Impact of Machine Perfusion on Biliary Complications after Liver Transplantation
Abstract
:1. Introduction
2. Overview of Biliary Injury and Underlying Mechanism in the Setting of Liver Transplantation
3. Risk Factors for Development of Biliary Complications after Liver Transplantation
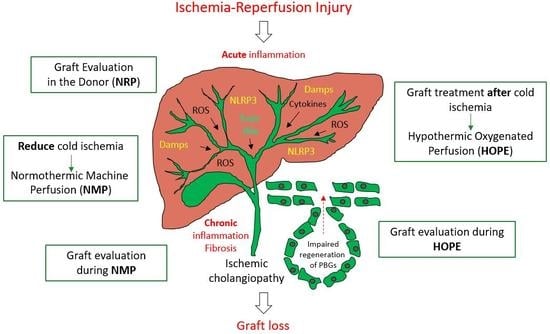
4. General Strategies of Biliary Tree Protection
5. Machine Perfusion
6. Suggested Decisive Mechanisms of Machine Liver Perfusion Techniques Against Biliary Injury
7. Prediction of Biliary Complications in Liver Transplantation
8. Conclusions and Future Perspective
Author Contributions
Conflicts of Interest
Abbreviations
| ABO | A and B represent cellular antigens, which initiate production of antithetical circulating antibodies in plasma |
| ADP | Adenosine diphosphate |
| ATP | Adenosine triphosphate |
| AS | Anastomotic strictures |
| AST | Aspartate-Aminotransferase |
| CCR5-Δ 32 | C-C-Motiv-Chemokin-Receptor 5 Δ 32 |
| DAMP’s | Danger associated molecular pattern’s |
| DBD | Donation after brain death |
| DCD | Donation after circulatory death |
| D-HOPE | Dual Hypothermic oxygenated perfusion |
| DWIT | Donor warm ischemia time |
| EAD | Early allograft dysfunction |
| ECD | Extended Criteria Donor |
| ECMO | Extracorporeal membrane oxygenation |
| HA | Hepatic artery |
| HAT | Hepatic artery thrombosis |
| HMP | Hypothermic machine perfusion |
| HOPE | Hypothermic oxygenated perfusion |
| IC | Ischemic cholangiopathy |
| KC’s | Kupffer cells |
| MELD | Model of end stage liver disease |
| MPT pore | Mitochondria permeability transition pore |
| NAS | Non-anastomotic stenosis |
| NRP | Normothermic regional perfusion |
| NMP | Normothermic machine perfusion |
| PNF | Primary non function |
| PV | Portal vein |
| ROS | Reactive oxygen species |
| SEC | Sinusoidal endothelial cells |
| TLR-4 | Toll-like-receptor-4 |
References
- Dubbeld, J.; Hoekstra, H.; Farid, W.; Ringers, J.; Porte, R.J.; Metselaar, H.J.; Baranski, A.G.; Kazemier, G.; van den Berg, A.P.; van Hoek, B. Similar liver transplantation survival with selected cardiac death donors and brain death donors. Br. J. Surg. 2010, 97, 744–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemes, B.; Gaman, G.; Polak, W.G.; Gelley, F.; Hara, T.; Ono, S.; Baimakhanov, Z.; Piros, L.; Eguchi, S. Extended criteria donors in liver transplantation Part I: Reviewing the impact of determining factors. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Kalisvaart, M.; Scalera, I.; Laing, R.; Mergental, H.; Mirza, D.; Perera, T.; Isaac, J.; Dutkowski, P.; Muiesan, P. The UK DCD Risk Score: A new proposal to define futility in donation-after-circulatory-death liver transplantation. J. Hepatol. 2018, 68, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.A.; Kalisvaart, M.; Muiesan, P. Machine perfusion in liver transplantation: An essential treatment or just an expensive toy? Minerva. Anestesiol. 2018, 84, 236–245. [Google Scholar] [PubMed]
- Karimian, N.; Westerkamp, A.C.; Porte, R.J. Biliary complications after orthotopic liver transplantation. Current Opinion in Organ Transplantation. Curr. Opin. Organ. Transplant. 2014, 19, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Urdazpal, L.; Gores, G.J.; Ward, E.M.; Maus, T.P.; Wahlstrom, H.E.; Moore, S.B.; Wiesner, R.H.; Krom, R.A. Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology 1992, 16, 49–53. [Google Scholar] [CrossRef] [PubMed]
- DeOliveira, M.L.; Jassem, W.; Valente, R.; Khorsandi, S.E.; Santori, G.; Prachalias, A.; Srinivasan, P.; Rela, M.; Heaton, N. Biliary complications after liver transplantation using grafts from donors after cardiac death: Results from a matched control study in a single large volume center. Ann. Surg. 2011, 254, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Op Den Dries, S.; Westerkamp, A.C.; Karimian, N.; Gouw, A.S.H.; Bruinsma, B.G.; Markmann, J.F.; Lisman, T.; Yeh, H.; Uygun, K.; Martins, P.N.; et al. Injury to peribiliary glands and vascular plexus before liver transplantation predicts formation of non-anastomotic biliary strictures. J. Hepatol. 2014, 60, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Op Den Dries, S.; Sutton, M.E.; Lisman, T.; Porte, R.J. Protection of bile ducts in liver transplantation: Looking beyond ischemia. Transplantation 2011, 92, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; Hollemann, D.; Pitton, M.B.; Heise, M.; Hoppe-Lotichius, M.; Schuchmann, M.; Kirkpatrick, C.J.; Otto, G. Histological examination and evaluation of donor bile ducts received during orthotopic liver transplantation-a morphological clue to ischemic-type biliary lesion? Virchows Arch. 2012, 461, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Karimian, N.; Op Den Dries, S.; Porte, R.J. The origin of biliary strictures after liver transplantation: Is it the amount of epithelial injury or insufficient regeneration that counts? J. Hepatol. 2013, 58, 1065–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Urdazpal, L.; Batts, K.P.; Gores, G.J.; Moore, S.B.; Sterioff, S.; Wiesner, R.H.; Krom, R.A. Increased bile duct complications in liver transplantation across the ABO barrier. Ann. Surg. 1993, 218, 152–158. [Google Scholar] [CrossRef] [PubMed]
- op den Dries, S.; Buis, C.I.; Adelmeijer, J.; Van der Jagt, E.J.; Haagsma, E.B.; Lisman, T.; Porte, R.J. The combination of primary sclerosing cholangitis and CCR5-Δ32 in recipients is strongly associated with the development of nonanastomotic biliary strictures after liver transplantation. Liver Int. 2011, 31, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Yska, M.J.; Buis, C.I.; Monbaliu, D.; Schuurs, T.A.; Gouw, A.S.H.; Kahmann, O.N.H.; Visser, D.S.; Pirenne, J.; Porte, R.J. The role of bile salt toxicity in the pathogenesis of bile duct injury after non-heart-beating porcine liver transplantation. Transplantation 2008, 85, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Beuers, U.; Hohenester, S.; de Buy Wenniger, L.J.M.; Kremer, A.E.; Jansen, P.L.M.; Elferink, R.P.J.O. The biliary HCO(3)(−) umbrella: A unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology 2010, 52, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Muller, X.; Marcon, F.; Sapisochin, G.; Marquez, M.; Dondero, F.; Rayar, M.; Doyle, M.M.B.; Callans, L.; Li, J.; Nowak, G.; et al. Defining Benchmarks in Liver Transplantation: A Multicenter Outcome Analysis Determining Best Achievable Results. Ann. Surg. 2017, 267, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.S.; Karp, S.J.; McCauley, M.E.; Markmann, J.F.; Croome, K.P.; Taner, C.B.; Heimbach, J.K.; Leise, M.D.; Fryer, J.P.; Bohorquez, H.E.; et al. Interpreting Outcomes in DCDD Liver Transplantation: First Report of the Multicenter IDOL Consortium. Transplantation 2017, 101, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Stahl, J.E.; Kreke, J.E.; Malek, F.A.; Schaefer, A.J.; Vacanti, J. Consequences of cold-ischemia time on primary nonfunction and patient and graft survival in liver transplantation: A meta-analysis. PLoS ONE 2008, 3, e2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcon, F.; Schlegel, A.; Bartlett, D.; Bishop, D.; Mergental, H.; Roberts, K.; Roberts, K.; Mirza, D.; Isaac, J.; Muiesan, P.; et al. Utilisation of declined liver grafts yields comparable transplant outcomes and previous decline should not be a deterrent to graft use. Transplantation 2018. [Google Scholar] [CrossRef] [PubMed]
- Croome, K.P.; Lee, D.D.; Perry, D.K.; Burns, J.M.; Nguyen, J.H.; Keaveny, A.P.; Taner, C.B. Comparison of longterm outcomes and quality of life in recipients of donation after cardiac death liver grafts with a propensity-matched cohort. Liver Transplant. 2017, 23, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Foley, D.P.; Fernandez, L.A.; Leverson, G.; Anderson, M.; Mezrich, J.; Sollinger, H.W.; D’Alessandro, A. Biliary complications after liver transplantation from donation after cardiac death donors: An analysis of risk factors and long-term outcomes from a single center. Ann. Surg. 2011, 253, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Calzadilla, L.B.; Adams, L. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016, 17, 774. [Google Scholar] [CrossRef] [PubMed]
- Dasari, B.V.M.; Schlegel, A.; Mergental, H.; Perera, M.T.P.R. The use of old donors in liver transplantation. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Goodrich, N.P.; Bragg-Gresham, J.L.; Dykstra, D.M.; Punch, J.D.; DebRoy, M.A.; Greenstein, S.M.; Merion, R.M. Characteristics associated with liver graft failure: The concept of a donor risk index. Am. J. Transplant. 2006, 6, 783–790. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J. Hepatol. 2016, 64, 433–485. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Muller, X.; Dutkowski, P. Hypothermic Liver Perfusion. Curr. Opin. Organ. Transplant. 2017, 22, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Giorgakis, E.; Khorsandi, S.; Jassem, W.; Heaton, N. Minimization of Ischemic Cholangiopathy in Donation After Cardiac Death Liver Transplantation: Is It Thrombolytic Therapy or Warm Ischemic Time Stringency and Donor Bile Duct Flush? Am. J. Transplant. 2017, 18, 274–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorsandi, S.; Giorgakis, E.; Vilca-Melendez, H.; O’Grady, J.; Heneghan, M.; Aluvihare, V.; Suddle, A.; Agarwal, K.; Menon, K.; Prachalias, A.; et al. Developing a donation after cardiac death risk index for adult and pediatric liver transplantation. World J. Transplant. 2017, 7, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Moench, C.; Moench, K.; Lohse, A.W.; Thies, J.; Otto, G. Prevention of ischemic-type biliary lesions by arterial back-table pressure perfusion. Liver Transplant. 2003, 9, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Scalera, I.; Perera, M.; Kalisvaart, M.; Mergental, H.; Mirza, D.; John, I.; Paolo, M. Impact of donor age in donation after cardiac death liver transplantation: Is the cut-off “60” still of relevance? Liver Transplant. 2017. [Google Scholar] [CrossRef]
- Manzini, G.; Kremer, M.; Houben, P.; Gondan, M.; Bechstein, W.O.; Becker, T.; Berlakovich, G.A.; Friess, H.; Guba, M.; Hohenberger, W.; et al. Reperfusion of liver graft during transplantation: Techniques used in transplant centres within Eurotransplant and meta-analysis of the literature. Transplant. Int. 2013, 26, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Orci, L.A.; Lacotte, S.; Delaune, V.; Slits, F.; Oldani, G.; Lazarevic, V.; Rossetti, C.; Rubbia-Brandt, L.; Morel, P.; Toso, C. Effects of the gut–liver axis on ischaemia-mediated hepatocellular carcinoma recurrence in the mouse liver. J. Hepatol. 2018, 68, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Oniscu, G.C.; Randle, L.V.; Muiesan, P.; Butler, A.J.; Currie, I.S.; Perera, M.T.P.R.; Forsythe, J.L.; Watson, C.J. In situ normothermic regional perfusion for controlled donation after circulatory death—The United Kingdom experience. Am. J. Transplant. 2014, 14, 2846–2854. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, J.; Reddy, S.; Coussios, C.; Pigott, D.; Guirriero, D.; Hughes, D.; Morovat, A.; Roy, D.; Winter, L.; Friend, P.J. Normothermic perfusion: A new paradigm for organ preservation. Ann. Surg. 2009, 250, 1–6. [Google Scholar] [CrossRef] [PubMed]
- op den Dries, S.; Karimian, N.; Westerkamp, A.C.; Sutton, M.E.; Kuipers, M.; Wiersema-Buist, J.; Ottens, P.J.; Kuipers, J.; Giepmans, B.N.; Leuvenink, H.G.; et al. Normothermic machine perfusion reduces bile duct injury and improves biliary epithelial function in rat donor livers. Liver Transplant. 2016, 22, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Nassar, A.; Farias, K.; Buccini, L.; Baldwin, W.; Mangino, M.; Bennett, A.; O’Rourke, C.; Okamoto, T.; Uso, T.D.; et al. Sanguineous normothermic machine perfusion improves hemodynamics and biliary epithelial regeneration in donation after cardiac death porcine livers. Liver Transplant. 2014, 20, 987–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; García-Valdecasas, J.C.; Heaton, N.; et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Bral, M.; Gala-Lopez, B.; Bigam, D.; Kneteman, N.; Malcolm, A.; Livingstone, S.; Andres, A.; Emamaullee, J.; Russell, L.; Coussios, C.; et al. Preliminary Single-Center Canadian Experience of Human Normothermic Ex Vivo Liver Perfusion: Results of a Clinical Trial. Am. J. Transplant. 2017, 17, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Goldaracena, N.; Echeverri, J.; Spetzler, V.N.; Kaths, J.M.; Barbas, A.S.; Louis, K.S.; Adeyi, O.A.; Grant, D.R.; Selzner, N.; Selzner, M. Anti-inflammatory signaling during ex vivo liver perfusion improves the preservation of pig liver grafts before transplantation. Liver Transplant. 2016, 22, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Spetzler, V.N.; Goldaracena, N.; Echiverri, J.; Kaths, J.M.; Louis, K.S.; Adeyi, O.A.; Yip, P.M.; Grant, D.R.; Selzner, N.; Selzner, M. Subnormothermic ex vivo liver perfusion is a safe alternative to cold static storage for preserving standard criteria grafts. Liver Transplant. 2016, 22, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Fontes, P.; Lopez, R.; Van Der Plaats, A.; Vodovotz, Y.; Minervini, M.; Scott, V.; Soltys, K.; Shiva, S.; Paranjpe, S.; Sadowsky, D.; et al. Liver preservation with machine perfusion and a newly developed cell-free oxygen carrier solution under subnormothermic conditions. Am. J. Transplant. 2015, 15, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Knaak, J.M.; Spetzler, V.N.; Goldaracena, N.; Louis, K.S.; Selzner, N.; Selzner, M. Technique of subnormothermic ex vivo liver perfusion for the storage, assessment, and repair of marginal liver grafts. J. Vis. Exp. 2014, 13, e51419. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Graf, R.; Clavien, P.A.; Dutkowski, P. Hypothermic oxygenated perfusion (HOPE) protects from biliary injury in a rodent model of DCD liver transplantation. J. Hepatol. 2013, 59, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Banan, B.; Xiao, Z.; Watson, R.; Xu, M.; Jia, J.; Upadhya, G.A.; Mohanakumar, T.; Lin, Y.; Chapman, W. Novel strategy to decrease reperfusion injuries and improve function of cold-preserved livers using normothermic ex vivo liver perfusion machine. Liver Transplant. 2016, 22, 333–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerkamp, A.C.; Mahboub, P.; Meyer, S.L.; Hottenrott, M.; Ottens, P.J.; Wiersema-Buist, J.; Gouw, A.S.; Lisman, T.; Leuvenink, H.G.; Porte, R.J. End-ischemic machine perfusion reduces bile duct injury in donation after circulatory death rat donor livers independent of the machine perfusion temperature. Liver Transplant. 2015, 21, 1300–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Op Den Dries, S.; Sutton, M.E.; Karimian, N.; De Boer, M.T.; Wiersema-Buist, J.; Gouw, A.S.H.; Leuvenink, H.G.; Lisman, T.; Porte, R.J. Hypothermic oxygenated machine perfusion prevents arteriolonecrosis of the peribiliary plexus in pig livers donated after circulatory death. PLoS ONE 2014, 9, e88521. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.; Hunt, F.; Butler, A.; Sutherland, A.; Upponi, S.; Currie, I.; Large, S.; Terrace, J.; Messer, S.; Oniscu, G.; et al. Normothermic regional perfusion (NRP) for DCD liver transplantation in the UK: Better graft survival with no cholangiopathy. In Proceedings of the 2018 Joint International Congress of ILTS, ELITA & LICAGE, Lisbon, Portugal, 23–26 May 2018. [Google Scholar]
- Hessheimer, A.; Coll, E.; Valdivieso, A.; Gómez, M.; Santoyo, J.; Ramírez, P.; Gómez-Bravo, M.Á.; López-Andujar, R.; Villar, J.; Jiménez, C.; et al. Superior outcomes using normothermic regional perfusion in cDCD liver transplantation. In Proceedings of the 2018 Joint International Congress of ILTS, ELITA & LICAGE, Lisbon, Portugal, 23–26 May 2018. [Google Scholar]
- Watson, C.; Kosmoliaptsis, V.; Pley, C.; Randle, L.; Fear, C.; Crick, K.; Gimson, A.E.; Allison, M.; Upponi, S.; Brais, R.; et al. Observations on the ex situ perfusion of livers for transplantation. Am. J. Transplant. 2018, 18, 2005–2020. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Muller, X.; Kalisvaart, M.; Muellhaupt, B.; Perera, M.; Isaac, J.; Clavien, P.A.; Muiesan, P.; Dutkowski, P. Outcomes of liver transplantations from donation after circulatory death (DCD) treated by hypothermic oxygenated perfusion (HOPE) before implantation. J Hepatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- De Carlis, R.; Di Sandro, S.; Lauterio, A.; Ferla, F.; Belli, L.; De Carlis, L. Donation after cardiac death liver transplantation with normothermic regional perfusion and hypothermic machine perfusion: Follow-up of the first Italian series. In Proceedings of the 2018 Joint International Congress of ILTS, ELITA & LICAGE, Lisbon, Portugal, 23–26 May 2018. [Google Scholar]
- van Rijn, R.; van Leeuwen, O.; Matton, A.; Burlage, L.; Wiersema-Buist, J.; van den Heuvel, M.C.; de Kleine, R.H.J.; de Boer, M.T.; Gouw, A.S.H.; Porte, R.J. Hypothermic oxygenated machine perfusion reduces bile duct reperfusion injury after transplantation of donation after circulatory death livers. Liver Transplant. 2018, 24, 655–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rijn, R.; Karimian, N.; Matton, A.; Burlage, L.; Wetserkamp, A.; Van den Berg, A.; de Kleine, R.H.J.; de Boer, M.T.; Lisman, T.; Porte, R.J. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br. J. Surg. 2017, 104, 907–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutkowski, P.; Polak, W.G.; Muiesan, P.; Schlegel, A.; Verhoeven, C.J.; Scalera, I.; DeOliveira, M.L.; Kron, P.; Clavien, P.A. First Comparison of Hypothermic Oxygenated PErfusion Versus Static Cold Storage of Human Donation After Cardiac Death Liver Transplants: An International-matched Case Analysis. Ann. Surg. 2015, 262, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, J.V.; Henry, S.D.; Samstein, B.; Reznik, E.; Musat, C.; Lukose, T.I.; Ratner, L.E.; Brown, R.S., Jr.; Kato, T.; Emond, J.C. Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am. J. Transplant. 2015, 15, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Tsui, S.S.L.; Oniscu, G.C. Extending normothermic regional perfusion to the thorax in donors after circulatory death. Curr. Opin. Organ Transplant. 2017, 22, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Kron, P.; Schlegel, A.; de Rougemont, O.; Oberkofler, C.E.; Clavien, P.-A.; Dutkowski, P. Short, Cool, and Well Oxygenated—HOPE for Kidney Transplantation in a Rodent Model. Ann. Surg. 2016, 264, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niatsetskaya, Z.V.; Sosunov, S.A.; Matsiukevich, D.; Utkina-Sosunova, I.V.; Ratner, V.I.; Starkov, A.A.; Ten, V.S. The Oxygen Free Radicals Originating from Mitochondrial Complex I Contribute to Oxidative Brain Injury Following Hypoxia-Ischemia in Neonatal Mice. J. Neurosci. 2012, 32, 3235–3244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kron, P.; Schlegel, A.; Mancina, L.; Clavien, P.A.; Dutkowski, P. Hypothermic oxygenated perfusion (HOPE) for fatty liver grafts in rats and humans. J. Hepatol. 2018, 68, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Boteon, Y.; Laing, R.; Schlegel, A.; Wallace, L.; Smith, A.; Attard, J.; Bhogal, R.H.; Neil, D.A.; Hübscher, S.; Perera, M.T.P.; et al. Combined Hypothermic and Normothermic Machine Perfusion Improves Functional Recovery of Extended Criteria Donor Livers. Liver Transplant. 2018. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Kron, P.; Graf, R.; Dutkowski, P.; Clavien, P.A. Warm vs. cold perfusion techniques to rescue rodent liver grafts. J. Hepatol. 2014, 61, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Lazeyras, F.; Buhler, L.; Vallee, J.P.; Hergt, M.; Nastasi, A.; Ruttimann, R.; Morel, P.; Buchs, J.B. Detection of ATP by “in line”31P magnetic resonance spectroscopy during oxygenated hypothermic pulsatile perfusion of pigs’ kidneys. MAGMA 2012, 25, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Giordano, D.M.; Maroni, L.; Marzioni, M. Role of inflammation and proinflammatory cytokines in cholangiocyte pathophysiology. Biochim. Biophys. Acta Mol. Basis. Dis. 2017, 1864, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.E.; Op Den Dries, S.; Karimian, N.; Weeder, P.D.; De Boer, M.T.; Wiersema-Buist, J.; Gouw, A.S.; Leuvenink, H.G.; Lisman, T.; Porte, R.J. Criteria for Viability Assessment of Discarded Human Donor Livers during Ex Vivo Normothermic Machine Perfusion. PLoS ONE 2014, 9, e110642. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.E.; Jochmans, I. From “Gut Feeling” to Objectivity: Machine Preservation of the Liver as a Tool to Assess Organ Viability. Curr. Transplant. Rep. 2018, 5, 72–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhoeven, C.J.; Farid, W.R.R.; De Ruiter, P.E.; Hansen, B.E.; Roest, H.P.; De Jonge, J.; Kwekkeboom, J.; Metselaar, H.J.; Tilanus, H.W.; Kazemier, G.; et al. MicroRNA profiles in graft preservation solution are predictive of ischemic-type biliary lesions after liver transplantation. J. Hepatol. 2013, 59, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Faitot, F.; Besch, C.; Battini, S.; Ruhland, E.; Onea, M.; Addeo, P.; Woehl-Jaeglé, M.L.; Ellero, B.; Bachellier, P.; Namer, I.J. Impact of real-time metabolomics in liver transplantation: Graft evaluation and donor-recipient matching. J. Hepatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Verhelst, X.; Geerts, A.; Jochmans, I.; Vanderschaeghe, D.; Paradissis, A.; Vanlander, A.; Berrevoet, F.; Dahlqvist, G.; Nevens, F.; Pirenne, J.; et al. Glycome Patterns of Perfusate in Livers Before Transplantation Associate with Primary Nonfunction. Gastroenterology. 2018, 154, 1361–1368. [Google Scholar] [CrossRef] [PubMed]



| Author | Year | Model | Species | Temp (°C) | Perfusion Duration (h) | Perfusion Route | OLT | Endpoints | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Goldaracena et al. [39] | 2016 | DBD | Pig | 33, 37 | 4 | PV + HA | Yes | Ischemia-Reperfusion Injury, bile duct injury, liver function (3 day follow up) | Addition of anti-inflammatory substances during sub-and normothermic perfusion improve all endpoints and may reduce biliary injury |
| Spetzler et al. [40] | 2016 | DBD | Pig | 33 | 4 | PV + HA | Yes | Ischemia-Reperfusion Injury, bile duct injury, liver function, histology | Subnormothermic perfusion improves outcomes after transplantation |
| Fontes et al. [41] | 2015 | Pig | 21 | PV + HA | Yes | Liver function and injury, markers for biliary injury, inflammation and animal survival | Subnormothermic perfusion improves liver function, bile procudetion and survival and reduces the inflammation after liver transplantation, documentation of mediator release (regenerative pathways and inflammation) during subnormothermic perfusion | ||
| Knaak et al. [42] | 2014 | DCD | Pig | 33 | 3 | PV + HA | Yes | Endothelial and biliary injury and liver function, animal survival | Endischemic subnormothermic perfusion reduces biliary and endothelial cell injury after transplantation |
| Schlegel et al. [43] | 2013 | DCD | Rat | 4 | 1 | PV | Yes | Ischemia-Reperfusion Injury, graft function, animal survival, biliary parameters and histology 4 weeks after OLT | HOPE treated DCD livers showed significantly less biliary cirrhosis and fibrosis within 4 weeks after liver transplantation. Such reduced injury is mediated through less reperfusion injury after HOPE treatment |
| Banan et al. [44] | 2016 | DBD | Pig | 38, gradual rewarming + 38 | 4–8 | PV + HA | No | Markers of hepatocyte injury and biliary tree injury | Reduced biliary epithelial cell injury in gradually rewarmed grafts (rewarming + normotherm better preservation of biliary tree compared to direct normotherm perfusion) |
| Op den Dries et al. [35] | 2016 | DBD + DCD | Rat | 37 | 3 | PV + HA | No | Markers of biliary function and injury, histology | Normothermic perfusion protects bile ducts when performed instead of cold storage |
| Westerkamp et al. [45] | 2015 | DCD | Rat | 10, 20, COR | 2 | PV + HA | No | Markers of biliary function and injury, histology | Less injury of large bile duct epithelium compared to cold storage alone |
| Liu et al. [36] | 2014 | DCD | Pig | 38 | 10 | PV + HA | No | Markers of biliary function and injury, histology | Normothermic perfusion instead of cold storage improves regeneration of biliary epithelial cells |
| Op den Dries et al. [46] | 2014 | DBD + DCD | Pig | 10 | 4 | PV + HA | No | Markers of biliary function and injury, histology | Hypothermic perfusion prevents ateriolonecrosis of the peribiliary vascular plexus of the bile ducts |
| Author | Year | Model | n | Technique | Temp (°C) | Device | Perfusion Duration (h) | Perfusion Route | Endpoints | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Watson et al. [47] | 2018 | DCD | 44 | NRP | 37 | Maquet/ECOPS | 2 | NRP | Peak ALT, graft function, biliary complications, 90-day survival | NRP is a successful selection tool, DCD livers recovered with NRP showed significant less biliary complications (AS and NAS), no differences in graft survival |
| Hessheimer et al. [48] | 2018 | DCD | 97 | NRP | 37 | ECMO | 2 | NRP | graft function, biliary complications, 1-year graft survival | NRP is a successful selection tool, DCD livers recovered with NRP showed significant less biliary complications (AS and NAS), no significant differences in graft survival |
| Nasralla et al. ✫ [37] | 2018 | DBD + DCD | 121 (34 DCD) | NMP | 37 | Organox metra | 9.1 | PV + HA | AST release and 1-year survival after liver transplantation | No difference in biliary complications (AS, NAS), reduced AST release after reperfusion |
| Watson et al. [49] | 2018 | DBD + DCD | 22 (16 DCD) | NMP | 37 | Liver Assist | 4–6 | PV + HA | Post-Reperfusion syndrome, graft function, rate of PNF, biliary complication, bile duct histology and graft survival | 25% of transplanted DCD livers developed a NAS, Bile pH during NMP is currently the best predictor for biliary complications at the cutoff 7.5 |
| Bral et al. ✢ [38] | 2017 | DBD + DCD | 9 (4 DCD) | NMP | 37 | Organox Metra | 11.5 | PV + HA | Graft function and injury, biliary complications, graft survival | Longer ITU and hospital stay in NMP group |
| Schlegel et al. [50] | 2018 | DCD | 50 | HOPE | 10 | Liver Assist | 2 | PV | Post-Reperfusion syndrome, graft function, rate of PNF, HAT and ischemic cholangiopathy, 5-year graft survival | HOPE treated extended DCD liver grafts showed significant improved 5-year graft survival due to less PNF, HAT and ischemic cholangiopathy |
| De Carlis et al. [51] | 2018 | DCD (II, III) | 15 | ECMO + HOPE | 37, 10 | ECMO/Liver Assist | 2, 2 | ECMO, PV + HA | Liver function, biliary complications, 1-year survival | No significant differences in biliary complications compared to DBD matching group, 2 NAS (endoscopically treated), no significant differences in survival |
| Van Rijn et al. [52] | 2018 | DCD | 20 | DHOPE | 10 | Liver Assist | 2 | PV + HA | Markers of biliary injury including histology of bile ducts | D-HOPE treatment restored hepatic ATP and protects the biliary tree from reperfusion injury and complications |
| Van Rijn et al. [53] | 2017 | DCD | 10 | DHOPE | 10 | Liver Assist | 2 | PV + HA | Liver function, ATP content, biliary complications, graft- and patient survival | D-HOPE treatment protect from reperfusion injury and improved 6 and 12 month graft survival and reduced biliary complications |
| Dutkowski et al. [54] | 2015 | DCD | 25 | HOPE | 10 | Liver Assist | 1–2 | PV | Graft function, EAD, biliary complications, graft and patient survival | HOPE treated extended DCD liver grafts showed comparable outcomes to matched low-risk primary DBD transplants, biliary complications were reduced compared to untreated DCD liver transplants |
| Guarrera et al. [55] | 2015 | ECD (no DCD) | 20 | HMP | 4-8 | Medtronic | 4–7 | PV + HA | Incidence of PNF, EAD, vascular and biliary complication, 1-year graft and patient survival | HMP showed significantly less biliary complications |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlegel, A.; Dutkowski, P. Impact of Machine Perfusion on Biliary Complications after Liver Transplantation. Int. J. Mol. Sci. 2018, 19, 3567. https://doi.org/10.3390/ijms19113567
Schlegel A, Dutkowski P. Impact of Machine Perfusion on Biliary Complications after Liver Transplantation. International Journal of Molecular Sciences. 2018; 19(11):3567. https://doi.org/10.3390/ijms19113567
Chicago/Turabian StyleSchlegel, Andrea, and Philipp Dutkowski. 2018. "Impact of Machine Perfusion on Biliary Complications after Liver Transplantation" International Journal of Molecular Sciences 19, no. 11: 3567. https://doi.org/10.3390/ijms19113567
APA StyleSchlegel, A., & Dutkowski, P. (2018). Impact of Machine Perfusion on Biliary Complications after Liver Transplantation. International Journal of Molecular Sciences, 19(11), 3567. https://doi.org/10.3390/ijms19113567