1. Introduction
Immobilization is a powerful tool to improve enzyme properties. Enzymatic immobilization arose primarily as a response to the need of reusing expensive enzymes in industrial processes [
1]. However, currently this technology not only solves the problem associated with enzyme recovery, but also, if used properly, improves many other characteristics of enzymes, such as stability, selectivity, activity, resistance to inhibitors and purity, among others [
2,
3].
Lipases (triacylglycerol acylhydrolases EC 3.1.1.3) are known as one of the main biocatalysts with the greatest potential in lipid technology. Lipases catalyze the hydrolysis of triglycerides into diglycerides, monoglycerides, glycerol, and free fatty acids [
4]. At low water content, they also catalyze synthesis reactions generating a wide range of esters [
5]. The production of lipases is carried out by means of fermentation processes, with bacteria, filamentous fungi, or yeasts. Among yeasts,
Yarrowia lipolytica is the specie that stands out because it produces great amount of this enzyme and it has GRAS (generally recognized as safe) status, providing acceptance for use in food production [
6,
7].
Y. lipolytica is known to possess 16 paralogs of genes coding for lipase, out of which Lip2 is the main extracellular lipase secreted by this yeast [
8]. The structural feature of Lip2 is characterized by the presence of a mobile subdomain, called lid, whose conformational changes control the access of substrate molecules to the catalytic center [
9].
Lipases can be used in their free or immobilized forms. However, in industry, the immobilized form is preferable because it allows higher stability and, depending on the immobilization method, it can even increase lipase activity [
10]. Manoel et al. [
11] confirmed that lipases immobilized on octyl agarose presented their open form stabilized while the covalent preparation maintains a closing/opening equilibrium that may be modulated by altering the medium. According to the modes of interaction between enzymes and support carriers, enzyme immobilization methods can be classified into chemical or physical methods. Among the physical methods (adsorption or entrapment), entrapment can be performed by gel/fiber entrapping, by metal organic frameworks embedding and by microencapsulation [
12].
Microencapsulation consists of the entrapment of the enzyme within the network of a matrix or polymer membrane. In this method, the enzymes are retained in the nets while substrates and products pass through, which avoids the contamination of the leached enzyme in the substrate solution as observed when physical adsorption is used to immobilize enzymes. Additionally, it improves stability and allows the generation of enzymatic reactions [
12]. Alginate, an anionic linear copolymer composed of 1,4′-linked β-
d-mannuronic acid and α-
l-guluronic acid, is the most frequently used polymer for microencapsulation due to its mild gelling properties and non-toxicity [
13]. Another natural polymer that has been used for microencapsulation is chitosan, which is obtained by N-deacetylation of chitin and is the second most abundant naturally occurring polymer found in the exoskeleton of marine crustaceans [
14]. Chitosan has been used for several biomedical applications such as: drug, gene and vaccine delivery, tissue engineering and as biological iron chelator [
14]. It acts as a polycation in solution, is readily soluble in dilute acid solutions and was used before in core-shell microcapsule technology for enzyme immobilization [
15].
Even though several enzymes as well as lipases have been immobilized in alginate beads [
13,
16,
17], no results of optimization of microencapsulation conditions of
Y. lipolytica lipase have been found in literature. The optimization of microencapsulation conditions is essential to obtain high enzyme activity. Chitosan was tested during the formation of the microcapsules, a different approach from the coating method already used [
13]. In this context, statistical experimental design was used to obtain the best conditions (alginate, chitosan and calcium chloride concentrations and complexation time) to microencapsulate the lipase produced from residual oil with
Yarrowia lipolytica IMUFRJ 50682, analyzing immobilization yield and microcapsules′ lipase activity.
2. Results and Discussion
Gelation is obtained by cross-linking between the carboxyl group of the α-
l-guluronic acid of sodium alginate and Ca
2+ ions. The drop-wise addition of an aqueous solution of sodium alginate and the enzyme in a solution of Ca
2+ causes the droplets to precipitate and entraps the biocatalyst [
13]. Therefore, sodium alginate and CaCl
2 concentrations used in this process have a huge influence on the entrapment of the enzyme. Chitosan forms polyelectrolyte complexes with alginate, which results in a reinforced gel, reducing leakage and it is usually used as a coating material [
13]. In the present work, chitosan was added during gelation to increase its interaction with the matrix, leaving the enzyme to interact with the substrate. However, a high chitosan concentration can reduce mass transfer, decreasing the biocatalyst activity. Therefore, its concentration must also be adjusted. The time used for gelation (complexation time) can also influence the formation of the matrix as an increase in the contact period between these substances may form barriers that reduce diffusional effects. Taqieddin and Amiji [
15] reported that increasing the complexation time to more than 5 min did increase the mechanical strength of the beads, but a considerable loss of enzyme activity was observed. As all these variables (sodium alginate, chitosan and calcium chloride concentrations and complexation time) influence each other, a 2
4-1 fractional factorial design (FFD) was performed to investigate their influence on immobilization yield and on the hydrolytic activity in
p-nitrophelyl laurate (
p-NFL) of the microcapsules obtained (immobilized lipase activity—ImLipA). The data obtained are shown in
Table 1. The results show that sometimes high immobilization yield (IY) is not accompanied by a high activity of the biocatalyst. This is because it can be effective to retain the enzyme but the pores formed are too small for the substrate to diffuse and, therefore, a low activity is detected.
Analysis of variance (ANOVA) and the significance of the results shown in
Table 1 were verified using Fisher′s statistical test (
F-test) at 5% of significance. The results of the analyses can be observed in the Pareto diagrams (
Figure 1).
Chitosan was the independent variable with greater influence on immobilization yield and on immobilized lipase activity, with a positive effect (
Figure 1), which indicates that higher concentrations of chitosan can improve both responses. High IY were obtained for almost all conditions, but higher ImLipA was only obtained when chitosan was used. On the other hand, negative effects were observed for CaCl
2 concentration, indicating that its reduction tends to increase the IY and ImLipA. Regarding complexation time, a negative effect was observed for IY and a positive effect for ImLipA, evidencing that reducing the contact time of the reagents favors the immobilization yield, but it does not favor the activity of the biocatalyst. As for alginate concentration, it was found that increasing its concentration may improve IY, but tends to reduce ImLipA. Furthermore, all interactions tested for both responses were also statistically significant.
Since all variables significantly influenced the immobilization yield and the immobilized lipase activity, they were chosen for the optimization strategy using the central composite rotatable design (CCRD). For this design, higher concentrations of chitosan and sodium alginate were used as well as lower calcium chloride concentration and shorter complexation time.
Table 2 shows the results obtained and the real values of the levels studied in each experiment.
It is important to observe in
Table 2 that IY ranged from 91.76 to 99.77%. Both values were higher than the results from fractional factorial design (FFD) (
Table 1), showing the improvement of this response by the statistical designs. The activity of the biocatalyst (ImLipA) ranged from 4.12 to 273.33 U/g, showing also an increase in relation to the FFD, with the highest value (273.33 U/g) almost 14 times higher than for FFD (19.89 U/g). From the experimental values presented, statistical adjustments were made with the purpose of generating significant models. The analysis of variance was performed and the significance of the model verified by Fisher’s statistical test (
F-test). Tests were performed for the significance of variable effects and for the lack of fit. The complete model was not significant (data not shown) and, therefore, the non-significant terms were eliminated. The results of the variance analysis for the reduced model with IY as response are presented in
Table 3.
A significant lack of fit (
p < 0.05) observed in
Table 3 may not be important in the development of a predictive model, according to Rodrigues & Iemma [
18], when the pure error, which is associated with the variability of the central points, presents very low value, which is the case here (0.027). Therefore, a mathematical model with the real variables is proposed to represent the immobilization yield (IY) for the studied conditions (Equaion (1)).
where SA is sodium alginate concentration, CTS is chitosan concentration, CaCl
2 is calcium chloride concentration and CT is complexation time.
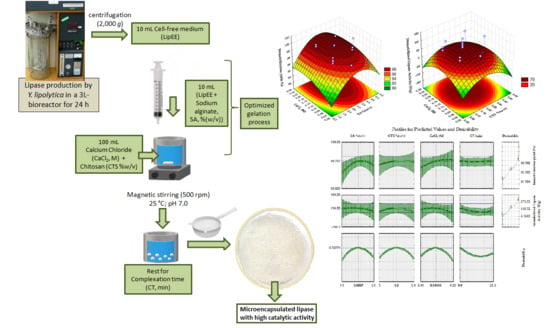
From the presented model the response surface plots were obtained (
Figure 2). The surface plot in
Figure 2 shows that in the whole range tested, intermediate values of SA are necessary to increase IY when intermediate to high values of CTS and CaCl
2 are used (
Figure 2a–c). Considering the complexation time, if intermediate values of SA are used (
Figure 2d), as well as high values of CaCl
2 (
Figure 2e), high IY are reached independent of the CT chosen. However, high CTS is needed to achieve high values of IY and this condition is obtained with high CT.
Optimum conditions for maximum immobilization yield are: SA, 3.7%(w/v); CTS, 0.3%(w/v); CaCl2, 0.17 M and CT, 5.4 min. On these conditions, lipase immobilization yield predicted by the model is 100%.
Sodium alginate concentration is determinant for immobilization yield. At low concentrations (<1%) the viscosity of this polymer is low, which can cause internal mixing of the components during complexation, delaying the formation of a semipermeable surface that reduces retention [
19]. In the present study higher concentrations (>1%) of this polymer, increased retention (IY), however a small reduction in immobilization yield was observed for SA close to 4%(
w/
v) or more, which is associated with viscosity increase. High viscosity makes it difficult to extrude the solution through the syringe needle, preventing uniform formation of microcapsules. Another hypothesis is that when the drops come into contact with the calcium chloride solution, they are influenced by the surface tension difference, forming imperfect microcapsules [
20]. These results corroborate studies by Fontes et al. [
21] when evaluating the variation of alginate concentration in the microencapsulation of penicillin G in alginate and starch matrix.
Chitosan concentration also exerts a strong influence on the retention of materials by the capsules. Chitosan was used as an alternative to improve immobilization yield by changing the composition of the cationic solution. Chitosan interacts with alginate as a cation reacts with an anion. In our study, chitosan was added to the calcium chloride gelling solution and a remarkable increase in immobilization yield was observed, especially when low alginate concentrations were used. The alginate/chitosan matrix reduced the leaching effect of enzymes owing to the physical and ionic interaction between the enzyme and support, compared to that of using alginate alone. This is because the carboxyl groups of alginate and amino and carboxyl groups of chitosan along with their good hydrophilicity and high porosity create a link with enzymes [
22]. According to Peña-Montes et al. [
23] the ionic interactions between the enzyme and the support improves the conformational stability of the immobilized enzyme, thereby providing longer shelf life. However, at chitosan concentrations above 0.3% the gelling solution was very viscous for application, causing agglomeration or aggregation of the surface of the microcapsules during formation. Anjani et al. [
24] encapsulated flavorzyme and found that when 2.0%(
w/
v) alginate and 0.1 M calcium chloride were employed, the immobilization yield was about 18.6%, but when encapsulated in 2.0% alginate and in calcium chloride solution containing chitosan, the immobilization yield increased to 72%, 76% and 84% with 0.1%, 0.2% and 0.3%(
w/
v) of chitosan in the cationic solution, respectively.
Calcium chloride is of great importance because it crosslinks with alginate leading to gelation. However, higher Ca2+ can reduce the intensity of the ion exchange between chitosan and sodium alginate, reducing the binding capacity of chitosan. It was observed that small concentrations of CaCl2 reduce the immobilization yield, which is associated with the small availability of Ca2+ ions necessary to the formation of cohesive interactions with alginate, leading to matrix formation. Another variable with great importance on IY is complexation time. CT corresponds to the time required for the diffusion of calcium ions into the microcapsules to promote the crosslinking with the alginate and consequently the formation of the matrix. In our study, it was verified that 5.4 min would be ideal to obtain the maximum efficiency of lipase encapsulation, and it is observed in very long exposure times the leaching of the encapsulated lipase to the calcium chloride solution.
As it was already pointed out, for enzymatic microencapsulation, immobilization yield can be high, but if the capsules do not allow the substrate to diffuse inside, this new biocatalyst presents low activity with no application. Therefore, the analysis of variance was also performed for immobilized lipase activity (ImLipA), as
Table 4 shows.
The results presented in
Table 4 show that there was no lack of fit (
p > 0.05), which indicates that the model obtained is adequate for the explanation of the process. In Equation (2) the mathematical model is presented with the real variables, which is proposed to represent the hydrolytic activity of the capsules in
p-NFL (immobilized lipase activity, ImLipA) under the studied conditions.
This mathematical model was used to obtain the surface response plots (
Figure 3). The hydrolytic activity of the biocatalyst in
p-NFL is enhanced by intermediate concentrations of CaCl
2 and CTS (
Figure 3a) and at those conditions, SA must be lower (
Figure 3b,c). From
Figure 3d–f it can be seen that an intermediate complexation time reduces ImLipA, in the range studied.
The increase of sodium alginate concentration may cause conformational changes in the entrapped enzyme and/or can limit substrate mass transfer to the microcapsules [
25]. Won et al. [
13] studied the immobilization of lipase from
Candida rugosa and observed a reduction in immobilization yield while increasing alginate concentration. Betigeri and Neau [
16] reported that alginate-enzyme interaction reduced lipase activity in relation to beads formed with chitosan. It is possible that the interactions between both polymers (alginate and chitosan) favor enzyme-support interactions that fix the enzyme in the open form, increasing its activity, as for some immobilization strategies reported by Mateo et al. [
10].
For chitosan, best results for immobilized lipase activity could be obtained with 0.22%(w/v). Despite the use of higher CTS to favor immobilization yield, the increase in the amount of this polymer tends to form a diffusional barrier on the surface of the microcapsule, which creates a resistance to the mass transfer of the substrate into the microcapsules, reducing the reaction between enzyme and substrate, directly affecting the immobilized lipase activity.
As for calcium chloride, an optimum value for the hydrolytic activity of the encapsulated enzyme was found when 0.14 M CaCl2 was used, which was similar to the one found for the immobilization yield (0.17 M). However, the effect of CaCl2 on immobilized lipase activity was small in the range tested (0.05–0.25 M), which may be due to the fact that excess Ca2+ to a certain level does not affect matrix formation in the gelation process.
Although better results were observed for CT close to zero, it is worth noting that the contact time of the microcapsules with the cationic solution should be sufficient for the available junction areas to be crosslinked, promoting effective bonds between the alginate and the calcium ions. Roger et al. [
26], studying natural magnetic films composed of alginate and maghemite nanoparticles, verified that the ion exchange process is fast, evidencing that half of the conversion takes place in the first 5 min, and after 10 min the concentration of calcium in the film remains constant.
As both responses (IY and ImLipA) are important for a good biocatalyst, the desirability function was used to optimize the two responses simultaneously. This function is based on a numerical interval that defines the desirability of the analyst in relation to the optimal condition of the process. The range is 0.0–1.0, where (0.0) means unwanted and (1.0) means desired, and the definitions allow to select the most efficient condition for the process in question [
27].
Figure 4 shows the results for the optimization of the CCRD through desirability function.
Optimum conditions to obtain 99.8% of immobilization yield and 150.7 U/g of immobilized lipase activity are: sodium alginate concentration of 3.1%(w/v), chitosan concentration of 0.2%(w/v), calcium chloride concentration of 0.14 M, and complexation time of 1 min.
A new microencapsulation procedure was performed in those conditions and an IY of 96.7% and ImLipA of 140.0 U/g were obtained for these microcapsules (
Appendix A,
Figure A1). Considering that 309 U of enzyme activity was encapsulated (by the difference of activity in solution—
Appendix A,
Table A2) and that 1.78 g (dry weight) of microspheres was produced, an immobilization efficiency of 80.4% was achieved. This biocatalyst was washed and tested for leakage and no significant amount of protein was found in the washing solution or in the leaking solution (
Appendix A,
Table A2). This biocatalyst presents a higher stability at reaction conditions (37 °C, pH 7.0) than the free enzyme (LipEE), as shown in
Figure 5.
C. antartica lipase B immobilized on green coconut fiber was over 2 times more stable than the free enzyme at 50 °C [
28].
This is the first report in literature of of microencapsulation optimization by gelification of lipase from Y. lipolytica. The results show the importance of this study since an increase in Immobilization yield to almost 100% and an increase up to 280 U/g of lipase activity of the microcapsules were achieved by using the experimental design optimization tool.
The reuse of immobilized enzymes is always taken into account for industrial application in order to reduce costs. Thus, the reuse of lipase immobilized by microencapsulation was performed by checking the relative activity of this enzyme over 5 reaction cycles. The microcapsules used for this study were obtained under optimal conditions.
Figure 6 shows the observed recycling stability.
The results show that immobilized lipase activity decreased 52% as early as the second reaction cycle and remained with only, approximately, 20% of its initial activity after five reaction cycles. One explanation for this is the leakage of the enzyme from the structure of the microcapsules to the reaction medium. Stolarzewicz et al. [
29] reported leakage of cell-bond lipase immobilized in alginate, but in the second cycle only 25% of activity was detected. These microcapsules were tested in a leaking buffer for protein and lipase activity and no protein was detected and just a non-significant activity was determined (
Table A2,
Appendix A). The measurement of the product (
p-nitrophenol) considering a 10 min reaction shows that in the second cycle 100% of product is obtained, which reduces with the successive reactions, but not as much as in activity. This indicates that there is a reduction of reaction rate, which might be due to substrate or product retained in the active site. Despite the considerable reduction of the ImLipA, the microcapsules are an interesting alternative for the immobilization of enzymes, since besides protecting them from the external environment, they also have a great advantage in the recovery process. Comparing to other immobilization techniques, alginate microcapsules are easily recycled by simple filtration, dispensing the need to use centrifugation or vacuum filtration.