Metallothionein and Superoxide Dismutase—Antioxidative Protein Status in Fullerene-Doxorubicin Delivery to MCF-7 Human Breast Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Biophysical Characterization of C60–DOX (Fullerene-Doxorubicin) Complexes
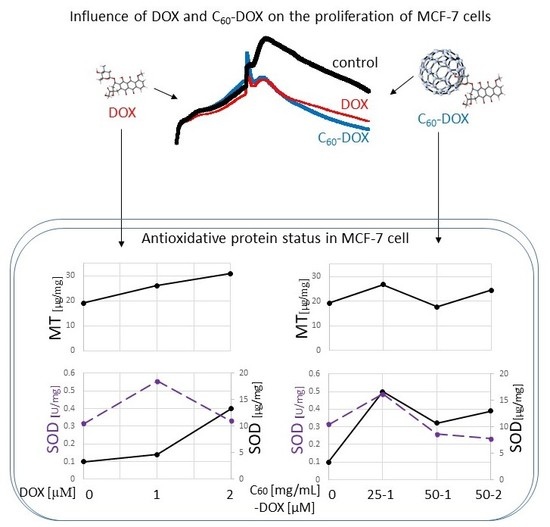
2.2. Proliferation Status of MCF-7 Human Breast Cancer Cells Treated by C60–DOX Complexes
2.3. The Effect of Complexes of C60–Doxorubicin on Metallothionein Concentration
2.4. Influence of C60 on the Concentration and Activity of SOD (Superoxide Dismutase) in MCF-7 Treatment by DOX
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Fullerene-Doxorubicin Complexes
4.3. Scanning Electron Microscopy
4.4. Dynamic Light Scattering
4.5. Fluorescence Measurements of DOX
4.6. Growth Measurement
4.7. Cell lysis and Total Protein Concentration Analysis
4.8. Metallothionein Concentration
4.9. Concentration of SOD1 and Cu/Zn SOD Activity
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| C60 | fullerene |
| DOX | doxorubicin |
| EE | entrapment efficiency |
| MT | metallothionein |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
References
- Mahalingaiah, P.K.S.; Singh, K.P. Chronic oxidative stress increases growth and tumorigenic potential of MCF-7 breast cancer cells. PLoS ONE 2014, 9, e87371. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladyshev, V.N. The free radical theory of aging is dead. Long live the damage theory! Antioxid. Redox Signal. 2014, 20, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Kizek, R.; Adam, V.; Hrabeta, J.; Eckschlager, T.; Smutny, S.; Burda, J.V.; Frei, E.; Stiborova, M. Anthracyclines and ellipticines as DNA-damaging anticancer drugs: Recent advances. Pharmacol. Ther. 2012, 133, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Heger, Z.; Rodrigo, M.A.M.; Krizkova, S.; Ruttkay-Nedecky, B.; Zalewska, M.; Del Pozo, E.M.P.; Pelfrene, A.; Pourrut, B.; Stiborova, M.; Eckschlager, T.; et al. Metallothionein as a scavenger of free radicals—New cardioprotective therapeutic agent or initiator of tumor chemoresistance? Curr. Drug Targets 2016, 17, 1438–1451. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sarkar, S.; Scott, L.; Danelisen, I.; Trush, M.A.; Jia, Z.; Li, Y.R. Doxorubicin redox biology: Redox cycling, topoisomerase inhibition, and oxidative stress. React. Oxyg. Species 2016, 1, 189–198. [Google Scholar] [CrossRef]
- Skalickova, S.; Loffelmann, M.; Gargulak, M.; Kepinska, M.; Docekalova, M.; Uhlirova, D.; Stankova, M.; Fernandez, C.; Milnerowicz, H.; Ruttkay-Nedecky, B.; et al. Zinc-modified nanotransporter of doxorubicin for targeted prostate cancer delivery. Nanomaterials 2017, 7, 435. [Google Scholar] [CrossRef] [PubMed]
- Prylutska, S.; Grynyuk, I.; Matyshevska, O.; Prylutskyy, Y.; Evstigneev, M.; Scharff, P.; Ritter, U. C60 Fullerene as synergistic agent in tumor-inhibitory doxorubicin treatment. Drugs R D 2014, 14, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kepinska, M.; Kizek, R.; Milnerowicz, H. Fullerene as a doxorubicin nanotransporter for targeted breast cancer therapy: Capillary electrophoresis analysis. Electrophoresis 2018, 39, 2370–2379. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ma, L.; Liu, Y.; Chen, C. Applications of functionalized fullerenes in tumor theranostics. Theranostics 2012, 2, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Milnerowicz, H.; Jabłonowska, M.; Bizoń, A. Change of zinc, copper, and metallothionein concentrations and the copper-zinc superoxide dismutase activity in patients with pancreatitis. Pancreas 2009, 38, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 14, 6044–6066. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, M.; Trefon, J.; Milnerowicz, H. The role of metallothionein interactions with other proteins. Proteomics 2014, 14, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Bizoń, A.; Jędryczko, K.; Milnerowicz, H. The role of metallothionein in oncogenesis and cancer treatment. Postepy Hig. Med. Dosw. 2017, 71, 98–109. [Google Scholar] [CrossRef]
- Krizkova, S.; Kepinska, M.; Emri, G.; Eckschlager, T.; Stiborova, M.; Pokorna, P.; Heger, Z.; Adam, V. An insight into the complex roles of metallothioneins in malignant diseases with emphasis on (sub)isoforms/isoforms and epigenetics phenomena. Pharmacol. Ther. 2018, 183, 90–117. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Manfredi, G.; Germain, D. SOD1, an unexpected novel target for cancer therapy. Genes Cancer 2014, 5, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P. Redox cycling of radical anion metabolites of toxic chemicals and drugs and the Marcus theory of electron transfer. Environ. Health Perspect. 1990, 87, 237–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panchuk, R.R.; Prylutska, S.V.; Chumakl, V.V.; Skorokhyd, N.R.; Lehka, L.V.; Evstigneev, M.P.; Prylutskyy, Y.I.; Berger, W.; Heffeter, P.; Scharff, P.; et al. Application of C60 fullerene-doxorubicin complex for tumor cell treatment in vitro and in vivo. J. Biomed. Nanotechnol. 2015, 11, 1139–1152. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, H.; Lu, W.; Zhou, Y.; Zhang, W.; Chen, Z.; Hu, Y.; Huang, P. Mitochondrial dysfunction and reactive oxygen species imbalance promote breast cancer cell motility through a CXCL14-mediated mechanism. Cancer Res. 2009, 69, 2375–2383. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Peng, Q.; Ruttkay-Nedecky, B.; Milnerowicz, H.; Kizek, R. Carbon nanomaterials for targeted cancer therapy drugs: A critical review. Chem. Rec. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bakry, R.; Vallant, R.M.; Najam-ul-Haq, M.; Rainer, M.; Szabo, Z.; Huck, C.W.; Bonn, G.K. Medicinal applications of fullerenes. Int. J. Nanomed. 2007, 2, 639–649. [Google Scholar]
- Chistyakov, V.A.; Smirnova, Y.O.; Prazdnova, E.V.; Soldatov, A.V. Possible mechanisms of fullerene C60 antioxidant action. BioMed Res. Int. 2013, 2013, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, N.; Pressac, M.; Hadchouel, M.; Szwarc, H.; Wilson, S.R.; Moussa, F. Fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005, 5, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Injac, R.; Perse, M.; Boskovic, M.; Djordjevic-Milic, V.; Djordjevic, A.; Hvala, A.; Cerar, A.; Strukelj, B. Cardioprotective effects of fullerenol C60(OH)24 on a single dose doxorubicin-induced cardiotoxicity in rats with malignant neoplasm. Technol. Cancer Res. Treat. 2008, 7, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Montellano, A.; Da Ros, T.; Bianco, A.; Prato, M. Fullerene C₆₀ as a multifunctional system for drug and gene delivery. Nanoscale 2011, 3, 4035–4041. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Xing, G.; Sun, B.; Zhao, F.; Lei, H.; Li, W.; Song, Y.; Chen, Z.; Yuan, H.; Wang, X.; et al. Potent angiogenesis inhibition by the particulate form of fullerene derivatives. ACS Nano 2010, 4, 2773–2783. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Wang, G.W.; Klein, J.B.; Kang, Y.J. Metallothionein inhibits doxorubicin-induced mitochondrial cytochrome c release and caspase-3 activation in cardiomyocytes. J. Pharmacol. Exp. Ther. 2001, 298, 461–468. [Google Scholar] [PubMed]
- Wierzowiecka, B.; Gomulkiewicz, A.; Cwynar-Zajac, L.; Olbromski, M.; Grzegrzolka, J.; Kobierzycki, C.; Podhorska-Okolow, M.; Dziegiel, P. Expression of metallothionein and vascular endothelial growth factor isoforms in breast cancer cells. In Vivo 2016, 30, 271–278. [Google Scholar] [PubMed]
- Cherian, M.G.; Jayasurya, A.; Bay, B.-H. Metallothioneins in human tumors and potential roles in carcinogenesis. Mutat. Res. 2003, 533, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Kim, J.Y.; Han, E.H.; Hwang, Y.P.; Choi, J.H.; Park, B.H.; Jeong, H.G. Metallothionein-2A overexpression increases the expression of matrix metalloproteinase-9 and invasion of breast cancer cells. FEBS Lett. 2011, 585, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageed, A.; Agrawal, K.C. Antisense down-regulation of metallothionein induces growth arrest and apoptosis in human breast carcinoma cells. Cancer Gene Ther. 1997, 4, 199–207. [Google Scholar] [PubMed]
- Jin, R.; Chow, V.T.-K.; Tan, P.-H.; Dheen, S.T.; Duan, W.; Bay, B.-H. Metallothionein 2A expression is associated with cell proliferation in breast cancer. Carcinogenesis 2002, 23, 81–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, D.; Jocelyn, K.M.-X.; Yip, G.W.-C.; Bay, B.-H. Silencing the Metallothionein-2A gene inhibits cell cycle progression from G1- to S-phase involving ATM and cdc25A signaling in breast cancer cells. Cancer Lett. 2009, 276, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Lim, D.; Tan, P.-H.; Leung, T.K.-C.; Yip, G.W.-C.; Bay, B.-H. Silencing the metallothionein-2A gene induces entosis in adherent MCF-7 breast cancer cells. Anat. Rec. 2010, 293, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Flora, S.J. Possible role of metal redistribution, hepatotoxicity and oxidative stress in chelating agents induced hepatic and renal metallothionein in rats. Food Chem. Toxicol. 2001, 39, 1029–1038. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, Z.; Kang, Y.J. Attenuation of doxorubicin chronic toxicity in metallothionein-overexpressing transgenic mouse heart. Cancer Res. 2001, 61, 3382–3387. [Google Scholar] [PubMed]
- Jacob, C.; Maret, W.; Vallee, B.L. Control of zinc transfer between thionein, metallothionein, and zinc proteins. Proc. Natl. Acad. Sci. USA 1998, 95, 3489–3494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, X.; Wu, H.; Chen, Y.; Kang, Y.J. Induction of antioxidants by adriamycin in mouse heart. Biochem. Pharmacol. 1998, 56, 87–93. [Google Scholar] [CrossRef]
- Doroshow, J.H. Role of hydrogen peroxide and hydroxyl radical formation in the killing of Ehrlich tumor cells by anticancer quinones. Proc. Natl. Acad. Sci. USA 1986, 83, 4514–4518. [Google Scholar] [CrossRef] [PubMed]
- Timur, M.; Akbas, S.H.; Ozben, T. The effect of Topotecan on oxidative stress in MCF-7 human breast cancer cell line. Acta Biochim. Pol. 2005, 52, 897–902. [Google Scholar] [PubMed]
- Doroshow, J.H.; Akman, S.; Esworthy, S.; Chu, F.F.; Burke, T. Doxorubicin resistance conferred by selective enhancement of intracellular glutathione peroxidase or superoxide dismutase content in human MCF-7 breast cancer cells. Free Radic. Res. Commun. 1991, 13, 779–781. [Google Scholar] [CrossRef]
- Ravid, A.; Rocker, D.; Machlenkin, A.; Rotem, C.; Hochman, A.; Kessler-Icekson, G.; Liberman, U.A.; Koren, R. 1,25-Dihydroxyvitamin D3 enhances the susceptibility of breast cancer cells to doxorubicin-induced oxidative damage. Cancer Res. 1999, 59, 862–867. [Google Scholar] [PubMed]
- Awasthi, K.K.; John, P.J.; Awasthi, A.; Awasthi, K. Multi walled carbon nano tubes induced hepatotoxicity in Swiss albino mice. Micron 2013, 44, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Prylutska, S.V.; Grynyuk, I.I.; Matyshevska, O.P.; Prylutskyy, Y.I.; Ritter, U.; Scharff, P. Anti-oxidant properties of C60 fullerenes in vitro. Fuller. Nanotub. Car. Nanostr. 2008, 16, 698–705. [Google Scholar] [CrossRef]
- Kaliszewski, M.; Kennedy, A.K.; Blaes, S.L.; Shaffer, R.S.; Knott, A.B.; Song, W.; Hauser, H.A.; Bossy, B.; Huang, T.-T.; Bossy-Wetzel, E. SOD1 Lysine 123 acetylation in the adult central nervous system. Front. Cell. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Hitchler, M.J.; Domann, F.E. Regulation of CuZnSOD and its redox signaling potential: Implications for amyotrophic lateral sclerosis. Antioxid. Redox Signal. 2014, 20, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.C.; Girouard, J.B.; Ulloa, J.L.; Subramaniam, J.R.; Wong, P.C.; Valentine, J.S.; Culotta, V.C. Mechanisms for activating Cu- and Zn-containing superoxide dismutase in the absence of the CCS Cu chaperone. Proc. Natl. Acad. Sci. USA 2004, 101, 5964–5969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culotta, V.C.; Yang, M.; O’Halloran, T.V. Activation of superoxide dismutases: Putting the metal to the pedal. Biochim. Biophys. Acta Mol. Cell Res. 2006, 1763, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Vonk, W.I.M.; Wijmenga, C.; Berger, R.; van de Sluis, B.; Klomp, L.W.J. Cu,Zn Superoxide dismutase maturation and activity are regulated by COMMD1. J. Biol. Chem. 2010, 285, 28991–29000. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, A.; Durdagi, S.; Doostdar, N.; Strom, T.A.; Barron, A.R.; Supuran, C.T. Nanoscale enzyme inhibitors: Fullerenes inhibit carbonic anhydrase by occluding the active site entrance. Bioorg. Med. Chem. 2010, 18, 2822–2828. [Google Scholar] [CrossRef] [PubMed]
- Vapa, I.; Torres, V.M.; Djordjevic, A.; Vasovic, V.; Srdjenovic, B.; Simic, V.D.; Popovic, J.K. Effect of fullerenol C60(OH)24 on lipid peroxidation of kidneys, testes and lungs in rats treated with doxorubicine. Eur. J. Drug Metab. Pharmacokinet. 2012, 37, 301. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.K.; Ziegler, Y.S.; McLeod, I.X.; Yates, J.R.; Nardulli, A.M. Effects of Cu/Zn superoxide dismutase on estrogen responsiveness and oxidative stress in human breast cancer cells. Mol. Endocrinol. 2008, 22, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Glasauer, A.; Sena, L.A.; Diebold, L.P.; Mazar, A.P.; Chandel, N.S. Targeting SOD1 reduces experimental non–small-cell lung cancer. J. Clin. Investig. 2014, 124, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Milnerowicz, H.; Bizoń, A. Determination of metallothionein in biological fluids using enzyme-linked immunoassay with commercial antibody. Acta Biochim. Pol. 2010, 57, 99–104. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]




| DOX/C60 | SOD1 Concentration (µg/mg of Total Protein) | SOD Activity (U/mg of Total Protein) | SOD Activity/SOD1 Concentration (U/µg SOD1) |
|---|---|---|---|
| 0 | 0.10 | 10.44 | 104.4 |
| 1 µM DOX | 0.14 | 18.56 | 132.5 |
| 2 µM DOX | 0.40 | 11.03 | 27.6 |
| 25 mg/mL C60 | 0.54 | 9.85 | 18.2 |
| 50 mg/mL C60 | 0.31 | 5.85 | 18.9 |
| 25 mg/mL C60 +1 µM DOX | 0.50 | 16.23 | 32.5 |
| 50 mg/mL C60 +1 µM DOX | 0.32 | 8.65 | 27.0 |
| 50 mg/mL C60+2 µM DOX | 0.39 | 7.77 | 19.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kepinska, M.; Kizek, R.; Milnerowicz, H. Metallothionein and Superoxide Dismutase—Antioxidative Protein Status in Fullerene-Doxorubicin Delivery to MCF-7 Human Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3253. https://doi.org/10.3390/ijms19103253
Kepinska M, Kizek R, Milnerowicz H. Metallothionein and Superoxide Dismutase—Antioxidative Protein Status in Fullerene-Doxorubicin Delivery to MCF-7 Human Breast Cancer Cells. International Journal of Molecular Sciences. 2018; 19(10):3253. https://doi.org/10.3390/ijms19103253
Chicago/Turabian StyleKepinska, Marta, Rene Kizek, and Halina Milnerowicz. 2018. "Metallothionein and Superoxide Dismutase—Antioxidative Protein Status in Fullerene-Doxorubicin Delivery to MCF-7 Human Breast Cancer Cells" International Journal of Molecular Sciences 19, no. 10: 3253. https://doi.org/10.3390/ijms19103253
APA StyleKepinska, M., Kizek, R., & Milnerowicz, H. (2018). Metallothionein and Superoxide Dismutase—Antioxidative Protein Status in Fullerene-Doxorubicin Delivery to MCF-7 Human Breast Cancer Cells. International Journal of Molecular Sciences, 19(10), 3253. https://doi.org/10.3390/ijms19103253