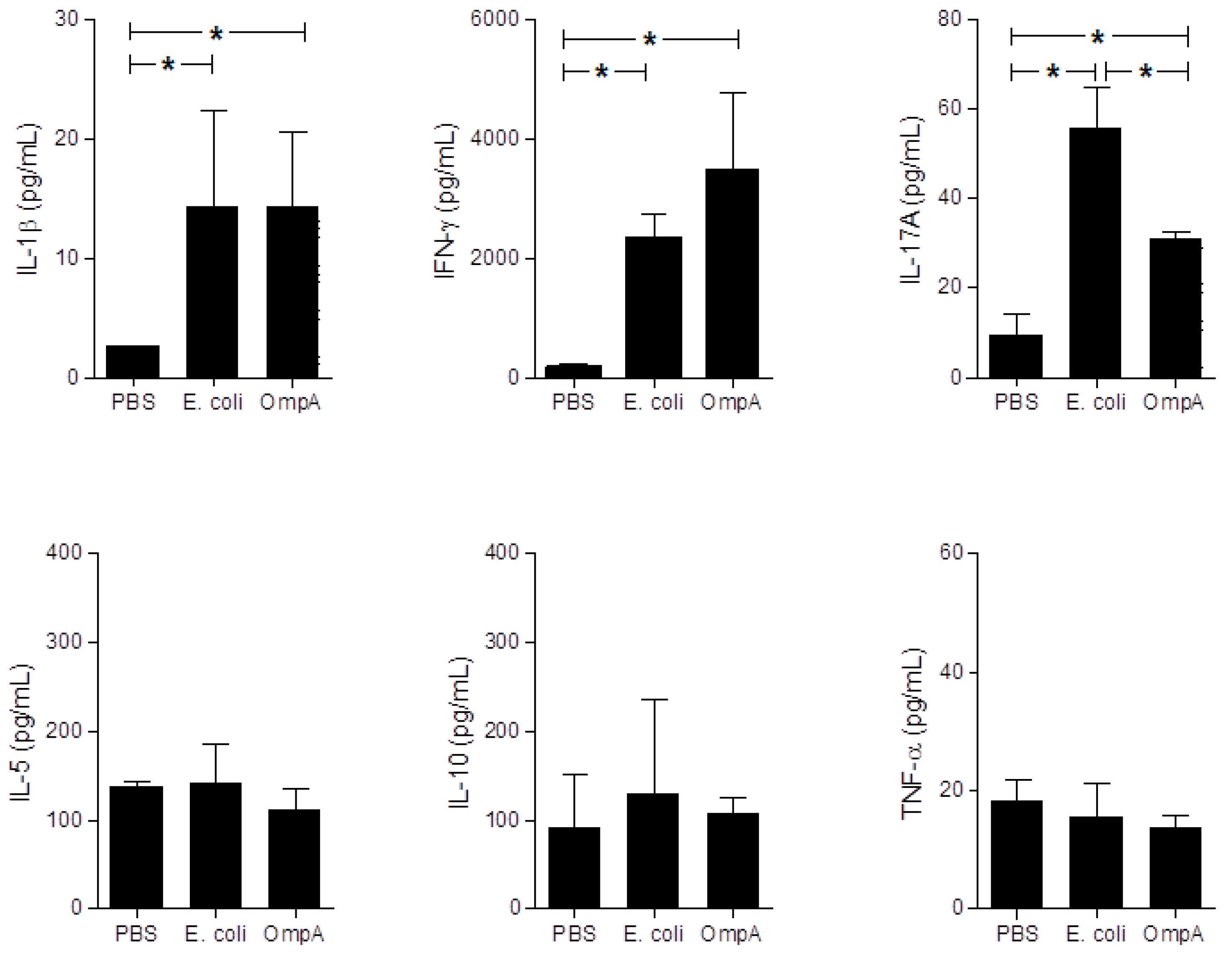
2.6. Cytokine Production in Sera of Mice with Extra-Intestinal Inflammation Induced by OmpA
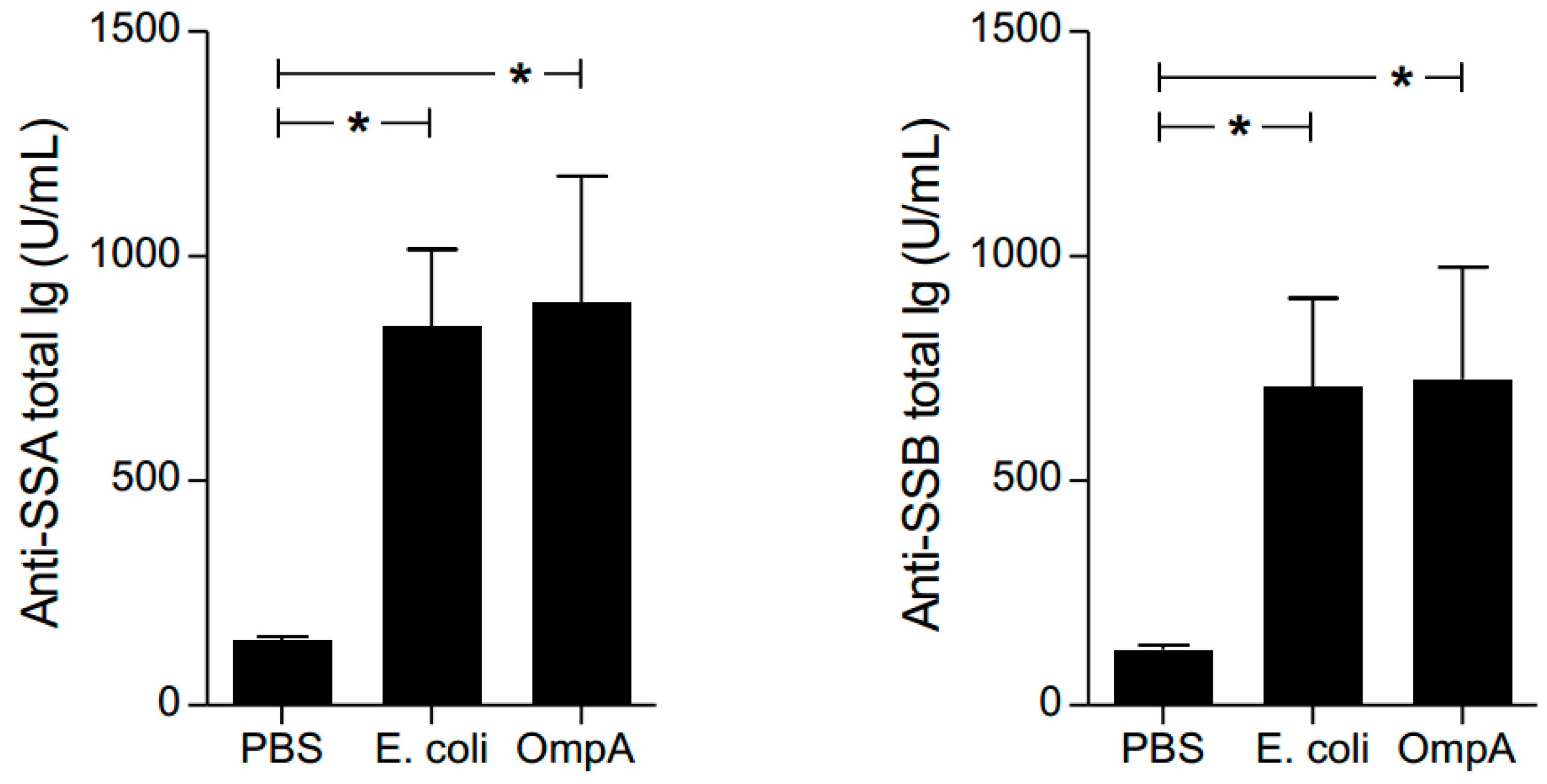
2.7. Effect of Autoantibody Production by OmpA
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Culture Conditions and Bacteria-Derived Components
4.2. Mice
4.3. Treatment of Mice with Bacteria and Bacterial Components
4.4. Isolation of Extra-Intestinal Gland Tissues
4.5. Sera Collection
4.6. Adoptive Transfer Experiments
4.7. Histology
4.8. Focus Scoring
4.9. Two Dimensional Polyacrylamide Gel Electrophoresis (2-D PAGE) of E. coli Cell Surface Proteins
4.10. Western Blotting of Immunoreactive E. coli Surface Proteins
4.11. Identification of Proteins Using MALDI-TOF-MS
4.12. Recombinant OmpA Protein Purification and Anti-OmpA Antibody Measurement
4.13. Bioinformatic Analysis of the OmpA Protein
4.14. Enzyme-Linked Immunosorbent Assay (ELISA)
4.15. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial community variation in human body habitats across space and time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Duriez, P.; Clermont, O.; Bonacorsi, S.; Bingen, E.; Chaventre, A.; Elion, J.; Picard, B.; Denamur, E. Commensal Escherichia coli isolates are phylogenetically distributed among geographically distinct human populations. Microbiology 2001, 147, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Tomas, J.; Reygner, J.; Mayeur, C.; Ducroc, R.; Bouet, S.; Bridonneau, C.; Cavin, J.B.; Thomas, M.; Langella, P.; Cherbuy, C. Early colonizing Escherichia coli elicits remodeling of rat colonic epithelium shifting toward a new homeostatic state. ISME J. 2015, 9, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the human infant intestinal microbiota. PLoS Biol. 2007, 5, e177. [Google Scholar] [CrossRef] [PubMed]
- Secher, T.; Brehin, C.; Oswald, E. Early settlers: Which E. coli strains do you not want at birth? Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G123–G129. [Google Scholar] [CrossRef] [PubMed]
- Lundell, A.C.; Bjornsson, V.; Ljung, A.; Ceder, M.; Johansen, S.; Lindhagen, G.; Tornhage, C.J.; Adlerberth, I.; Wold, A.E.; Rudin, A. Infant B cell memory differentiation and early gut bacterial colonization. J. Immunol. 2012, 188, 4315–4322. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Conway, T.; Cohen, P.S. Commensal and pathogenic Escherichia coli metabolism in the gut. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Spees, A.M.; Wangdi, T.; Lopez, C.A.; Kingsbury, D.D.; Xavier, M.N.; Winter, S.E.; Tsolis, R.M.; Baumler, A.J. Streptomycin-induced inflammation enhances Escherichia coli gut colonization through nitrate respiration. mBio 2013, 4, e00430-13. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Yang, Y.; Luo, Z.; Zhu, W. Temporal microbiota changes of high-protein diet intake in a rat model. Anaerobe 2017, 47, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Elson, C.O. Adaptive immune education by gut microbiota antigens. Immunology 2018, 154, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Lindskog Jonsson, A.; Caesar, R.; Akrami, R.; Reinhardt, C.; Fåk Hållenius, F.; Borén, J.; Bäckhed, F. Impact of gut microbiota and diet on the development of atherosclerosis in Apoe(−/−) Mice. Arterioscler. Thromb. Vasc. Biol. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Szymula, A.; Rosenthal, J.; Szczerba, B.M.; Bagavant, H.; Fu, S.M.; Deshmukh, U.S. T cell epitope mimicry between Sjögren’s syndrome Antigen A (SSA)/Ro60 and oral, gut, skin and vaginal bacteria. Clin. Immunol. 2014, 152, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Haruta, I.; Shibata, N.; Yanagisawa, N.; Yagi, J. Flagellar filament structural protein induces Sjögren’s syndrome-like sialadenitis in mice. Oral Dis. 2017, 23, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Yoshikawa, K.; Yokoyama, T.; Nonogaki, T.; Iwasaki, S.; Mitsui, T.; Niwa, S. Role of enteric bacteria in the pathogenesis of rheumatoid arthritis: Evidence for antibodies to enterobacterial common antigens in rheumatoid sera and synovial fluids. Ann. Rheum. Dis. 1996, 55, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Marcoux, J.; Politis, A.; Rinehart, D.; Marshall, D.P.; Wallace, M.I.; Tamm, L.K.; Robinson, C.V. Mass spectrometry defines the C-terminal dimerization domain and enables modeling of the structure of full-length OmpA. Structure 2014, 22, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.N.; Gotschlich, E.C. Outer membrane protein A (OmpA) contributes to serum resistance and pathogenicity of Escherichia coli K-1. Infect. Immun. 1991, 59, 2252–2258. [Google Scholar] [PubMed]
- Smith, S.G.; Mahon, V.; Lambert, M.A.; Fagan, R.P. A molecular Swiss army knife: OmpA structure, function and expression. FEMS Microbiol. Lett. 2007, 273, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Rinehart, D.; Szabo, G.; Tamm, L.K. Refolded outer membrane protein A of Escherichia coli forms ion channels with two conductance states in planar lipid bilayers. J. Biol. Chem. 2000, 275, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Prasadarao, N.V.; Blom, A.M.; Villoutreix, B.O.; Linsangan, L.C. A novel interaction of outer membrane protein A with C4b binding protein mediates serum resistance of Escherichia coli K1. J. Immunol. 2002, 169, 6352–6360. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Belaaouaj, A.A.; Dahlgren, C.; Bylund, J. Outer membrane protein A deficient Escherichia coli activates neutrophils to produce superoxide and shows increased susceptibility to antibacterial peptides. Microbes Infect. 2003, 5, 781–788. [Google Scholar] [CrossRef]
- Vila-Farres, X.; Parra-Millan, R.; Sanchez-Encinales, V.; Varese, M.; Ayerbe-Algaba, R.; Bayo, N.; Guardiola, S.; Pachon-Ibanez, M.E.; Kotev, M.; Garcia, J.; et al. Combating virulence of Gram-negative bacilli by OmpA inhibition. Sci. Rep. 2017, 7, 14683. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.P. The harderian gland: A tercentennial review. J. Anat. 1994, 185, 1–49. [Google Scholar] [PubMed]
- Terao, M.; Kurosaki, M.; Barzago, M.M.; Fratelli, M.; Bagnati, R.; Bastone, A.; Giudice, C.; Scanziani, E.; Mancuso, A.; Tiveron, C.; et al. Role of the molybdoflavoenzyme aldehyde oxidase homolog 2 in the biosynthesis of retinoic acid: Generation and characterization of a knockout mouse. Mol. Cell. Biol. 2009, 29, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Haruta, I.; Yanagisawa, N.; Yagi, J. Mouse monoclonal antibody specific for outer membrane protein A of Escherichia coli. Monoclon. Antib. Immunodiagn. Immunother. 2013, 32, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Battistuzzi, F.U.; Billing-Ross, P.; Murillo, O.; Filipski, A.; Kumar, S. Estimating divergence times in large molecular phylogenies. Proc. Natl. Acad. Sci. USA 2012, 109, 19333–19338. [Google Scholar] [CrossRef] [PubMed]
- Oke, V.; Wahren-Herlenius, M. The immunobiology of Ro52 (TRIM21) in autoimmunity: A critical review. J. Autoimmun. 2012, 39, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, N.C.; Kapsogeorgou, E.K.; Tzioufas, A.G. A comprehensive review of autoantibodies in primary Sjögren’s syndrome: Clinical phenotypes and regulatory mechanisms. J. Autoimmun. 2014, 51, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Haruta, I.; Yanagisawa, N.; Kawamura, S.; Furukawa, T.; Shimizu, K.; Kato, H.; Kobayashi, M.; Shiratori, K.; Yagi, J. A mouse model of autoimmune pancreatitis with salivary gland involvement triggered by innate immunity via persistent exposure to avirulent bacteria. Lab. Investig. 2010, 90, 1757–1769. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, C.S.; Jones, D.B.; Stern, M.E.; Bian, F.; Moore, Q.L.; Corbiere, S.; Streckfus, C.F.; Hutchinson, D.S.; Ajami, N.J.; Petrosino, J.F.; et al. Altered mucosal microbiome diversity and disease severity in Sjögren syndrome. Sci. Rep. 2016, 6, 23561. [Google Scholar] [CrossRef] [PubMed]
- Tsigalou, C.; Stavropoulou, E.; Bezirtzoglou, E. Current insights in microbiome shifts in Sjögren’s syndrome and possible therapeutic interventions. Front. Immunol. 2018, 9, 1106. [Google Scholar] [CrossRef] [PubMed]
- Greiling, T.M.; Dehner, C.; Chen, X.; Hughes, K.; Iniguez, A.J.; Boccitto, M.; Ruiz, D.Z.; Renfroe, S.C.; Vieira, S.M.; Ruff, W.E.; et al. Commensal orthologs of the human autoantigen Ro60 as triggers of autoimmunity in lupus. Sci. Transl. Med. 2018, 10, eaan2306. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Kurashige, Y.; Nishimura, M.; Yamazaki, M.; Sato, J.; Saitoh, M.; Selimovic, D.; Abiko, Y. Involvement of toll-like receptors in autoimmune sialoadenitis of the non-obese diabetic mouse. J. Oral Pathol. Med. 2012, 41, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Van Aalst, S.; Ludwig, I.S.; van der Zee, R.; van Eden, W.; Broere, F. Bystander activation of irrelevant CD4+ T cells following antigen-specific vaccination occurs in the presence and absence of adjuvant. PLoS ONE 2017, 12, e0177365. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yasui, N.; Ishimaru, N.; Arakaki, R.; Hayashi, Y. Development of autoimmune arthritis with aging via bystander T cell activation in the mouse model of Sjögren’s syndrome. Arthritis Rheum. 2004, 50, 3974–3984. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, N.; Haruta, I.; Shimizu, K.; Furukawa, T.; Higuchi, T.; Shibata, N.; Shiratori, K.; Yagi, J. Identification of commensal flora-associated antigen as a pathogenetic factor of autoimmune pancreatitis. Pancreatology 2014, 14, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Jeannin, P.; Renno, T.; Goetsch, L.; Miconnet, I.; Aubry, J.P.; Delneste, Y.; Herbault, N.; Baussant, T.; Magistrelli, G.; Soulas, C.; et al. OmpA targets dendritic cells, induces their maturation and delivers antigen into the MHC class I presentation pathway. Nat. Immunol. 2000, 1, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.G.; Li, Y.; Tutt, C.B.; Xin, L.; Eaves-Pyles, T.; Soong, L. Outer membrane protein A of Escherichia coli O157:H7 stimulates dendritic cell activation. Infect. Immun. 2006, 74, 2676–2685. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Jung, I.D.; Lee, C.M.; Park, J.W.; Chun, S.H.; Jeong, S.K.; Ha, T.K.; Shin, Y.K.; Kim, D.J.; Park, Y.M. Outer membrane protein a of Salmonella enterica serovar Typhimurium activates dendritic cells and enhances Th1 polarization. BMC Microbiol. 2010, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Liao, Y.; Zhang, J.; Wang, Y.; Liu, Z.; Cheng, P.; Wang, X.; Zou, Q.; Gu, J. Rational design and evaluation of an artificial Escherichia coli K1 protein vaccine candidate based on the structure of OmpA. Front. Cell. Infect. Microbiol. 2018, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Rainard, P.; Reperant-Ferter, M.; Gitton, C.; Gilbert, F.B.; Germon, P. Cellular and humoral immune response to recombinant Escherichia coli OmpA in cows. PLoS ONE 2017, 12, e0187369. [Google Scholar] [CrossRef] [PubMed]
- Pore, D.; Mahata, N.; Chakrabarti, M.K. Outer membrane protein A (OmpA) of Shigella flexneri 2a links innate and adaptive immunity in a TLR2-dependent manner and involvement of IL-12 and nitric oxide. J. Biol. Chem. 2012, 287, 12589–12601. [Google Scholar] [CrossRef] [PubMed]
- Jeannin, P.; Bottazzi, B.; Sironi, M.; Doni, A.; Rusnati, M.; Presta, M.; Maina, V.; Magistrelli, G.; Haeuw, J.F.; Hoeffel, G.; et al. Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity 2005, 22, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.M.; Raybourne, R.B. Demonstration of cross-reactivity between bacterial antigens and class I human leukocyte antigens by using monoclonal antibodies to Shigella flexneri. Infect. Immun. 1990, 58, 1774–1781. [Google Scholar] [PubMed]
- Barr, J.Y.; Wang, X.; Meyerholz, D.K.; Lieberman, S.M. CD8 T cells contribute to lacrimal gland pathology in the nonobese diabetic mouse model of Sjögren syndrome. Immunol. Cell Biol. 2017, 95, 684–694. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanagisawa, N.; Ueshiba, H.; Abe, Y.; Kato, H.; Higuchi, T.; Yagi, J. Outer Membrane Protein of Gut Commensal Microorganism Induces Autoantibody Production and Extra-Intestinal Gland Inflammation in Mice. Int. J. Mol. Sci. 2018, 19, 3241. https://doi.org/10.3390/ijms19103241
Yanagisawa N, Ueshiba H, Abe Y, Kato H, Higuchi T, Yagi J. Outer Membrane Protein of Gut Commensal Microorganism Induces Autoantibody Production and Extra-Intestinal Gland Inflammation in Mice. International Journal of Molecular Sciences. 2018; 19(10):3241. https://doi.org/10.3390/ijms19103241
Chicago/Turabian StyleYanagisawa, Naoko, Hidehiro Ueshiba, Yoshihiro Abe, Hidehito Kato, Tomoaki Higuchi, and Junji Yagi. 2018. "Outer Membrane Protein of Gut Commensal Microorganism Induces Autoantibody Production and Extra-Intestinal Gland Inflammation in Mice" International Journal of Molecular Sciences 19, no. 10: 3241. https://doi.org/10.3390/ijms19103241
APA StyleYanagisawa, N., Ueshiba, H., Abe, Y., Kato, H., Higuchi, T., & Yagi, J. (2018). Outer Membrane Protein of Gut Commensal Microorganism Induces Autoantibody Production and Extra-Intestinal Gland Inflammation in Mice. International Journal of Molecular Sciences, 19(10), 3241. https://doi.org/10.3390/ijms19103241



