Development of Inducible CD19-CAR T Cells with a Tet-On System for Controlled Activity and Enhanced Clinical Safety
Abstract
:1. Introduction
2. Results
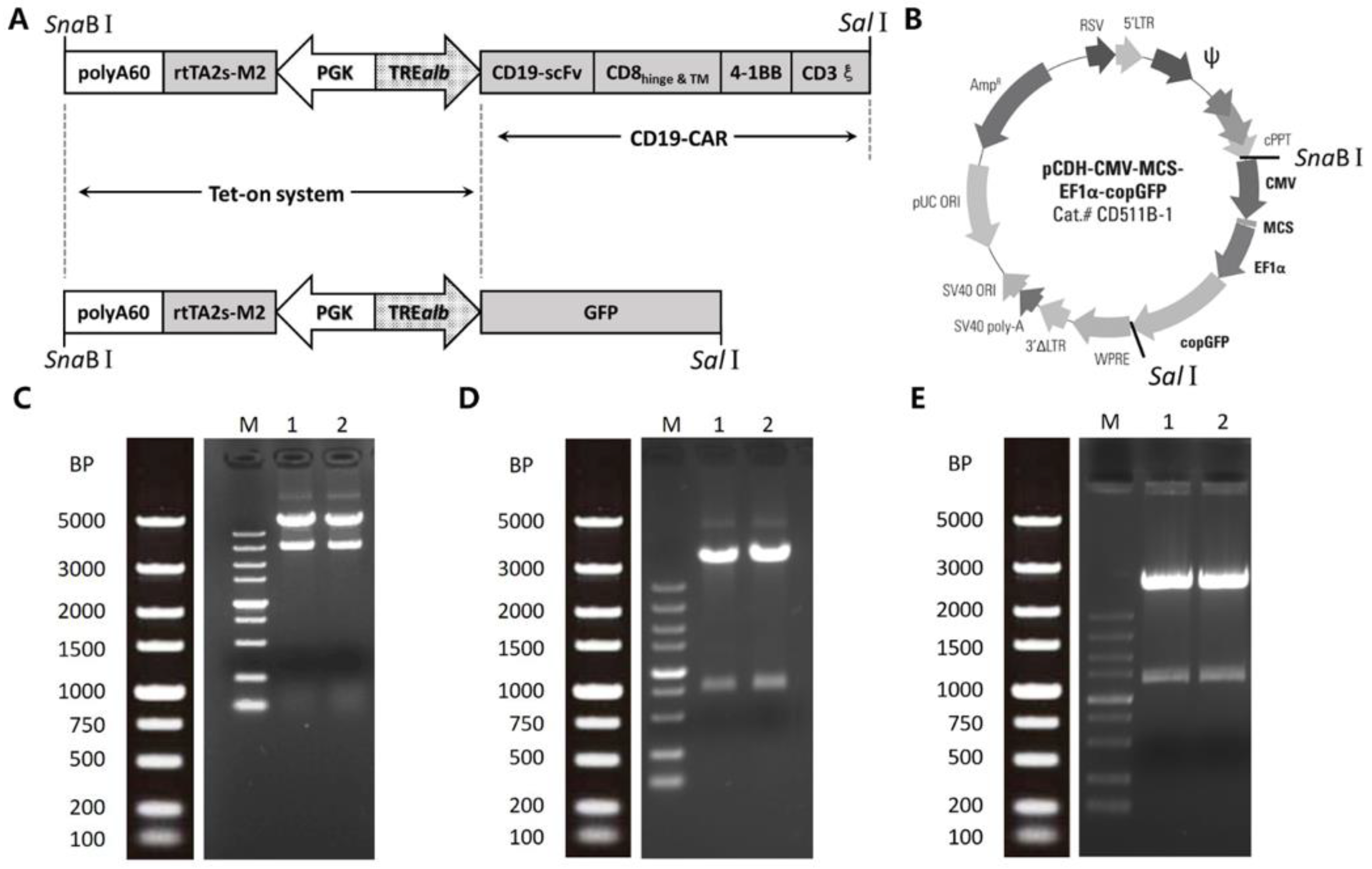
2.1. Construction of Inducible Lentiviral Vectors
2.2. Quality Testing
2.3. Dox-Induced GFP Expression in HEK293T Cells
2.4. Dox-Dependent CAR Expression in CAR T Cells
2.5. Cell Proliferation and Cytokine Secretion
2.6. Cytotoxicity Assays
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Vector Constructs
4.3. Lentiviral Vector Production
4.4. Lentiviral Vector Quality Testing
4.4.1. Microbiological Tests
4.4.2. Titration
4.4.3. Replication-Competent Recombinant Lentivirus
4.4.4. Endotoxin
4.4.5. pH
4.5. HEK293T Cell Transduction
4.6. PBMC Transduction
4.7. Quantitative Real-Time PCR
4.8. Cell Proliferation and Cytokine Secretion Assays
4.9. Cytotoxicity Assays
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| iCAR19 | inducible CD19CAR |
| CAR | chimeric antigen receptor |
| CRS | cytokine release syndrome |
| Dox | doxycycline |
| TRE | tetracycline-responsive element |
| TREalb | Tet-responsive promoter for albumin |
| GMP | Good Manufacturing Practice |
| FBS | fetal bovine serum |
| iGFP | inducible GFP |
| PBMCs | peripheral blood mononuclear cells |
| IL-2 | interleukin-2 |
| IFNγ | interferon-gamma |
| CFSE | carboxyfluorescein succinimidyl ester |
| scFv | single-chain variable fragment |
References
- Maude, S.L.; Teachey, D.T.; Porter, D.L.; Grupp, S.A. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 2015, 125, 4017–4023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makita, S.; Yoshimura, K.; Tobinai, K. Clinical development of anti-CD19 chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma. Cancer Sci. 2017, 108, 1109–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, D.L.; Levine, B.L.; Kalos, M.; Bagg, A.; June, C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011, 365, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Kochenderfer, J.N.; Dudley, M.E.; Feldman, S.A.; Wilson, W.H.; Spaner, D.E.; Maric, I.; Stetler-Stevenson, M.; Phan, G.Q.; Hughes, M.S.; Sherry, R.M.; et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012, 119, 2709–2720. [Google Scholar] [CrossRef] [PubMed]
- Lamers, C.H.; Sleijfer, S.; van Steenbergen, S.; van Elzakker, P.; van Krimpen, B.; Groot, C.; Vulto, A.; den Bakker, M.; Oosterwijk, E.; Debets, R.; et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: Clinical evaluation and management of on-target toxicity. Mol. Ther. 2013, 21, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Ruella, M.; Kenderian, S.S.; Shestova, O.; Klichinsky, M.; Melenhorst, J.J.; Wasik, M.A.; Lacey, S.F.; June, C.H.; Gill, S.I. Kinase Inhibitor Ibrutinib Prevents Cytokine-Release Syndrome after Anti-CD19 Chimeric Antigen Receptor T Cells (CART) for B Cell Neoplasms. Blood 2016, 128, 2159. [Google Scholar]
- Budde, L.E.; Berger, C.; Lin, Y.; Wang, J.; Lin, X.; Frayo, S.E.; Brouns, S.A.; Spencer, D.M.; Till, B.G.; Jensen, M.C.; et al. Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PLoS ONE 2013, 8, e82742. [Google Scholar] [CrossRef] [PubMed]
- Bonini, C.; Ferrari, G.; Verzeletti, S.; Servida, P.; Zappone, E.; Ruggieri, L.; Ponzoni, M.; Rossini, S.; Mavilio, F.; Traversari, C.; et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science 1997, 276, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, K.; Jamil, M.O.; Al-Obaidi, M.; Pereboeva, L.; Salzman, D.; Erba, H.P.; Lamb, L.S.; Bhatia, R.; Mineishi, S.; Di Stasi, A. In Vitro Pre-Clinical Validation of Suicide Gene Modified Anti-CD33 Redirected Chimeric Antigen Receptor T-Cells for Acute Myeloid Leukemia. PLoS ONE 2016, 11, e0166891. [Google Scholar] [CrossRef] [PubMed]
- Lamartina, S.; Roscilli, G.; Rinaudo, C.D.; Sporeno, E.; Silvi, L.; Hillen, W.; Bujard, H.; Cortese, R.; Ciliberto, G.; Toniatti, C. Stringent control of gene expression in vivo by using novel doxycycline-dependent trans-activators. Hum. Gene Ther. 2002, 13, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Zabala, M.; Wang, L.; Hernandez-Alcoceba, R.; Hillen, W.; Qian, C.; Prieto, J.; Kramer, M.G. Optimization of the Tet-on system to regulate interleukin 12 expression in the liver for the treatment of hepatic tumors. Cancer Res. 2004, 64, 2799–2804. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Kramer, M.G.; Fernandez-Ruiz, V.; Kawa, M.P.; Huang, X.; Liu, Z.; Prieto, J.; Qian, C. Development of Endothelial-Specific Single Inducible Lentiviral Vectors for Genetic Engineering of Endothelial Progenitor Cells. Sci. Rep. 2015, 5, 17166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, D.; Xiong, Y.; Wu, D.; Nlle, V.; Schmitz, S.; Haso, W.; Kaiser, A.; Dropulic, B.; Orentas, R.J. A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. J. Immunother. Cancer 2017, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y. Equipping CAR-modified T cells with a brake to prevent chronic adverse effects. Curr. Gene Ther. 2012, 12, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, R.S.; Petukhov, A.V.; Knorre, V.D.; Maschan, M.A.; Stepanov, A.V.; Gabibov, A.G. Molecular Approaches to Safe and Controlled Engineered T-cell Therapy. Acta Naturae 2018, 10, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Drent, E.; Poels, R.; Mulders, M.J.; van de Donk, N.; Themeli, M.; Lokhorst, H.M.; Mutis, T. Feasibility of controlling CD38-CAR T cell activity with a Tet-on inducible CAR design. PLoS ONE 2018, 13, e0197349. [Google Scholar] [CrossRef] [PubMed]
- Sakemura, R.; Terakura, S.; Watanabe, K.; Julamanee, J.; Takagi, E.; Miyao, K.; Koyama, D.; Goto, T.; Hanajiri, R.; Nishida, T.; et al. A Tet-On Inducible System for Controlling CD19-Chimeric Antigen Receptor Expression upon Drug Administration. Cancer Immunol. Res. 2016, 4, 658–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casucci, M.; Bondanza, A. Suicide gene therapy to increase the safety of chimeric antigen receptor-redirected T lymphocytes. J. Cancer 2011, 2, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Knochelmann, H.M.; Smith, A.S.; Dwyer, C.J.; Wyatt, M.M.; Mehrotra, S.; Paulos, C.M. CAR T Cells in Solid Tumors: Blueprints for Building Effective Therapies. Front. Immunol. 2018, 9, 1740. [Google Scholar] [CrossRef] [PubMed]
- Hacein-Bey-Abina, S.; von Kalle, C.; Schmidt, M.; Le Deist, F.; Wulffraat, N.; McIntyre, E.; Radford, I.; Villeval, J.L.; Fraser, C.C.; Cavazzana-Calvo, M.; et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003, 348, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Olsen, P.; Lee, D.H.; Kenoyer, A.L.; Budde, L.E.; O’Steen, S.; Green, D.J.; Heimfeld, S.; Jensen, M.C.; Riddell, S.R.; et al. Preclinical Optimization of a CD20-specific Chimeric Antigen Receptor Vector and Culture Conditions. J. Immunother. 2018, 41, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Tran, R.; Myers, D.R.; Shields, J.E.; Ahn, B.; Qiu, Y.; Hansen, C.; Sakurai, Y.; Moot, R.; Spencer, H.T.; Doering, C.B.; et al. Improving Lentiviral Transduction Efficiency with Microfluidic Systems. Blood 2015, 126, 4415. [Google Scholar]
- Gonzalez-Zorn, B.; Escudero, J.A. Ecology of antimicrobial resistance: Humans, animals, food and environment. Int. Microbiol. 2012, 15, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Breger, L.; Wettergren, E.E.; Quintino, L.; Lundberg, C. Regulated Gene Therapy. In Gene Therapy for Neurological Disorders; Manfredsson, F.P., Ed.; Springer: New York, NY, USA, 2016; Volume 1382, p. 60. ISBN 978-1-4939-3271-9. [Google Scholar]
- June, C.H. Remote Controlled CARs: Towards a Safer Therapy for Leukemia. Cancer Immunol. Res. 2016, 4, 643. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Flowers, M.E.; Warren, E.H.; Riddell, S.R. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood 2006, 107, 2294–2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginhoux, F.; Turbant, S.; Gross, D.A.; Poupiot, J.; Marais, T.; Lone, Y.; Lemonnier, F.A.; Firat, H.; Perez, N.; Danos, O.; et al. HLA-A*0201-restricted cytolytic responses to the rtTA transactivator dominant and cryptic epitopes compromise transgene expression induced by the tetracycline on system. Mol. Ther. 2004, 10, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Sternberg, M.R.; Kottiri, B.J.; McQuillan, G.M.; Lee, F.K.; Nahmias, A.J.; Berman, S.M.; Markowitz, L.E. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 2006, 296, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Stieger, K.; Belbellaa, B.; Le Guiner, C.; Moullier, P.; Rolling, F. In vivo gene regulation using tetracycline-regulatable systems. Adv. Drug Deliv. Rev. 2009, 61, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chang, Q.A.; Virag, T.; West, N.C.; George, D.; Castro, M.G.; Bohn, M.C. Lack of humoral immune response to the tetracycline (Tet) activator in rats injected intracranially with Tet-off rAAV vectors. Gene Ther. 2010, 17, 616–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lhériteau, E.; Libeau, L.; Mendes-Madeira, A.; Deschamps, J.Y.; Weber, M.; Le Meur, G.; Provost, N.; Guihal, C.; Moullier, P.; Rolling, F. Regulation of retinal function but nonrescue of vision in RPE65-deficient dogs treated with doxycycline-regulatable AAV vectors. Mol. Ther. 2010, 18, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Lizee, G.; Aerts, J.L.; Gonzales, M.I.; Chinnasamy, N.; Morgan, R.A.; Topalian, S.L. Real-time quantitative reverse transcriptase-polymerase chain reaction as a method for determining lentiviral vector titers and measuring transgene expression. Hum. Gene Ther. 2003, 14, 497–507. [Google Scholar] [CrossRef] [PubMed]




| Test | Method | Requirement | Report |
|---|---|---|---|
| Bacteria | Blood agar plates | No growth | No growth |
| Fungi | Sabouraud glucose agar plates | No growth | No growth |
| Mycoplasma | PCR detection kit | Negative | Negative |
| Titration | Real-time PCR | Report TU/mL | 1.5 × 107 TU/mL |
| RCR test | p24 ELISA kit | Negative | Negative |
| Endotoxin | Chromogenic LAL assay kit | <10 EU/mL | <0.03125 EU/mL |
| pH | pH meter | 6.9–7.8 | 7.25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, X.; He, D.; Li, C.; Wang, H.; Yang, G. Development of Inducible CD19-CAR T Cells with a Tet-On System for Controlled Activity and Enhanced Clinical Safety. Int. J. Mol. Sci. 2018, 19, 3455. https://doi.org/10.3390/ijms19113455
Gu X, He D, Li C, Wang H, Yang G. Development of Inducible CD19-CAR T Cells with a Tet-On System for Controlled Activity and Enhanced Clinical Safety. International Journal of Molecular Sciences. 2018; 19(11):3455. https://doi.org/10.3390/ijms19113455
Chicago/Turabian StyleGu, Xingjian, Dongyang He, Caixin Li, Hua Wang, and Guanghua Yang. 2018. "Development of Inducible CD19-CAR T Cells with a Tet-On System for Controlled Activity and Enhanced Clinical Safety" International Journal of Molecular Sciences 19, no. 11: 3455. https://doi.org/10.3390/ijms19113455
APA StyleGu, X., He, D., Li, C., Wang, H., & Yang, G. (2018). Development of Inducible CD19-CAR T Cells with a Tet-On System for Controlled Activity and Enhanced Clinical Safety. International Journal of Molecular Sciences, 19(11), 3455. https://doi.org/10.3390/ijms19113455





