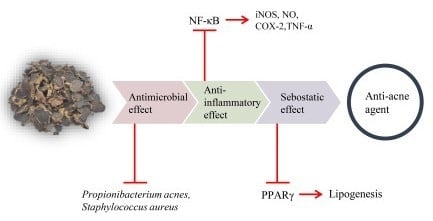
Kaempferia parviflora Extract as a Potential Anti-Acne Agent with Anti-Inflammatory, Sebostatic and Anti-Propionibacterium acnes Activity
Abstract
:1. Introduction
2. Results
2.1. Anti-P. acnes Activity of K. parviflora Extract
2.2. Anti-Inflammatory Effect of K. parviflora Extract
2.3. Sebostatic Effect of K. parviflora Extracts
3. Discussion
3.1. K. parviflora Extract Inhibits the Growth of Skin Bacteria
3.2. K. parviflora Extract Inhibits Inflammatory Responses
3.3. The Anti-Lipogenesis Effect of K. parviflora Extract in Sebocytes
4. Materials and Methods
4.1. Preparation of K. parviflora Extracts in Sebocytes
4.2. Microbial Cultivation
4.3. Bacterial Inactivation by K. parviflora Extract
4.4. Cell Culture
4.5. Nitrite Determination
4.6. Cytokine Measurement
4.7. Western Blotting Analysis
4.8. Confocal Microscope Analysis
4.9. Sebocyte Culture and Triglyceride Assay
4.10. Oil Red O Staining
4.11. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bojar, R.A.; Holland, K.T. Acne and Propionibacterium acnes. Clin. Dermatol. 2004, 22, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.D.; Ingham, E. Acne: Inflammation. Clin. Dermatol. 2004, 22, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Eady, A.; Philpott, M.; Goldsmith, L.A.; Orfanos, C.; Cunliffe, W.C.; Rosenfield, R. What is the pathogenesis of acne? Exp. Dermatol. 2005, 14, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Pawin, H.; Beylot, C.; Chivot, M.; Faure, M.; Poli, F.; Revuz, J.; Dréno, B. Physiopathology of acne vulgaris: Recent data, new understanding of the treatments. Eur. J. Dermatol. 2004, 14, 4–12. [Google Scholar] [PubMed]
- Downing, D.T.; Stewart, M.E.; Wertz, P.W.; Strauss, J.S. Essential fatty acids and acne. J. Am. Acad. Dermatol. 1986, 14, 221–225. [Google Scholar] [CrossRef]
- Bergfeld, W.F. The pathophysiology of acne vulgaris in children and adolescents, Part 1. Cutis 2004, 74, 92–97. [Google Scholar] [PubMed]
- Makrantonaki, E.; Ganceviciene, R.; Zouboulis, C. An update on the role of the sebaceous gland in the pathogenesis of acne. Dermato-Endocrinology 2011, 3, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasri, H.; Bahmani, M.; Shahinfard, N.; Nafchi, A.M.; Saberianpour, S.; Kopaei, M.R. Medicinal plants for the treatment of acne vulgaris: A review of recent evidences. Jundishapur J. Microbiol. 2015, 8, e25580. [Google Scholar] [CrossRef] [PubMed]
- Pitakpawasutthi, Y.; Palanuvej, C.; Ruangrungsi, N. Quality evaluation of Kaempferia parviflora rhizome with reference to 5,7-dimethoxyflavone. J. Adv. Pharm. Technol. Res. 2018, 9, 26–31. [Google Scholar] [PubMed]
- Mekjaruskul, C.; Jay, M.; Sripanidkulchai, B. Pharmacokinetics, bioavailability, tissue distribution, excretion, and metabolite identification of methoxyflavones in Kaempferia parviflora extract in rats. Drug Metab. Dispos. 2012, 40, 2342–2353. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Hitoe, S.; Takeda, S.; Shimoda, H. Black ginger extract increases physical fitness performance and muscular endurance by improving inflammation and energy metabolism. Heliyon 2016, 2, e00115. [Google Scholar] [CrossRef] [PubMed]
- Potikanond, S.; Sookkhee, S.; Na Takuathung, M.; Mungkornasawakul, P.; Wikan, N.; Smith, D.R.; Nimlamool, W. Kaempferia parviflora extract exhibits anti-cancer activity against HeLa cervical cancer cells. Front. Pharmacol. 2017, 8, 630. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baviera, G.; Leoni, M.C.; Capra, L.; Cipriani, F.; Longo, G.; Maiello, N.; Ricci, G.; Galli, E. Microbiota in healthy skin and in atopic eczema. BioMed Res. Int. 2014, 2014, 436921. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, K.; Sato, T.; Akimoto, N.; Noguchi, N.; Sasatsu, M.; Nishijima, S.; Kurokawa, I.; Ito, A. Involvement of Propionibacterium acnes in the augmentation of lipogenesis in hamster sebaceous glands in vivo and in vitro. J. Investig. Dermatol. 2009, 129, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Han, H.M.; Song, P.I.; Armstrong, C.A.; Park, Y. Suppression of Propionibacterium acnes infection and the associated inflammatory response by the antimicrobial peptide P5 in mice. PLoS ONE 2015, 10, e0132619. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C. Propionibacterium acnes and sebaceous lipogenesis: A love-hate relationship? J. Investig. Dermatol. 2009, 129, 2093–2096. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, Y.; Aisa, H.A. Anti-inflammatory effect of pomegranate flower in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages. Pharm. Biol. 2017, 55, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.M.; Lee, H.S.; Jeong, D.; Oh, H.K.; Ra, K.H.; Lee, M.Y. Antimicrobial photodynamic therapy using chlorin e6 with halogen light for acne bacteria-induced inflammation. Life Sci. 2015, 124, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, K.H.; Kwag, E.H.; Seol, Y.J.; Lee, Y.M.; Ku, Y.; Rhyuays, I.C. Magnoliae Cortex and maize modulate Porphyromonas gingivalis-induced inflammatory reactions. J. Period. Implant Sci. 2018, 48, 70–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uto, T.; Suangkaew, N.; Morinaga, O.; Kariyazono, H.; Oiso, S.; Shoyama, Y. Eriobotryae folium extract suppresses LPS-induced iNOS and COX-2 expression by inhibition of NF-κB and MAPK activation in murine macrophages. Am. J. Chin. Med. 2010, 38, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Song, H.Y.; Ju, S.M.; Lee, S.J.; Kwon, H.J.; Eum, W.S.; Jang, S.H.; Choi, S.Y.; Park, J.S. Differential regulation of inducible nitric oxide synthase and cyclooxygenase-2 expression by superoxide dismutase in lipopolysaccharide stimulated RAW 264.7 cells. Exp. Mol. Med. 2009, 41, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.E.; Kim, O.S.; Yoo, S.R.; Seo, C.S.; Kim, Y.; Shin, H.K.; Jeong, S.J. Anti-inflammatory effect and action mechanisms of traditional herbal formula Gamisoyo-san in RAW 264.7 macrophages. BMC Complement. Altern. Med. 2016, 16, 219. [Google Scholar] [CrossRef] [PubMed]
- Needleman, P.; Manning, P.T. Interactions between the inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) pathways: Implications for therapeutic intervention in osteoarthritis. Osteoarthr. Cartil. 1999, 7, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Jourdan, E.; Picardo, M. Acne is an inflammatory disease and alterations of sebum composition initiate acne lesions. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Lovászi, M.; Szegedi, A.; Zouboulis, C.C.; Törőcsik, D. Sebaceous-immunobiology is orchestrated by sebum lipids. Dermato-Endocrinology 2017, 9, e1375636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahlhoff, M.; Camera, E.; Ludovici, M.; Picardo, M.; Müller, U.; Leonhardt, H.; Zouboulis, C.C.; Schneider, M.R. EGFR/ERBB receptors differentially modulate sebaceous lipogenesis. FEBS Lett. 2015, 589, 1376–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picardo, M.; Ottaviani, M.; Camera, E.; Mastrofrancesco, A. Sebaceous gland lipids. Dermato-Endocrinology 2009, 1, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determination by the use of a stable free radical. Nature 1958, 181, 1190–1200. [Google Scholar] [CrossRef]
- Leelarungrayub, N.; Rattanapanone, V.; Chanarat, N.; Gebicki, J.M. Quantitative evaluation of the antioxidant properties of garlic and shallot preparations. Nutrition 2006, 22, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Shangguan, Z.S.; Chen, C.; Zhang, H.J.; Lin, Y. Anti-inflammatory effects of guggulsterone on murine macrophage by inhibiting LPS-induced inflammatory cytokines in NF-κB signaling pathway. Drug Des. Dev. Ther. 2016, 10, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S.; Rawat, R. Peroxisome proliferator-activated receptors (PPARs) and PPAR agonists: The ‘future’ in dermatology therapeutics? Arch. Dermatol. Res. 2015, 307, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Hur, H.J.; Lee, K.W.; Lee, H.J. Anti-inflammatory effects of recombinant arginine deiminase originating from Lactococcus lactis ssp. lactis ATCC 7962. J. Microbiol. Biotechnol. 2007, 17, 1491–1497. [Google Scholar] [PubMed]
- Masure, J.; Bonnel, D.; Stauber, J.; Hunt, D.; Hofland, H.E. 528 DRM01, a novel, topical sebum inhibitor for the treatment of acne. J. Investig. Dermatol. 2016, 136, S93. [Google Scholar] [CrossRef]
- Jin, S.; Lee, M.Y. The ameliorative effect of hemp seed hexane extracts on the Propionibacterium acnes-induced inflammation and lipogenesis in sebocytes. PLoS ONE 2018, 13, e0202933. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.M.; Cha, J.D.; Jang, E.J.; Kim, G.U.; Lee, K.Y. Sophoraflavanone G prevents Streptococcus mutans surface antigen I/II-induced production of NO and PGE2 by inhibiting MAPK-mediated pathways in RAW 264.7 macrophages. Arch. Oral Biol. 2016, 68, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Lee, K.G.; Yeo, J.H.; Baek, H.J.; Park, K.K. Antibacterial and anti-inflammatory effects of honeybee (Apis mellifera) venom against acne-inducing bacteria. J. Med. Plants Res. 2010, 4, 459–464. [Google Scholar]
- Wang, Y.Y.; Ryu, A.R.; Jin, S.; Jeon, Y.M.; Lee, M.Y. Chlorin e6-mediated photodynamic therapy suppresses P. acnes-induced inflammatory response via NF-κB and MAPKs signaling pathway. PLoS ONE 2017, 12, e0170599. [Google Scholar]
- Oh, J.E.; Kim, R.H.; Shin, K.H.; Park, N.H.; Kang, M.K. ΔNp63α protein triggers epithelial-mesenchymal transition and confers stem cell properties in normal human keratinocytes. J. Biol. Chem. 2011, 286, 38757–38767. [Google Scholar] [CrossRef] [PubMed]
- Sae-Wong, C.; Matsuda, H.; Tewtrakul, S.; Tansakul, P.; Nakamura, S.; Nomura, Y.; Yoshikawa, M. Suppressive effects of methoxyflavonoids isolated from Kaempferia parviflora on inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells. J. Ethnopharmacol. 2011, 136, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Park, H.R.; Kim, H.; Choi, Y.; Jun, Y.-H.; Park, C.-S.; Ahn, H.-J.; Cho, J.-J.; Park, Y.S. Effect of crotonaldehyde on the induction of COX-2 expression in human endothelial cells. Mol. Cell Toxicol. 2017, 13, 345–350. [Google Scholar] [CrossRef]
- Benetti, E.; Mastrocola, R.; Rogazzo, M.; Chiazza, F.; Aragno, M.; Fantozzi, R.; Collino, M.; Minetto, M.A. High sugar intake and development of skeletal muscle insulin resistance and inflammation in mice: A protective role for PPAR-δ agonism. Mediat. Inflamm. 2013, 2013, 509502. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Li, Y.; Kuang, Y.; Cui, H.; Yang, Y.; Sun, W.; Liu, K.; Chen, D.; Yan, Q.; Wen, L. PKCδ silencing alleviates saturated fatty acid induced ER stress by enhancing SERCA activity. Biosci. Rep. 2017, 37, BSR20170869. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Lee, M.-Y. Kaempferia parviflora Extract as a Potential Anti-Acne Agent with Anti-Inflammatory, Sebostatic and Anti-Propionibacterium acnes Activity. Int. J. Mol. Sci. 2018, 19, 3457. https://doi.org/10.3390/ijms19113457
Jin S, Lee M-Y. Kaempferia parviflora Extract as a Potential Anti-Acne Agent with Anti-Inflammatory, Sebostatic and Anti-Propionibacterium acnes Activity. International Journal of Molecular Sciences. 2018; 19(11):3457. https://doi.org/10.3390/ijms19113457
Chicago/Turabian StyleJin, Solee, and Mi-Young Lee. 2018. "Kaempferia parviflora Extract as a Potential Anti-Acne Agent with Anti-Inflammatory, Sebostatic and Anti-Propionibacterium acnes Activity" International Journal of Molecular Sciences 19, no. 11: 3457. https://doi.org/10.3390/ijms19113457
APA StyleJin, S., & Lee, M.-Y. (2018). Kaempferia parviflora Extract as a Potential Anti-Acne Agent with Anti-Inflammatory, Sebostatic and Anti-Propionibacterium acnes Activity. International Journal of Molecular Sciences, 19(11), 3457. https://doi.org/10.3390/ijms19113457