Neuroimmunological Implications of Subclinical Lipopolysaccharide from Salmonella Enteritidis
Abstract
:1. Introduction
- (1)
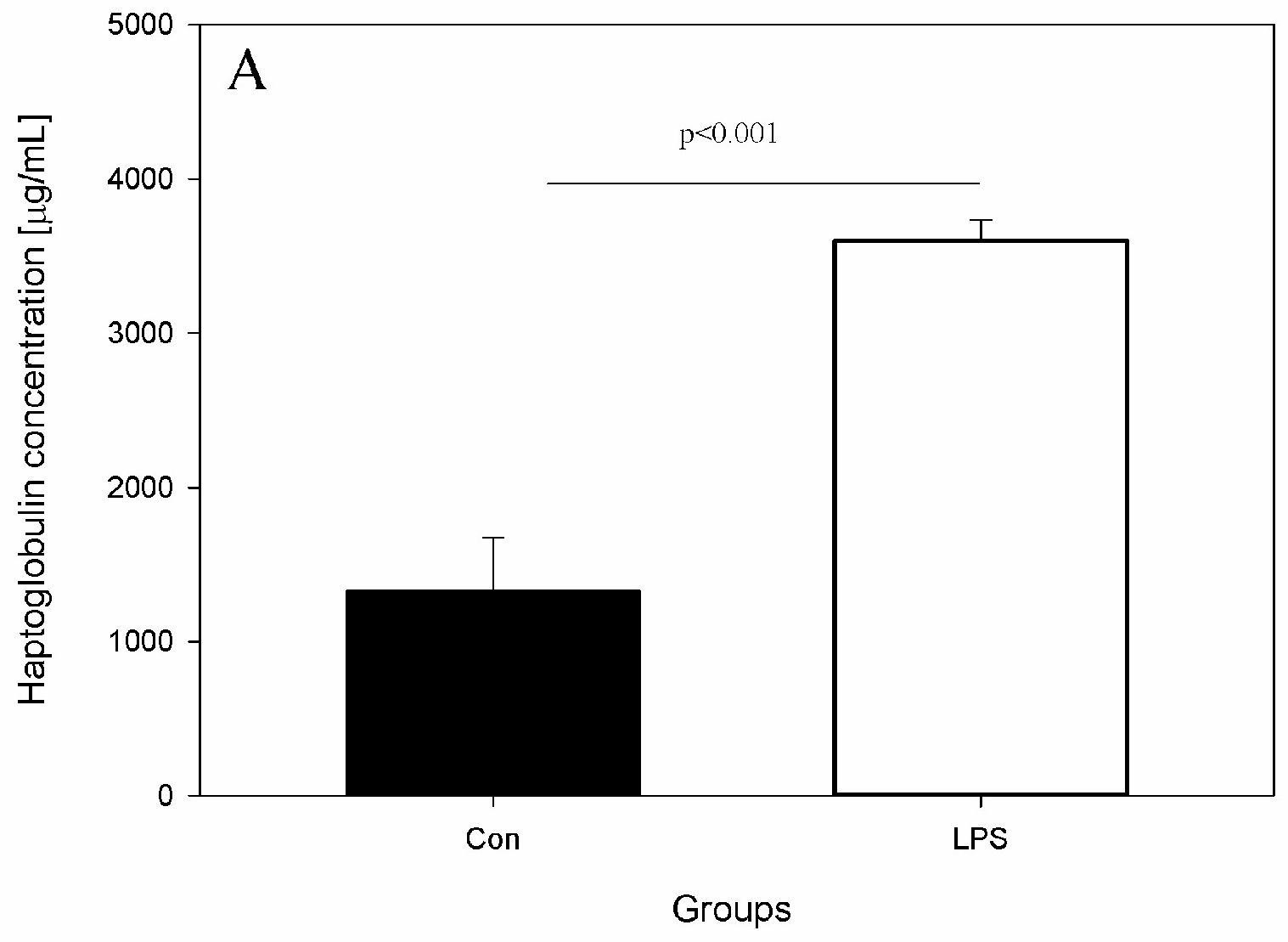
- Peripheral blood levels of CD4 T-lymphocytes, CD8 T-lymphocytes, IL-6, TNF-α, and Hp
- (2)
- cLN levels of CD4 T-lymphocytes, CD8 T-lymphocytes and neuropeptides such as GAL, NPY, SP and VIP
- (3)
- SN and PFC levels of DA
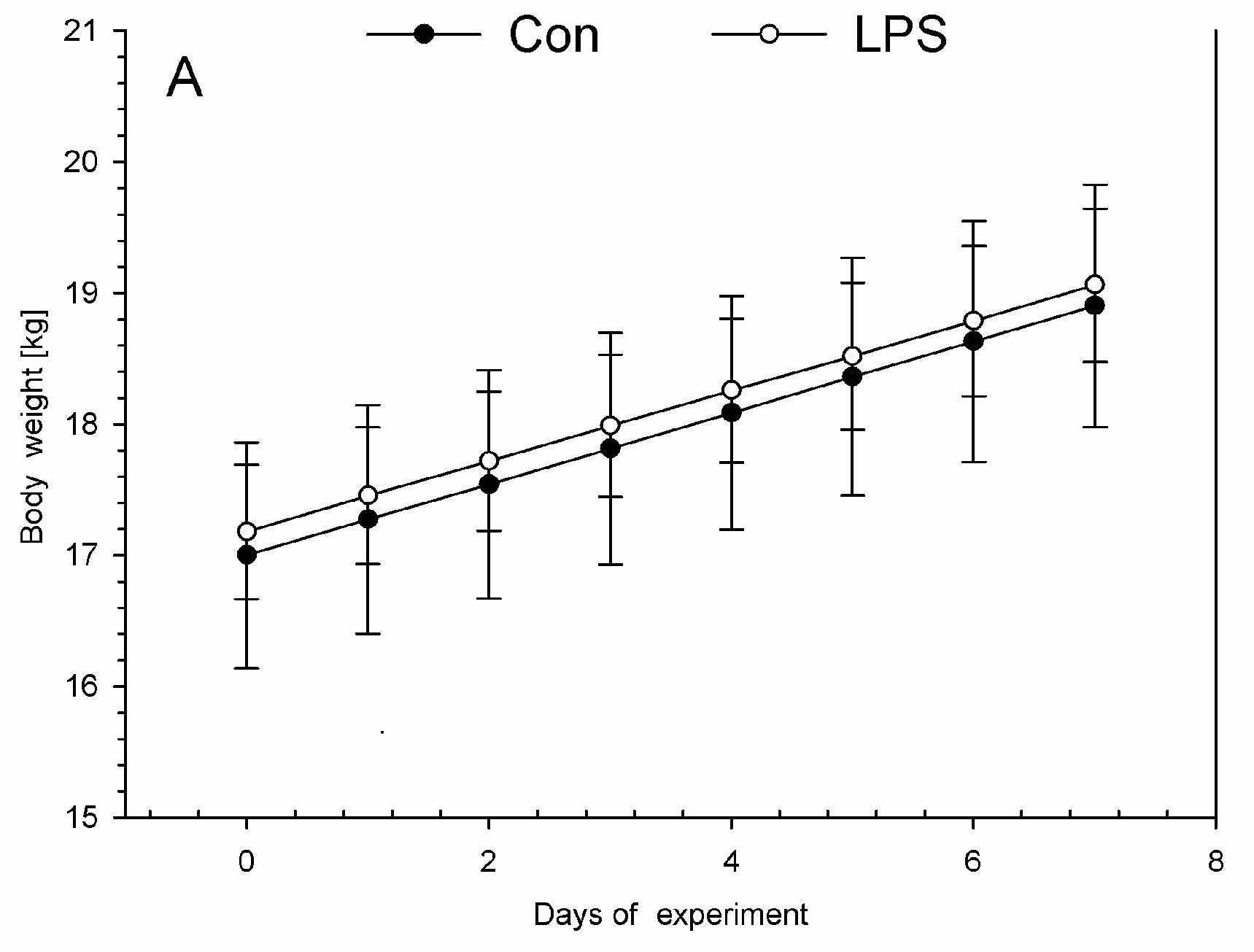
2. Results
3. Discussion
4. Materials and Methods
4.1. Animal Housing, Health Status, and LPS Administration
4.2. Sample Collection
4.3. Lymphocytes Isolation Phenotyping
4.3.1. Isolation of Peripheral Blood Mononuclear Cells (PBMCs) from Blood
4.3.2. Lymphocytes Isolation from the scLN
4.3.3. Lymphocyte Phenotyping
4.4. Measurement of Serum Concentration of Hp, IL-6, and TNF-α
- -
- Pig Haptoglobin ELISA (HAPT-9, Life Diagnostic Inc., West Chester, PA, USA); sensitivity range from 18.75 to 300 ng/mL
- -
- Quantizing® ELISA Porcine IL-6 (P6000B, R&D Systems, Minneapolis, MN, USA); sensitivity range from 0.68 to 4.30 pg/mL
- -
- Quantizing® ELISA Porcine TNF-α (PTA00, R&D Systems, Minneapolis, MN, USA); sensitivity range from 2.8 to 5.0 pg/mL
4.5. Brain Sample Preparation and Determination of DA Concentrations
4.6. Purification and Determination of Neuropeptide Levels from scLN
4.6.1. Sample Preparation and High-Temperature Extraction
4.6.2. Solid-Phase Extraction (SPE) and Concentration
4.6.3. Enzyme-Linked Immunosorbent Assay for Quantitative Determination of GAL, NPY, SP, and VIP in scLN
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| BBB | blood brain barrier |
| BCSFB | blood-cerebrospinal fluid barrier |
| cLN | cervical lymph nodes |
| CSF | cerebrospinal fluid |
| DA | dopamine |
| dcLN | deep cervical lymph nodes |
| GAL | galanin |
| Hp | haptoglobin |
| IL-6 | interleukin-6 |
| LN | lymph nodes |
| LPS | lipopolysaccharide |
| NPY | neuropeptide Y |
| PD | Parkinson’s disease |
| PFC | prefrontal cortex |
| scLN | superficial cervical lymph nodes |
| SN | substantia nigra |
| SP | substance P |
| TNF-α | tumour necrosis factor α |
| VIP | vasoactive intestinal peptide |
References
- De Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Mesri, E.A.; Feitelson, M.A.; Munger, K. Human viral oncogenesis: A cancer hallmarks analysis. Cell Host Microbe 2014, 15, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Vedham, V.; Divi, R.L.; Starks, V.L.; Verma, M. Multiple Infections and Cancer: Implications in Epidemiology. Technol. Cancer Res. Treat. 2014, 13, 177–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikołajczyk, A. Invited Brief Commentary on the Article “Breast Cancer Association with Cytomegalo Virus—A Tertiary Center Case-Control Study” Is Cytomegalo Virus a Breast Cancer Etiologic Risk Factor? J. Investig. Surg. 2017, 30, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Zloza, A. Viruses, bacteria, and parasites—Oh my! A resurgence of interest in microbial-based therapy for cancer. J. Immunother. Cancer 2018, 6, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, G. Gram-positive and gram-negative bacterial toxins in sepsis. Virulence 2014, 5, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Bing, G. Lipopolysaccharide animal models for Parkinson’s disease. Parkinsons. Dis. 2011, 2011, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hoban, D.B.; Connaughton, E.; Connaughton, C.; Hogan, G.; Thornton, C.; Mulcahy, P.; Moloney, T.C.; Dowd, E. Further characterisation of the LPS model of Parkinson’s disease: A comparison of intra-nigral and intra-striatal lipopolysaccharide administration on motor function, microgliosis and nigrostriatal neurodegeneration in the rat. Brain Behav. Immun. 2013, 27, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Nehru, B. Characterization of the lipopolysaccharide induced model of Parkinson’s disease: Role of oxidative stress and neuroinflammation. Neurochem. Int. 2015, 87, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Liu, J.; Ju, C.; Yang, D.; Chen, G.; Xu, S.; Zeng, Y.; Yan, X.; Wang, W.; Liu, D.; et al. Licochalcone A prevents the loss of dopaminergic neurons by inhibiting microglial activation in lipopolysaccharide (LPS)-induced Parkinson’s disease models. Int. J. Mol. Sci. 2017, 18, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.D. Exacerbation of Motor Neuron Disease by Chronic Stimulation of Innate Immunity in a Mouse Model of Amyotrophic Lateral Sclerosis. J. Neurosci. 2004, 24, 1340–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.; Liu, M.; Hunter, R.L.; Cass, W.A.; Pandya, J.D.; Patrick, G.; Shin, E.; Kim, H.; Gash, D.M.; Bing, G. Striatal Neuroinflammation Promotes Parkinsonism in Rats. PLoS ONE 2009, 4, e5482. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Ren, Q.; Yang, C.; Zhang, J.C.; Yao, W.; Dong, C.; Ohgi, Y.; Futamura, T.; Hashimoto, K. Antidepressant effects of combination of brexpiprazole and fluoxetine on depression-like behavior and dendritic changes in mice after inflammation. Psychopharmacology 2017, 234, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Cao, J.B.; Zhang, L.M.; Li, Y.F.; Mi, W.D. Deferoxamine attenuates lipopolysaccharide-induced neuroinflammation and memory impairment in mice. J. Neuroinflamm. 2015, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hawkesworth, S.; Moore, S.E.; Fulford, A.J.C.; Barclay, G.R.; Darboe, A.A.; Mark, H.; Nyan, O.A.; Prentice, A.M. Evidence for metabolic endotoxemia in obese and diabetic Gambian women. Nutr. Diabetes 2013, 3, e83–e86. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Stamova, B.; Jin, L.W.; DeCarli, C.; Phinney, B.; Sharp, F.R. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 2016, 87, 2324–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pretorius, E.; Page, M.J.; Mbotwe, S.; Kell, D.B. Lipopolysaccharide-binding protein (LBP) can reverse the amyloid state of fibrin seen or induced in Parkinson’s disease. PLoS ONE 2018, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jaber, V.; Lukiw, W.J. Secretory Products of the Human GI Tract Microbiome and Their Potential Impact on Alzheimer’s Disease (AD): Detection of Lipopolysaccharide (LPS) in AD Hippocampus. Front. Cell. Infect. Microbiol. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Gray, A.M.; Erickson, M.A.; Salameh, T.S.; Damodarasamy, M.; Sheibani, N.; Meabon, J.S.; Wing, E.E.; Morofuji, Y.; Cook, D.G.; et al. Lipopolysaccharide-induced blood-brain barrier disruption: Roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J. Neuroinflamm. 2015, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Robinson, S.M. Minimal penetration of lipopolysaccharide across the murine blood-brain barrier. Brain Behav. Immun. 2010, 24, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.; Sousa, J.C.; Coppola, G.; Falcao, A.M.; Rodrigues, A.J.; Geschwind, D.H.; Sousa, N.; Correia-Neves, M.; Palha, J.A. Kinetic profile of the transcriptome changes induced in the choroid plexus by peripheral inflammation. J. Cereb. Blood Flow Metab. 2009, 29, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.; Sousa, J.C.; Coppola, G.; Geschwind, D.H.; Sousa, N.; Palha, J.A.; Correia-Neves, M. The choroid plexus response to peripheral inflammatory stimulus. BMC Neurosci. 2009, 10, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niehaus, I.; Lange, J.H. Endotoxin: Is it an environmental factor in the cause of Parkinson’s disease? Occup. Environ. Med. 2003, 60, 378. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, I. In vivo Radiodetoxification of Salmonella minnesota Lipopolysaccharides with radio-labeled Leucine Enkephalin cures sensory polyneuropathy: A Case report. Niger. Health J. 2010, 10, 26–33. [Google Scholar]
- Yao, Z.; Mates, J.M.; Cheplowitz, A.M.; Hammer, L.P.; Phillips, G.S.; Wewers, M.D.; Rajaram, M.V.S.; John, M.; Anderson, C.L.; Ganesan, L.P.; et al. Blood-Borne Lipopolysaccharide Is Rapidly Eliminated by Liver Sinusoidal Endothelial Cells via High-Density Lipoprotein. J. Immunol. 2016, 197, 2390–2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, R.F.; de Jonge, E.; Dekkers, P.E.; Timmerman, J.J.; Spek, C.A.; van Deventer, S.J.; van Deursen, P.; van Kerkhoff, L.; van Gemen, B.; ten Cate, H.; et al. The in vivo kinetics of tissue factor messenger RNA expression during human endotoxemia: Relationship with activation of coagulation. Blood 2000, 96, 554–559. [Google Scholar] [PubMed]
- Maxwell, J.R.; Ruby, C.; Kerkvliet, N.I.; Vella, A.T. Contrasting the Roles of Costimulation and the Natural Adjuvant Lipopolysaccharide during the Induction of T Cell Immunity. J. Immunol. 2002, 168, 4372–4381. [Google Scholar] [CrossRef] [PubMed]
- Haudek, S.B.; Natmessnig, B.E.; Fürst, W.; Bahrami, S.; Schlag, G.; Redl, H. Lipopolysaccharide dose response in baboons. Shock 2003, 20, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Arredouani, M.; Matthijs, P.; Van Hoeyveld, E.; Kasran, A.; Baumann, H.; Ceuppens, J.L.; Stevens, E. Haptoglobin directly affects T cells and suppresses T helper cell type 2 cytokine release. Immunology 2003, 108, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arredouani, M.S.; Kasran, A.; Vanoirbeek, J.A.; Berger, F.G.; Baumann, H.; Ceuppens, J.L. Haptoglobin dampens endotoxin-induced inflammatory effects both in vitro and in vivo. Immunology 2005, 114, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikołajczyk, A.; Gonkowski, S.; Złotkowska, D. Modulation of the main porcine enteric neuropeptides by a single low-dose of lipopolysaccharide (LPS) Salmonella Enteritidis. Gut Pathog. 2017, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N. Immunologists getting nervous: Neuropeptides, dendritic cells and T cell activation. Respir. Res. 2001, 2, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Farzi, A.; Reichmann, F.; Holzer, P. The homeostatic role of neuropeptide Y in immune function and its impact on mood and behaviour. Acta Physiol. 2015, 213, 603–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasowicz, K.; Winnicka, A.; Kaleczyc, J.; Zalecki, M.; Podlasz, P.; Pidsudko, Z. Neuropeptides and lymphocyte populations in the porcine ileum and ileocecal lymph nodes during postnatal life. PLoS ONE 2018, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ganea, D.; Hooper, K.M.; Kong, W. The neuropeptide VIP: Direct effects on immune cells and involvement in inflammatory and autoimmune diseases. Acta Physiol. 2015, 213, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Madva, E.N.; Granstein, R.D. Nerve-derived Transmitters Including Peptides Influence Cutaneous Immunology. Brain Behav. Immun. 2013, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qiu, A.W.; Peng, Y.P.; Liu, Y.; Huang, H.W.; Qiu, Y.H. Roles of dopamine receptor subtypes in mediating modulation of T lymphocyte function. Neuroendocrinol. Lett. 2010, 31, 782–791. [Google Scholar] [PubMed]
- Sarkar, C.; Basu, B.; Chakroborty, D.; Dasgupta, P.S.; Basu, S. The immunoregulatory role of dopamine: An update and. Brain Behav. Immun. 2010, 24, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, R.; Contreras, F.; Zouali, M. The dopaminergic system in autoimmune diseases. Front. Immunol. 2014, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Slifstein, M.; Van De Giessen, E.; Van Snellenberg, J.; Thompson, J.L.; Narendran, R.; Gil, R.; Hackett, E.; Girgis, R.; Ojeil, N.; Moore, H.; et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry 2015, 72, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.H.; Marques, T.R.; Jauhar, S.; Nour, M.M.; Goodwin, G.M.; Young, A.H.; Howes, O.D. The dopamine hypothesis of bipolar affective disorder: The state of the art and implications for treatment. Mol. Psychiatry 2017, 22, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louveau, A.; Harris, T.H.; Kipnis, J. Revisiting the concept of CNS immune privilege Antoine. Trends Immunol. 2015, 36, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ni, Z.; Chen, Y.; Wang, D.; Qi, Y.; Zhang, Q.; Wang, S. Olfactory route for cerebrospinal fluid drainage into the cervical lymphatic system in a rabbit experimental model. Neural Regen. Res. 2012, 7, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Elias, G.; Yostos, M.P.; Stimec, B.; Fasel, J.; Murphy, K. Pathways of cerebrospinal fluid outflow: A deeper understanding of resorption. Neuroradiology 2015, 57, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Laman, J.D.; Weller, R.O. Drainage of cells and soluble antigen from the CNS to regional lymph nodes. J. Neuroimmune Pharmacol. 2013, 8, 840–856. [Google Scholar] [CrossRef] [PubMed]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J., Jr.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Bassols, A.; Costa, C.; Eckersall, P.D.; Osada, J.; Sabrià, J.; Tibau, J. The pig as an animal model for human pathologies: A proteomics perspective. Proteom. Clin. Appl. 2014, 8, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Kumar, P.; Cutler, C.W.; Mohamadzadeh, M.; Van Dyke, T.; Banchereau, J. Lipopolysaccharides from Distinct Pathogens Induce Different Classes of Immune Responses In Vivo. J. Immunol. 2001, 167, 5067–5076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedrebø, T.; Reed, R.K. Different serotypes of endotoxin (lipopolysaccharide) cause different increases in albumin extravasation in rats. Shock 2002, 18, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.E.; Spring, D.R.; Gangloff, M.; Gay, N.J. The molecular basis of the host response to lipopolysaccharide. Nat. Rev. Microbiol. 2010, 8, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Fedele, G.; Nasso, M.; Spensieri, F.; Palazzo, R.; Frasca, L.; Watanabe, M.; Ausiello, C.M. Lipopolysaccharides from Bordetella pertussis and Bordetella parapertussis Differently Modulate Human Dendritic Cell Functions Resulting in Divergent Prevalence of Th17-Polarized Responses. J. Immunol. 2008, 181, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Mikołajczyk, A.; Kozłowska, A.; Gonkowski, S. Distribution and Neurochemistry of the Porcine Ileocaecal Valve Projecting Sensory Neurons in the Dorsal Root Ganglia and the Influence of Lipopolysaccharide from Different Serotypes of Salmonella spp. on the Chemical Coding of DRG Neurons in the Cell Cultures. Int. J. Mol. Sci. 2018, 19, 2551. [Google Scholar] [CrossRef]
- Maciel, B.M.; Rezende, R.P.; Sriranganathan, N.K. Salmonella enterica: Latency. In Current Topics in Salmonella and Salmonellosis; Mares, M., Ed.; InTechOpen: Rijeka, Croatia, 2017; pp. 41–58. [Google Scholar]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Knapp, D.J.; Crews, F.T.; Hill, C.; Carolina, N.; Park, T. Systemic LPS Causes Chronic Neuroinflammation and Progressive Neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Calvano, S.E.; Coyle, S.M. Experimental Human Endotoxemia: A Model of the Systemic Inflammatory Response Syndrome? Surg. Infect. 2012, 13, 293–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webel, D.M.; Finck, B.N.; Baker, D.H.; Johnson, R.W. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J. Anim. Sci. 1997, 75, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Llamas Moya, S.; Boyle, L.; Lynch, P.B.; Arkins, S. Pro-inflammatory cytokine and acute phase protein responses to low-dose lipopolysaccharide (LPS) challenge in pigs. Anim. Sci. 2006, 82, 527–534. [Google Scholar] [CrossRef]
- Maes, M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Pan, J.Q.; Tang, X.E.; Li, B.; Liu, S.F.; Ma, W.L. The role of immune abnormality in depression and cardiovascular disease. J. Geriatr. Cardiol. 2017, 14, 703–710. [Google Scholar] [PubMed]
- Robertson, M.J.; Schacterle, R.S.; Mackin, G.A.; Wilson, S.N.; Bloomingdale, K.L.; Ritz, J.; Komaroff, A.L. Lymphocyte subset differences in patients with chronic fatigue syndrome, multiple sclerosis and major depression. Clin. Exp. Immunol. 2005, 141, 326–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juffermans, N.P.; Paxton, W.A.; Dekkers, P.E.; Verbon, A.; de Jonge, E.; Speelman, P.; van Deventer, S.J.; van der Poll, T. Up-regulation of HIV coreceptors CXCR4 and CCR5 on CD4(+) T cells during human endotoxemia and after stimulation with (myco)bacterial antigens: The role of cytokines. Blood 2000, 96, 2649–2654. [Google Scholar] [PubMed]
- Palmer, C.D.; Tomassilli, J.; Sirignano, M.; Tejeda, M.R.; Arnold, B.; Che, D.; Lauffenburger, D.A.; Jost, S.; Allen, T.; Mayer, K.H.; et al. Enhanced Immune Activation Linked to Endotoxemia in HIV-1 Seronegative Men who have Sex with Men. AIDS 2014, 28, 2162–2166. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, D.C.; Herrera, M.O.; Brizuela, N.O.; Mejía, G.B.; Jiménez, F.T.; García, E.H.; Olguín, H.J. Assessment of the effects of oseltamivir and indomethacin on dopamine, 5-HIAA, and some oxidative stress markers in stomach and brain of Salmonella typhimurium-infected rats. Neuroendocrinol. Lett. Vol. 2016, 37, 129–136. [Google Scholar]
- Gołembiowska, K.; Wardas, J.; Noworyta-Sokołowska, K.; Kamińska, K.; Górska, A. Effects of adenosine receptor antagonists on the in vivo lps-induced inflammation model of parkinson’s disease. Neurotox. Res. 2013, 24, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Noworyta-Sokolowska, K.; Gorska, A.; Golembiowska, K. LPS-induced oxidative stress and inflammatory reaction in the rat striatum. Pharmacol. Rep. 2013, 65, 863–869. [Google Scholar] [CrossRef]
- Booij, L.; Welfeld, K.; Leyton, M.; Dagher, A.; Boileau, I.; Sibon, I.; Baker, G.B.; Diksic, M.; Soucy, J.P.; Pruessner, J.C.; et al. Dopamine cross-sensitization between psychostimulant drugs and stress in healthy male volunteers. Transl. Psychiatry 2016, 6, e740. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.J.; Weinstein, J.J.; Chohan, M.O.; Slifstein, M.; Kegeles, L.S.; Moore, H.; Abi-dargham, A. Pathway-Specific Dopamine Abnormalities in Schizophrenia Review Pathway-Speci fi c Dopamine Abnormalities in Schizophrenia. Biol. Psychiatry 2017, 81, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, R.; Contreras, F.; Prado, C. Cells, molecules and mechanisms involved the neuro-immune interaction. In Cell Interaction; Gowder, S., Ed.; InTechOpen: Rijeka, Croatia, 2012; pp. 139–166. [Google Scholar]
- Delgado, M.; Ganea, D. VIP and PACAP inhibit activation induced apoptosis in T lymphocytes. Ann. N. Y. Acad. Sci. 2000, 921, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Bedoui, S.; Kawamura, N.; Straub, R.H.; Pabst, R.; Yamamura, T.; Von Hörsten, S. Relevance of neuropeptide Y for the neuroimmune crosstalk. J. Neuroimmunol. 2003, 134, 1–11. [Google Scholar] [CrossRef]
- Hauser, G.J.; Myers, A.K.; Dayao, E.K.; Zukowska-Grojec, Z. Neuropeptide Y infusion improves hemodynamics and survival in rat endotoxic shock. Am. J. Physiol. 1993, 265, H1416–H1423. [Google Scholar] [CrossRef] [PubMed]
- Bedoui, S.; von Hörsten, S.; Gebhardt, T. A role for neuropeptide Y (NPY) in phagocytosis: Implications for innate and adaptive immunity. Peptides 2007, 28, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Trejter, M.; Brelinska, R.; Warchol, J.B.; Butowska, W.; Neri, G.; Rebuffat, P.; Gottardo, L.; Malendowicz, L.K. Effects of galanin on proliferation and apoptosis of immature rat thymocytes. Int. J. Mol. Med. 2002, 10, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Mignini, F.; Streccioni, V.; Amenta, F. Autonomic innervation of immune organs and neuroimmune modulation. Auton. Autacoid Pharmacol. 2003, 23, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; Zakharov, A.; Papaiconomou, C.; Salmasi, G.; Armstrong, D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004, 1, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radjavi, A.; Smirnov, I.; Derecki, N.; Kipnis, J. Dynamics of the Meningeal CD4+ T-cell repertoire are defined by the cervical lymph nodes and facilitate cognitive task performance in mice. Mol. Psychiatry 2014, 19, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Brochard, V.; Combadière, B.; Prigent, A.; Laouar, Y.; Perrin, A.; Beray-Berthat, V.; Bonduelle, O.; Alvarez-Fischer, D.; Callebert, J.; Launay, J.M.; et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Investig. 2009, 119, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Schetters, S.T.T.; Gomez-Nicola, D.; Garcia-Vallejo, J.J.; Van Kooyk, Y. Neuroinflammation: Microglia and T cells get ready to tango. Front. Immunol. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McKenna, F.; McLaughlin, P.J.; Lewis, B.J.; Sibbring, G.C.; Cummerson, J.A.; Bowen-Jones, D.; Moots, R.J. Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: A flow cytometric study. J. Neuroimmunol. 2002, 132, 34–40. [Google Scholar] [CrossRef]
- Lucin, K.; Wyss-Coray, T. Immune activation in brain aging and neurodegeneration: Too much or too little? Neuron 2009, 64, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Greifenberg, V.; Ribechini, E.; Rößner, S.; Lutz, M.B. Myeloid-derived suppressor cell activation by combined LPS and IFN-γ treatment impairs DC development. Eur. J. Immunol. 2009, 39, 2865–2876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arreola, R.; Alvarez-Herrera, S.; Pérez-Sánchez, G.; Becerril-Villanueva, E.; Cruz-Fuentes, C.; Flores-Gutierrez, E.O.; Garcés-Alvarez, M.E.; De La Cruz-Aguilera, D.L.; Medina-Rivero, E.; Hurtado-Alvarado, G.; et al. Immunomodulatory Effects Mediated by Dopamine. J. Immunol. Res. 2016, 2016, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Kozina, E.; Sadasivan, S.; Jiao, Y.; Dou, Y.; Ma, Z.; Tan, H.; Kodali, K.; Shaw, T.; Peng, J.; Smeyne, R.J. Mutant LRRK2 mediates peripheral and central immune responses leading to neurodegeneration in vivo. Brain 2018, 141, 1753–1769. [Google Scholar] [CrossRef] [PubMed]
- Mikołajczyk, A. Safe and effective anaesthesiological protocols in domestic pig. Ann. Warsaw Univ. Life Sci. SGGW Anim. Sci. 2016, 55, 219–227. [Google Scholar]
- Fu, H.Q.; Yang, T.; Xiao, W.; Fan, L.; Wu, Y.; Terrando, N.; Wang, T.L. Prolonged neuroinflammation after lipopolysaccharide exposure in aged rats. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.C. LPS and neuroinflammation: A matter of timing. Inflammopharmacology 2016, 24, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Saar, L.I. Lymph Nodes of the Head, Neck and Shoulder Region of Swine. Iowa State Univ. Vet. Digit. Respir. 1962, 25, 120–134. [Google Scholar]
- Jelsing, J.; Hay-Schmidt, A.; Dyrby, T.; Hemmingsen, R.; Uylings, H.B.M.; Pakkenberg, B. The prefrontal cortex in the Göttingen minipig brain defined by neural projection criteria and cytoarchitecture. Brain Res. Bull. 2006, 70, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Waters, W.R.; Sacco, R.E.; Dorn, A.D.; Hontecillas, R.; Zuckermann, F.A.; Wannemuehler, M.J. Systemic and mucosal immune responses of pigs to parenteral immunization with a pepsin-digested Serpulina hyodysenteriae bacterin. Vet. Immunol. Immunopathol. 1999, 69, 75–87. [Google Scholar] [CrossRef]
- Mierzejewska, D.; Mitrowska, P.; Rudnicka, B.; Kubicka, E.; Kostyra, H. Effect of non-enzymatic glycosylation of pea albumins on their immunoreactive properties. Food Chem. 2008, 111, 127–131. [Google Scholar] [CrossRef]
- Jun, S.M.; Ochoa-Repáraz, J.; Zlotkowska, D.; Hoyt, T.; Pascual, D.W. Bystander-mediated stimulation of proteolipid protein-specific regulatory T (Treg) cells confers protection against experimental autoimmune encephalomyelitis (EAE) via TGF-β. J. Neuroimmunol. 2012, 245, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Wong, J.H.; Lu, G.; Antonio, G.E.; Yeung, D.K.; Ng, T.B.; Forster, L.E.; Yew, D.T. Gene expression of synaptosomal-associated protein 25 (SNAP-25) in the prefrontal cortex of the spontaneously hypertensive rat (SHR). Biochim. Biophys. Acta 2009, 1792, 766–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najmanová, V.; Rambousek, L.; Syslová, K.; Bubeníková, V.; Šlamberová, R.; Valeš, K.; Kačer, P. LC-ESI-MS-MS method for monitoring dopamine, serotonin and their metabolites in brain tissue. Chromatographia 2011, 73, 143–149. [Google Scholar] [CrossRef]
- Conlon, J.M. Purification of naturally occurring peptides by reversed-phase HPLC. Nat. Protoc. 2007, 2, 191–197. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikołajczyk, A.; Złotkowska, D. Neuroimmunological Implications of Subclinical Lipopolysaccharide from Salmonella Enteritidis. Int. J. Mol. Sci. 2018, 19, 3274. https://doi.org/10.3390/ijms19103274
Mikołajczyk A, Złotkowska D. Neuroimmunological Implications of Subclinical Lipopolysaccharide from Salmonella Enteritidis. International Journal of Molecular Sciences. 2018; 19(10):3274. https://doi.org/10.3390/ijms19103274
Chicago/Turabian StyleMikołajczyk, Anita, and Dagmara Złotkowska. 2018. "Neuroimmunological Implications of Subclinical Lipopolysaccharide from Salmonella Enteritidis" International Journal of Molecular Sciences 19, no. 10: 3274. https://doi.org/10.3390/ijms19103274
APA StyleMikołajczyk, A., & Złotkowska, D. (2018). Neuroimmunological Implications of Subclinical Lipopolysaccharide from Salmonella Enteritidis. International Journal of Molecular Sciences, 19(10), 3274. https://doi.org/10.3390/ijms19103274





