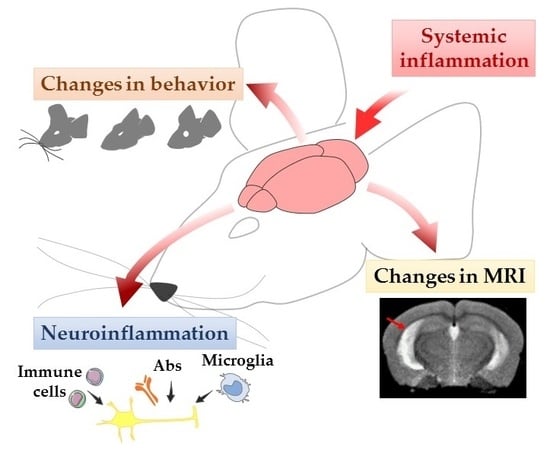
From Systemic Inflammation to Neuroinflammation: The Case of Neurolupus
Abstract
:1. Introduction
2. Systemic and NP Aspects of Lupus Disease
2.1. General Presentation of SLE
2.2. CNS Involvement in Human SLE: The Neuropsychiatric Lupus Disease (NPSLE)
2.3. Modelization of the Disease in Mice
2.3.1. Tests of Depression
2.3.2. Anxiety Tests
2.3.3. Cognitive Tests
2.3.4. Locomotor function
3. Neuroimaging in NPSLE
3.1. Magnetic Resonance Imaging Modalities
3.2. Brain Imaging and MRL/lpr Mice
4. Neuroinflammation in Lupus-Prone Mice
4.1. Characteristic Elements Occurring in the Brain of Lupus-Prone Mice
4.1.1. Pathogenic AutoAbs
4.1.2. Cytokines
4.1.3. Peripheral Immune Cell Infiltration
4.1.4. Glial Cells
4.2. Hippocampus as the Possible Primary Target of Neuroinflammatory Lupus
4.3. Potential Mechanisms of Neuroinflammation in NPSLE
4.4. Is NPSLE a Primarily CNS Disease?
5. Therapeutic Approach to NPSLE
5.1. Current Treatments
5.2. A Potential Therapeutic Option: The Hypothesis of Autophagy
6. Conclusive Remarks and Perspectives
7. Ethics Statement
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ab | Antibody |
| ACR | American College of Rheumatology |
| aPL | Antiphospholipid |
| BBB | Blood–brain barrier |
| BCSFB | Blood–cerebrospinal-fluid barrier |
| BOLD | Blood oxygen level-dependent |
| CD | Cluster of differentiation |
| CMA | Chaperone-mediated autophagy |
| CNS | Central nervous system |
| CP | Choroid plexus |
| CSF | Cerebrospinal fluid |
| Cyt c | Cytochrome c |
| dsDNA | Double stranded DNA |
| DTI | Diffusion tensor imaging |
| DWI | Diffusion-weighted imaging |
| ECs | Endothelial cells |
| Fas/APO-1/CD95 | Apoptosis stimulating fragment |
| HPA | Hypothalamic–pituitary–adrenal |
| HSCT | Hematopoietic stem cell transplantation |
| HSPA8 | Heat shock 70-kD protein A8 isoform 1 |
| Iba1 | Ionized calcium binding adaptor molecule 1 |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IFN | Interferon |
| IFNAR | IFN-α/β receptor |
| Ig | Immunoglobulin |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| LAP | LC3-associated phagocytosis |
| LC3 | Microtubule-associated protein light chain 3 |
| lpr | Lymphoproliferation |
| M-CSF | Macrophage colony-stimulating factor |
| MIF | Macrophage migration inhibitory factor |
| MPTP | Mitochondrial permeability transition pore |
| MRA | Magnetic resonance angiography |
| MRI | Magnetic resonance imaging |
| MRL | Murphy Roths Large |
| MRS | Magnetic resonance spectroscopy |
| MS | Multiple sclerosis |
| MTI | Magnetic transfer imaging |
| mTOR | Mechanistic/mammalian target of rapamycin |
| MTR | Magnetic transfer ratio |
| NAA | N-acetyl aspartate |
| NMDAR | N-methyl-D-aspartate receptor |
| NPSLE | Neuropsychiatric systemic lupus erythematosus |
| NSAIDs | Non-steroid anti-inflammatory drugs |
| NZW | New Zealand White |
| NZB | New Zealand Black |
| PAF | Paraformaldehyde |
| RARE | Rapid acquisition with relaxation enhancement |
| ROS | Reactive oxygen species |
| SQSTM1/p62 | Sequestosome-1/p62 |
| T1 | Longitudinal relaxation time |
| T2 | Transverse relaxation time |
| TE | Echo time |
| Tfh | T follicular helper |
| TNF | Tumor necrosis factor |
| TR | Repetition time |
| TWEAK | TNF-like weak inducer of apoptosis |
| VCAM-1 | Vascular cell adhesion protein 1 |
| vs. | versus |
References
- Suárez-Fueyo, A.; Bradley, S.J.; Tsokos, G.C. T cells in systemic lupus erythematosus. Curr. Opin. Immunol. 2016, 43, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, G.C. Systemic lupus erythematosus. N. Engl. J. Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; Cervera, R. Systemic lupus erythematosus. Best Pract. Res. Clin. Rheumatol. 2010, 24, 841–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, A.; Isenberg, D.A. Systemic lupus erythematosus. N. Engl. J. Med. 2008, 358, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Bove, R. Autoimmune diseases and reproductive aging. Clin. Immunol. 2013, 149, 251–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, M.; Kawasaki, A.; Ito, I.; Furuya, T.; Ohashi, J.; Kyogoku, C.; Ito, S.; Hayashi, T.; Matsumoto, I.; Kusaoi, M.; et al. Cumulative association of eight susceptibility genes with systemic lupus erythematosus in a Japanese female population. J. Hum. Genet. 2011, 56, 503–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubtsov, A.V.; Rubtsova, K.; Kappler, J.W.; Marrack, P. Genetic and hormonal factors in female-biased autoimmunity. Autoimmun. Rev. 2010, 9, 494–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zandman-Goddard, G.; Solomon, M.; Rosman, Z.; Peeva, E.; Shoenfeld, Y. Environment and lupus-related diseases. Lupus 2012, 21, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Hanly, J.G.; Urowitz, M.B.; Su, L.; Bae, S.C.; Gordon, C.; Wallace, D.J.; Clarke, A.; Bernatsky, S.; Isenberg, D.; Rahman, A.; et al. Prospective analysis of neuropsychiatric events in an international disease inception cohort of patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2010, 69, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Jeltsch-David, H.; Muller, S. Neuropsychiatric systemic lupus erythematosus: Pathogenesis and biomarkers. Nat. Rev. Neurol. 2014, 10, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Hanly, J.G.; Kozora, E.; Beyea, S.; Birnbaum, J. Nervous system disease in systemic lupus erythematosus: Current status and future directions. Arthritis Rheumatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Monahan, R.C.; Beaart-van de Voorde, L.J.J.; Steup-Beekman, G.M.; Magro-Checa, C.; Huizinga, T.W.J.; Hoekman, J.; Kaptein, A.A. Neuropsychiatric symptoms in systemic lupus erythematosus: Impact on quality of life. Lupus 2017, 26, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Magro-Checa, C.; Beaart-van de Voorde, L.J.J.; Middelkoop, H.A.M.; Dane, M.L.; van der Wee, N.J.; van Buchem, M.A.; Huizinga, T.W.J.; Steup-Beekman, G.M. Outcomes of neuropsychiatric events in systemic lupus erythematosus based on clinical phenotypes; prospective data from the Leiden NP SLE cohort. Lupus 2017, 26, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Zirkzee, E.; Huizinga, T.; Bollen, E.; van Buchem, M.; Middelkoop, H.; van der Wee, N.; le Cessie, S.; Steup-Beekman, G. Mortality in neuropsychiatric systemic lupus erythematosus (NPSLE). Lupus 2014, 23, 31–38. [Google Scholar] [CrossRef] [PubMed]
- ACR ad hoc committee The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999, 42, 599–608. [CrossRef]
- Hanly, J.G. Diagnosis and management of neuropsychiatric SLE. Nat. Rev. Rheumatol. 2014, 10, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Ainiala, H.; Loukkola, J.; Peltola, J.; Korpela, M.; Hietaharju, A. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology 2001, 57, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Ainiala, H.; Hietaharju, A.; Loukkola, J.; Peltola, J.; Korpela, M.; Metsänoja, R.; Auvinen, A. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: A population-based evaluation. Arthritis Rheum. 2001, 45, 419–423. [Google Scholar] [CrossRef]
- Bortoluzzi, A.; Scirè, C.A.; Govoni, M. Attribution of neuropsychiatric manifestations to systemic lupus erythematosus. Front. Med. 2018, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- Zandman-Goddard, G.; Chapman, J.; Shoenfeld, Y. Autoantibodies involved in neuropsychiatric SLE and antiphospholipid syndrome. Semin. Arthritis Rheum. 2007, 36, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Leslie, B.; Crowe, S.F. Cognitive functioning in systemic lupus erythematosus: A meta-analysis. Lupus 2018, 27, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Gelb, S.; Stock, A.D.; Anzi, S.; Putterman, C.; Ben-Zvi, A. Mechanisms of neuropsychiatric lupus: The relative roles of the blood-cerebrospinal fluid barrier versus blood-brain barrier. J. Autoimmun. 2018, 91, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Gerosa, M.; Poletti, B.; Pregnolato, F.; Castellino, G.; Lafronza, A.; Silani, V.; Riboldi, P.; Meroni, P.L.; Merrill, J.T. Antiglutamate receptor antibodies and cognitive impairment in primary antiphospholipid syndrome and systemic lupus erythematosus. Front. Immunol. 2016, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Ho, R.C.; Thiaghu, C.; Ong, H.; Lu, Y.; Ho, C.S.; Tam, W.W.; Zhang, M.W. A meta-analysis of serum and cerebrospinal fluid autoantibodies in neuropsychiatric systemic lupus erythematosus. Autoimmun. Rev. 2016, 15, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Dema, B.; Charles, N. Autoantibodies in SLE: Specificities, isotypes and receptors. Antibodies 2016, 5, 2. [Google Scholar] [CrossRef]
- Hirohata, S.; Sakuma, Y.; Matsueda, Y.; Arinuma, Y.; Yanagida, T. Role of serum autoantibodies in blood brain barrier damages in neuropsychiatric systemic lupus erythematosus. Clin. Exp. Rheumatol. 2018. [Google Scholar]
- Matsueda, Y.; Arinuma, Y.; Nagai, T.; Hirohata, S. Elevation of serum anti–glucose-regulated protein 78 antibodies in neuropsychiatric systemic lupus erythematosus. Lupus Sci. Med. 2018, 5, e000281. [Google Scholar] [CrossRef] [PubMed]
- de Boer, A.G.; Gaillard, P.J. Blood-brain barrier dysfunction and recovery. J. Neural Transm. 2006, 113, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Preston, J.E.; Joan Abbott, N.; Begley, D.J. Transcytosis of macromolecules at the blood-brain barrier. Adv. Pharmacol. 2014, 71, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Mendonça, L.L.F.; Dolman, D.E.M. The blood-brain barrier in systemic lupus erythematosus. Lupus 2003, 12, 908–915. [Google Scholar] [CrossRef] [PubMed]
- McLean, B.N.; Miller, D.; Thompson, E.J. Oligoclonal banding of IgG in CSF, blood-brain barrier function, and MRI findings in patients with sarcoidosis, systemic lupus erythematosus, and Behçet’s disease involving the nervous system. J. Neurol. Neurosurg. Psychiatry 1995, 58, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Fujii, T.; Yokoyama, T.; Fujita, Y.; Imura, Y.; Yukawa, N.; Kawabata, D.; Nojima, T.; Ohmura, K.; Usui, T.; et al. Anti-U1 RNP antibodies in cerebrospinal fluid are associated with central neuropsychiatric manifestations in systemic lupus erythematosus and mixed connective tissue disease. Arthritis Rheum. 2010, 62, 3730–3740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.H.; Buchanan, N.; Barker, G.; Morrissey, S.P.; Kendall, B.E.; Rudge, P.; Khamashta, M.; Hughes, G.R.; McDonald, W.I. Gadolinium-enhanced magnetic resonance imaging of the central nervous system in systemic lupus erythematosus. J. Neurol. 1992, 239, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Stock, A.D.; Gelb, S.; Pasternak, O.; Ben-Zvi, A.; Putterman, C. The blood brain barrier and neuropsychiatric lupus: New perspectives in light of advances in understanding the neuroimmune interface. Autoimmun. Rev. 2017, 16, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Gulinello, M.; Putterman, C. The MRL/lpr mouse strain as a model for neuropsychiatric systemic lupus erythematosus. J. Biomed. Biotechnol. 2011, 2011, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.; Sang, A.; Yin, Y.; Zheng, Y.-Y.; Morel, L. Murine models of systemic lupus erythematosus. J. Biomed. Biotechnol. 2011, 2011, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rekvig, O.P.; Putterman, C.; Casu, C.; Gao, H.-X.; Ghirardello, A.; Mortensen, E.S.; Tincani, A.; Doria, A. Autoantibodies in lupus: Culprits or passive bystanders? Autoimmun. Rev. 2012, 11, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Stanojcic, M.; Loheswaran, G.; Xu, L.; Hoffman, S.A.; Sakić, B. Intrathecal antibodies and brain damage in autoimmune MRL mice. Brain. Behav. Immun. 2010, 24, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Sidor, M.M.; Sakić, B.; Malinowski, P.M.; Ballok, D.A.; Oleschuk, C.J.; Macri, J. Elevated immunoglobulin levels in the cerebrospinal fluid from lupus-prone mice. J. Neuroimmunol. 2005, 165, 104–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeltsch-David, H.; Muller, S. Neuropsychiatric systemic lupus erythematosus and cognitive dysfunction: The MRL-lpr mouse strain as a model. Autoimmun. Rev. 2014, 13, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Umiker, B.R.; Kazimirova, A.A.; Fray, M.; Korgaonkar, P.; Selsing, E.; Imanishi-Kari, T. Expression of an anti-RNA autoantibody in a mouse model of SLE increases neutrophil and monocyte numbers as well as IFN-I expression. Eur. J. Immunol. 2014, 44, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Bialas, A.R.; Presumey, J.; Das, A.; van der Poel, C.E.; Lapchak, P.H.; Mesin, L.; Victora, G.; Tsokos, G.C.; Mawrin, C.; Herbst, R.; et al. Microglia-dependent synapse loss in type I interferon-mediated lupus. Nature 2017, 546, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Nestor, J.; Arinuma, Y.; Huerta, T.S.; Kowal, C.; Nasiri, E.; Kello, N.; Fujieda, Y.; Bialas, A.; Hammond, T.; Sriram, U.; et al. Lupus antibodies induce behavioral changes mediated by microglia and blocked by ACE inhibitors. J. Exp. Med. 2018, 215, 2554–2566. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.H.; Volpe, B.T.; Mackay, M.; Aranow, C.; Watson, P.; Kowal, C.; Storbeck, J.; Mattis, P.; Berlin, R.; Chen, H.; et al. Selective impairment of spatial cognition caused by autoantibodies to the N-Methyl-D-aspartate receptor. EBioMedicine 2015, 2, 755–764. [Google Scholar] [CrossRef] [PubMed]
- DeGiorgio, L.A.; Konstantinov, K.N.; Lee, S.C.; Hardin, J.A.; Volpe, B.T.; Diamond, B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 2001, 7, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Frauenknecht, K.; Leukel, P.; Weiss, R.; von Pein, H.D.; Katzav, A.; Chapman, J.; Sommer, C.J. Decreased hippocampal cell proliferation in mice with experimental antiphospholipid syndrome. Brain Struct. Funct. 2018, 223, 3463–3471. [Google Scholar] [CrossRef] [PubMed]
- Pikman, R.; Kivity, S.; Levy, Y.; Arango, M.-T.; Chapman, J.; Yonath, H.; Shoenfeld, Y.; Gofrit, S.G. Neuropsychiatric SLE: From animal model to human. Lupus 2017, 26, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.D.; Roths, J.B. A Y chromosome associated factor in strain BXSB producing accelerated autoimmunity and lymphoproliferation. Arthritis Rheum. 1979, 22, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.L.; Rao, J.K.; Gilkeson, G.S.; Ruiz, P.; Eicher, E.M.; Pisetsky, D.S.; Matsuzawa, A.; Rochelle, J.M.; Seldin, M.F. Genetic analysis of MRL-lpr mice: Relationship of the Fas apoptosis gene to disease manifestations and renal disease-modifying loci. J. Exp. Med. 1992, 176, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Drappa, J.; Brot, N.; Elkon, K.B. The Fas protein is expressed at high levels on CD4+CD8+ thymocytes and activated mature lymphocytes in normal mice but not in the lupus-prone strain, MRL lpr/lpr. Proc. Natl. Acad. Sci. USA 1993, 90, 10340–10344. [Google Scholar] [CrossRef] [PubMed]
- Theofilopoulos, A.N. Murine models of lupus. In Systemic Lupus Erythematosus; Lahita, R.G., Ed.; Churchill Livingstone: New York, NY, USA, 1992; pp. 121–194. [Google Scholar]
- Deacon, R.M.J. Shallow water (paddling) variants of water maze tests in mice. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D. Animal model of depression. Biomedicine 1979, 30, 139–140. [Google Scholar] [PubMed]
- Sakić, B.; Szechtman, H.; Talangbayan, H.; Denburg, S.D.; Carbotte, R.M.; Denburg, J.A. Disturbed emotionality in autoimmune MRL-lpr mice. Physiol. Behav. 1994, 56, 609–617. [Google Scholar] [CrossRef]
- Sakić, B.; Szechtman, H.; Keffer, M.; Talangbayan, H.; Stead, R.; Denburg, J.A. A behavioral profile of autoimmune lupus-prone MRL mice. Brain. Behav. Immun. 1992, 6, 265–285. [Google Scholar] [PubMed]
- Gao, H.-X.; Sanders, E.; Tieng, A.T.; Putterman, C. Sex and autoantibody titers determine the development of neuropsychiatric manifestations in lupus-prone mice. J. Neuroimmunol. 2010, 229, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sakić, B.; Szechtman, H.; Braciak, T.; Richards, C.; Gauldie, J.; Denburg, J.A. Reduced preference for sucrose in autoimmune mice: A possible role of interleukin-6. Brain Res. Bull. 1997, 44, 155–165. [Google Scholar] [CrossRef]
- Ballok, D.A.; Earls, A.M.; Krasnik, C.; Hoffman, S.A.; Sakić, B. Autoimmune-induced damage of the midbrain dopaminergic system in lupus-prone mice. J. Neuroimmunol. 2004, 152, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Ballok, D.A.; Szechtman, H.; Sakić, B. Taste responsiveness and diet preference in autoimmune MRL mice. Behav. Brain Res. 2003, 140, 119–130. [Google Scholar] [CrossRef]
- Sakić, B.; Denburg, J.A.; Denburg, S.D.; Szechtman, H. Blunted sensitivity to sucrose in autoimmune MRL-lpr mice: A curve-shift study. Brain Res. Bull. 1996, 41, 305–311. [Google Scholar] [CrossRef]
- Szechtman, H.; Sakić, B.; Denburg, J.A. Behaviour of MRL mice: An animal model of disturbed behaviour in systemic autoimmune disease. Lupus 1997, 6, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Misslin, R.; Belzung, C.; Vogel, E. Behavioural validation of a light/dark choice procedure for testing anti-anxiety agents. Behav. Process. 1989, 18, 119–132. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Pellow, S.; File, S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986, 24, 525–529. [Google Scholar] [CrossRef]
- Gao, H.-X.; Campbell, S.R.; Cui, M.-H.; Zong, P.; Hwang, J.-H.; Gulinello, M.; Putterman, C. Depression is an early disease manifestation in lupus-prone MRL/lpr mice. J. Neuroimmunol. 2009, 207, 45–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, D.M.; Crnic, L.S. Elevated plus maze behavior, auditory startle response, and shock sensitivity in predisease and in early stage autoimmune disease MRL/lpr mice. Brain. Behav. Immun. 2002, 16, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.N. Exploratory behavior models of anxiety in mice. Neurosci. Biobehav. Rev. 1985, 9, 37–44. [Google Scholar] [CrossRef]
- Simon, P.; Dupuis, R.; Costentin, J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav. Brain Res. 1994, 61, 59–64. [Google Scholar] [CrossRef]
- Carola, V.; D’Olimpio, F.; Brunamonti, E.; Mangia, F.; Renzi, P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav. Brain Res. 2002, 134, 49–57. [Google Scholar] [CrossRef]
- Archer, J. Rodent sex differences in emotional and related behavior. Behav. Biol. 1975, 14, 451–479. [Google Scholar] [CrossRef]
- Donner, N.C.; Lowry, C.A. Sex differences in anxiety and emotional behavior. Pflugers Arch. 2013, 465, 601–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, S.; Stock, A.; Macian, F.; Putterman, C. A distinct T follicular helper cell subset infiltrates the brain in murine neuropsychiatric lupus. Front. Immunol. 2018, 9, 487. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Cacucci, F.; Lever, C. Which memory task for my mouse? A systematic review of spatial memory performance in the Tg2576 Alzheimer’s mouse model. J. Alzheimers Dis. JAD 2011, 26, 105–126. [Google Scholar] [CrossRef] [PubMed]
- Ennaceur, A.; Neave, N.; Aggleton, J.P. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp. Brain Res. 1997, 113, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Aggleton, J.P.; Nelson, A.J.D. Why do lesions in the rodent anterior thalamic nuclei cause such severe spatial deficits? Neurosci. Biobehav. Rev. 2015, 54, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Sakić, B.; Szechtman, H.; Denburg, S.; Carbotte, R.; Denburg, J.A. Spatial learning during the course of autoimmune disease in MRL mice. Behav. Brain Res. 1993, 54, 57–66. [Google Scholar] [CrossRef]
- Vogelweid, C.M.; Wright, D.C.; Johnson, J.C.; Hewett, J.E.; Walker, S.E. Evaluation of memory, learning ability, and clinical neurologic function in pathogen-free mice with systemic lupus erythematosus. Arthritis Rheum. 1994, 37, 889–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramaniyan, S.; Heo, S.; Patil, S.; Li, L.; Hoger, H.; Pollak, A.; Lubec, G. A hippocampal nicotinic acetylcholine alpha 7-containing receptor complex is linked to memory retrieval in the multiple-T-maze in C57BL/6j mice. Behav. Brain Res. 2014, 270, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, M.; Falsafi, S.K.; Hoeger, H.; Lubec, G. Hippocampal levels of GluR1 and GluR2 complexes are modulated by training in the multiple T-Maze in C57BL/6J mice. Brain Struct. Funct. 2012, 217, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Pioli, E.Y.; Gaskill, B.N.; Gilmour, G.; Tricklebank, M.D.; Dix, S.L.; Bannerman, D.; Garner, J.P. An automated maze task for assessing hippocampus-sensitive memory in mice. Behav. Brain Res. 2014, 261, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, R. The neurobiological basis of spontaneous alternation. Neurosci. Biobehav. Rev. 2002, 26, 91–104. [Google Scholar] [CrossRef]
- Jeltsch-David, H.; Muller, S. Autoimmunity, neuroinflammation, pathogen load: A decisive crosstalk in neuropsychiatric SLE. J. Autoimmun. 2016, 74, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Brun, S.; René, F.; de Sèze, J.; Loeffler, J.P.; Jeltsch-David, H. Autophagy in neuroinflammatory diseases. Autoimmun. Rev. 2017, 16, 856–874. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.L.; Shields, C.N. Effects of sex on ethanol conditioned place preference, activity and variability in C57BL/6J and DBA/2J mice. Pharmacol. Biochem. Behav. 2018, 173, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Katzav, A.; Solodeev, I.; Brodsky, O.; Chapman, J.; Pick, C.G.; Blank, M.; Zhang, W.; Reichlin, M.; Shoenfeld, Y. Induction of autoimmune depression in mice by anti-ribosomal P antibodies via the limbic system. Arthritis Rheum. 2007, 56, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Kowal, C.; DeGiorgio, L.A.; Nakaoka, T.; Hetherington, H.; Huerta, P.T.; Diamond, B.; Volpe, B.T. Cognition and immunity: Antibody impairs memory. Immunity 2004, 21, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Huerta, P.T.; Kowal, C.; DeGiorgio, L.A.; Volpe, B.T.; Diamond, B. Immunity and behavior: Antibodies alter emotion. Proc. Natl. Acad. Sci. USA 2006, 103, 678–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshio, T.; Okamoto, H.; Hirohata, S.; Minota, S. IgG anti–NR2 glutamate receptor autoantibodies from patients with systemic lupus erythematosus activate endothelial cells. Arthritis Rheum. 2013, 65, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.D.; Parikh, N.U.; Woodruff, T.M.; Jarvis, J.N.; Lopez, M.; Hennon, T.; Cunningham, P.; Quigg, R.J.; Schwartz, S.A.; Alexander, J.J. C5a alters blood-brain barrier integrity in a human in vitro model of systemic lupus erythematosus. Immunology 2015, 146, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Ainiala, H.; Dastidar, P.; Loukkola, J.; Lehtimäki, T.; Korpela, M.; Peltola, J.; Hietaharju, A. Cerebral MRI abnormalities and their association with neuropsychiatric manifestations in SLE: A population-based study. Scand. J. Rheumatol. 2005, 34, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbon, B.M.; Fairhall, S.L.; Kirk, I.J.; Kalev-Zylinska, M.; Pui, K.; Dalbeth, N.; Keelan, S.; Robinson, E.; During, M.; McQueen, F.M. Functional MRI in NPSLE patients reveals increased parietal and frontal brain activation during a working memory task compared with controls. Rheumatology 2008, 47, 50–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibbitt, W.L., Jr.; Brooks, W.M.; Kornfeld, M.; Hart, B.L.; Bankhurst, A.D.; Roldan, C.A. Magnetic resonance imaging and brain histopathology in neuropsychiatric systemic lupus erythematosus. Semin. Arthritis Rheum. 2010, 40, 32–52. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, N.; Alobeidi, F.; Toledano, P.; Espinosa, G.; Giles, I.; Rahman, A.; Yousry, T.; Capurro, S.; Jäger, R.; Cervera, R.; Bargalló, N. Brain abnormalities in newly diagnosed neuropsychiatric lupus: Systematic MRI approach and correlation with clinical and laboratory data in a large multicenter cohort. Autoimmun. Rev. 2015, 14, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, N.; Toledano, P.; Calvo, A.; Roura, E.; Sarbu, M.I.; Espinosa, G.; Lladó, X.; Cervera, R.; Bargalló, N. Advanced MRI techniques: Biomarkers in neuropsychiatric lupus. Lupus 2017, 26, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Toledano, P.; Sarbu, N.; Espinosa, G.; Bargalló, N.; Cervera, R. Neuropsychiatric systemic lupus erythematosus: Magnetic resonance imaging findings and correlation with clinical and immunological features. Autoimmun. Rev. 2013, 12, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Appenzeller, S.; Pike, G.B.; Clarke, A.E. Magnetic resonance imaging in the evaluation of central nervous system manifestations in systemic lupus erythematosus. Clin. Rev. Allergy Immunol. 2008, 34, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Appenzeller, S.; Bonilha, L.; Rio, P.A.; Min Li, L.; Costallat, L.T.L.; Cendes, F. Longitudinal analysis of gray and white matter loss in patients with systemic lupus erythematosus. NeuroImage 2007, 34, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Postal, M.; Lapa, A.T.; Reis, F.; Rittner, L.; Appenzeller, S. Magnetic resonance imaging in neuropsychiatric systemic lupus erythematosus: Current state of the art and novel approaches. Lupus 2017, 26, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, N.; Corrêa, D.G.; Kubo, T.A.; Netto, T.M.; Pereira, D.B.; Fonseca, R.P.; Gasparetto, E.L. Global Cognitive Impairment in Systemic Lupus Erythematosus Patients: A Structural MRI Study. Clin. Neuroradiol. 2017, 27, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Luyendijk, J.; Steens, S.C.A.; Ouwendijk, W.J.N.; Steup-Beekman, G.M.; Bollen, E.L.E.M.; van der Grond, J.; Huizinga, T.W.J.; Emmer, B.J.; van Buchem, M.A. Neuropsychiatric systemic lupus erythematosus: Lessons learned from magnetic resonance imaging. Arthritis Rheum. 2011, 63, 722–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mak, A.; Ren, T.; Fu, E.H.; Cheak, A.A.; Ho, R.C. A prospective functional MRI study for executive function in patients with systemic lupus erythematosus without neuropsychiatric symptoms. Semin. Arthritis Rheum. 2012, 41, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.; Chen, X.; Wen, W. White matter hyperintensities in mid-adult life. Curr. Opin. Psychiatry 2008, 21, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.W.; Her, M.; Bae, J.S.; Kim, S.-K.; Lee, S.W.; Kim, H.K.; Kim, D.; Park, N.; Chung, W.T.; Lee, S.Y.; et al. Brain MRI in neuropsychiatric lupus: Associations with the 1999 ACR case definitions. Rheumatol. Int. 2015, 35, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Ercan, E.; Ingo, C.; Tritanon, O.; Magro-Checa, C.; Smith, A.; Smith, S.; Huizinga, T.; van Buchem, M.A.; Ronen, I. A multimodal MRI approach to identify and characterize microstructural brain changes in neuropsychiatric systemic lupus erythematosus. NeuroImage Clin. 2015, 8, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Abda, E.A.; Selim, Z.I.; Radwan, M.E.M.; Mahmoud, N.M.; Herdan, O.M.; Mohamad, K.A.; Hamed, S.A. Markers of acute neuropsychiatric systemic lupus erythematosus: A multidisciplinary evaluation. Rheumatol. Int. 2013, 33, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Zimny, A.; Szmyrka-Kaczmarek, M.; Szewczyk, P.; Bladowska, J.; Pokryszko-Dragan, A.; Gruszka, E.; Wiland, P.; Sasiadek, M. In vivo evaluation of brain damage in the course of systemic lupus erythematosus using magnetic resonance spectroscopy, perfusion-weighted and diffusion-tensor imaging. Lupus 2014, 23, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Nystedt, J.; Nilsson, M.; Jönsen, A.; Nilsson, P.; Bengtsson, A.; Lilja, Å.; Lätt, J.; Mannfolk, P.; Sundgren, P.C. Altered white matter microstructure in lupus patients: A diffusion tensor imaging study. Arthritis Res. Ther. 2018, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Costallat, B.L.; Ferreira, D.M.; Lapa, A.T.; Rittner, L.; Costallat, L.T.L.; Appenzeller, S. Brain diffusion tensor MRI in systematic lupus erythematosus: A systematic review. Autoimmun. Rev. 2018, 17, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Sled, J.G.; Spring, S.; van Eede, M.; Lerch, J.P.; Ullal, S.; Sakić, B. Time course and nature of brain atrophy in the MRL mouse model of central nervous system lupus. Arthritis Rheum. 2009, 60, 1764–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, H.E.; Powell, N.M.; Ma, D.; Ismail, O.; Harrison, I.F.; Wells, J.A.; Colgan, N.; O’Callaghan, J.M.; Johnson, R.A.; Murray, T.K.; et al. Comparison of in vivo and ex vivo MRI for the detection of structural abnormalities in a mouse model of tauopathy. Front. Neuroinformatics 2017, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- McKinney, A.M. Enlargement or asymmetry of the lateral ventricles simulating hydrocephalus. In Atlas of Normal Imaging Variations of the Brain, Skull, and Craniocervical Vasculature; Springer International Publishing: Cham, Switzerland, 2017; pp. 349–369. ISBN 978-3-319-39789-4. [Google Scholar]
- Wachinger, C.; Salat, D.H.; Weiner, M.; Reuter, M.; Alzheimer’s Disease Neuroimaging Initiative. Whole-brain analysis reveals increased neuroanatomical asymmetries in dementia for hippocampus and amygdala. Brain J. Neurol. 2016, 139, 3253–3266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malheiros, J.M.; Paiva, F.F.; Longo, B.M.; Hamani, C.; Covolan, L. Manganese-Enhanced MRI: Biological Applications in Neuroscience. Front. Neurol. 2015, 6, 161. [Google Scholar] [CrossRef] [PubMed]
- Kivity, S.; Tsarfaty, G.; Agmon-Levin, N.; Blank, M.; Manor, D.; Konen, E.; Chapman, J.; Reichlin, M.; Wasson, C.; Shoenfeld, Y.; et al. Abnormal olfactory function demonstrated by manganese-enhanced MRI in mice with experimental neuropsychiatric lupus. Ann. N. Y. Acad. Sci. 2010, 1193, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Van der Graaf, M. In vivo magnetic resonance spectroscopy: Basic methodology and clinical applications. Eur. Biophys. J. 2010, 39, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Lapteva, L.; Nowak, M.; Yarboro, C.H.; Takada, K.; Roebuck-Spencer, T.; Weickert, T.; Bleiberg, J.; Rosenstein, D.; Pao, M.; Patronas, N.; et al. Anti–N-methyl-D-aspartate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis Rheum. 2006, 54, 2505–2514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamond, B. Antibodies and the brain: Lessons from lupus. J. Immunol. 2010, 185, 2637–2640. [Google Scholar] [CrossRef] [PubMed]
- Kowal, C.; DeGiorgio, L.A.; Lee, J.Y.; Edgar, M.A.; Huerta, P.T.; Volpe, B.T.; Diamond, B. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc. Natl. Acad. Sci. USA 2006, 103, 19854–19859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maric, D.; Millward, J.M.; Ballok, D.A.; Szechtman, H.; Denburg, J.A.; Barker, J.L.; Sakić, B. Neurotoxic properties of cerebrospinal fluid from behaviorally impaired autoimmune mice. Brain Res. 2001, 920, 183–193. [Google Scholar] [CrossRef]
- Gharavi, A.E.; Mellors, R.C.; Elkon, K.B. IgG anti-cardiolipin antibodies in murine lupus. Clin. Exp. Immunol. 1989, 78, 233–238. [Google Scholar] [PubMed]
- Blatt, N.B.; Glick, G.D. Anti-DNA autoantibodies and systemic lupus erythematosus. Pharmacol. Ther. 1999, 83, 125–139. [Google Scholar] [CrossRef]
- Amoura, Z.; Chabre, H.; Koutouzov, S.; Lotton, C.; Cabrespines, A.; Bach, J.F.; Jacob, L. Nucleosome-restricted antibodies are detected before anti-dsDNA and/or antihistone antibodies in serum of MRL-Mp lpr/lpr and +/+ mice, and are present in kidney eluates of lupus mice with proteinuria. Arthritis Rheum. 1994, 37, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Elkon, K.B.; Parnassa, A.P.; Foster, C.L. Lupus autoantibodies target ribosomal P proteins. J. Exp. Med. 1985, 162, 459–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonfa, E.; Parnassa, A.P.; Rhoads, D.D.; Roufa, D.J.; Wool, I.G.; Elkon, K.B. Antiribosomal S10 antibodies in humans and MRL/lpr mice with systemic lupus erythematosus. Arthritis Rheum. 1989, 32, 1252–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, C.Y.; Wu, T.H.; Huang, S.F.; Sun, K.H.; Hsieh, S.C.; Han, S.H.; Yu, H.S.; Yu, C.L. Abnormal splenic and thymic IL-4 and TNF-alpha expression in MRL-lpr/lpr mice. Scand. J. Immunol. 1995, 41, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Holman, B.J.; Santoro, T.J. Aberrant cytokine gene expression in the hippocampus in murine systemic lupus erythematosus. Neurosci. Lett. 2001, 302, 129–132. [Google Scholar] [CrossRef]
- McHale, J.F.; Harari, O.A.; Marshall, D.; Haskard, D.O. TNF-alpha and IL-1 sequentially induce endothelial ICAM-1 and VCAM-1 expression in MRL/lpr lupus-prone mice. J. Immunol. 1999, 163, 3993–4000. [Google Scholar] [PubMed]
- Tomita, M.; Holman, B.J.; Williams, L.S.; Pang, K.C.; Santoro, T.J. Cerebellar dysfunction is associated with overexpression of proinflammatory cytokine genes in lupus. J. Neurosci. Res. 2001, 64, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.P.; Stott, D.I. Restoration of an early, progressive defect in responsiveness to T-cell activation in lupus mice by exogenous IL-2. Autoimmunity 1993, 15, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Simon-Stoos, K.; Puck, J.M. Hypo-active variant of IL-2 and associated decreased T cell activation contribute to impaired apoptosis in autoimmune prone MRL mice. Eur. J. Immunol. 2002, 32, 677–685. [Google Scholar] [CrossRef]
- Suzuki, H.; Yasukawa, K.; Saito, T.; Narazaki, M.; Hasegawa, A.; Taga, T.; Kishimoto, T. Serum soluble interleukin-6 receptor in MRL/lpr mice is elevated with age and mediates the interleukin-6 signal. Eur. J. Immunol. 1993, 23, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Matsuda, T.; Akira, S.; Nagata, N.; Ikehara, S.; Hirano, T.; Kishimoto, T. Age-associated increase in interleukin 6 in MRL/lpr mice. Int. Immunol. 1991, 3, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Cash, H.; Relle, M.; Menke, J.; Brochhausen, C.; Jones, S.A.; Topley, N.; Galle, P.R.; Schwarting, A. Interleukin 6 (IL-6) deficiency delays lupus nephritis in MRL-Faslpr mice: The IL-6 pathway as a new therapeutic target in treatment of autoimmune kidney disease in systemic lupus erythematosus. J. Rheumatol. 2010, 37, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Q.; Yang, X.; Li, M. Interleukin-9 is associated with elevated anti-double-stranded DNA antibodies in lupus-prone mice. Mol. Med. 2015, 21, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Bahtiyar, G.; Zhang, N.; Liu, L.; Zhu, P.; Robert, M.E.; McNiff, J.; Madaio, M.P.; Craft, J. IL-10 regulates murine lupus. J. Immunol. Baltim. Md 1950 2002, 169, 2148–2155. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Xiao, H.; Liu, X.; Zhu, G.; Yu, D.; Han, G.; Chen, G.; Hou, C.; Ma, N.; et al. Foxd3 suppresses interleukin-10 expression in B cells. Immunology 2017, 150, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.P.; Feng, G.J.; Lindop, G.; Stott, D.I.; Liew, F.Y. The role of interleukin 12 and nitric oxide in the development of spontaneous autoimmune disease in MRL/MP-lpr/lpr mice. J. Exp. Med. 1996, 183, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Li, T.; Zhang, K.; Wan, J.; Qi, X. CD4(+)B220(+)TCRγδ(+) T cells produce IL-17 in lupus-prone MRL/lpr mice. Int. Immunopharmacol. 2016, 38, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Favilli, F.; Anzilotti, C.; Martinelli, L.; Quattroni, P.; De Martino, S.; Pratesi, F.; Neumann, D.; Beermann, S.; Novick, D.; Dinarello, C.A.; et al. IL-18 activity in systemic lupus erythematosus. Ann. N. Y. Acad. Sci. 2009, 1173, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, E.; McInnes, I.B.; Lindop, G.; Huang, F.P.; Field, M.; Komai-Koma, M.; Wei, X.; Liew, F.Y. A proinflammatory role of IL-18 in the development of spontaneous autoimmune disease. J. Immunol. 2001, 167, 5338–5347. [Google Scholar] [CrossRef] [PubMed]
- Herber, D.; Brown, T.P.; Liang, S.; Young, D.A.; Collins, M.; Dunussi-Joannopoulos, K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J. Immunol. 2007, 178, 3822–3830. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Weng, Q.; Hu, L.; Yang, L.; Wang, X.; Xiang, X.; Hong, B.; Gong, X.; Wang, Q. Increased interleukin-22 levels in lupus nephritis and its associated with disease severity: A study in both patients and lupus-like mice model. Clin. Exp. Rheumatol. 2018. [Google Scholar]
- Yui, M.A.; Brissette, W.H.; Brennan, D.C.; Wuthrich, R.P.; Rubin-Kelley, V.E. Increased macrophage colony-stimulating factor in neonatal and adult autoimmune MRL-lpr mice. Am. J. Pathol. 1991, 139, 255–261. [Google Scholar] [PubMed]
- Leng, L.; Chen, L.; Fan, J.; Greven, D.; Arjona, A.; Du, X.; Austin, D.; Kashgarian, M.; Yin, Z.; Huang, X.R.; et al. A small-molecule macrophage migration inhibitory factor antagonist protects against glomerulonephritis in lupus-prone NZB/NZW F1 and MRL/lpr mice. J. Immunol. 2011, 186, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Santoro, T.J.; Benjamin, W.R.; Oppenheim, J.J.; Steinberg, A.D. The cellular basis for immune interferon production in autoimmune MRL-Ipr/Ipr mice. J. Immunol. 1983, 131, 265–268. [Google Scholar] [PubMed]
- Tomita, M.; Khan, R.L.; Blehm, B.H.; Santoro, T.J. The potential pathogenetic link between peripheral immune activation and the central innate immune response in neuropsychiatric systemic lupus erythematosus. Med. Hypotheses 2004, 62, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Ussin, L.; Cheng, X.; Murali, R.; Sullivan, K.E. TNFalpha inhibition in MRL/lpr mice ameliorates pulmonary but not renal disease. J. Autoimmun. 2002, 19, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Xia, Y.; Stock, A.; Michaelson, J.S.; Burkly, L.C.; Gulinello, M.; Putterman, C. Neuropsychiatric disease in murine lupus is dependent on the TWEAK/Fn14 pathway. J. Autoimmun. 2013, 43, 44–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dantzer, R. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. North Am. 2009, 29, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D. MRL/lpr lupus-prone mice show exaggerated ICAM-1-dependent leucocyte adhesion and transendothelial migration in response to TNF-. Rheumatology 2003, 42, 929–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballok, D.A.; Woulfe, J.; Sur, M.; Cyr, M.; Sakić, B. Hippocampal damage in mouse and human forms of systemic autoimmune disease. Hippocampus 2004, 14, 649–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banks, W.A.; Kastin, A.J.; Gutierrez, E.G. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci. Lett. 1994, 179, 53–56. [Google Scholar] [CrossRef]
- Brey, R.L.; Amato, A.A.; Kagan-Hallet, K.; Rhine, C.B.; Stallworth, C.L. Anti-Intercellular Adhesion Molecule-1 (ICAM-1) antibody treatment prevents central and peripheral nervous system disease in autoimmune-prone mice. Lupus 1997, 6, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Bernhagen, J.; Calandra, T.; Mitchell, R.A.; Martin, S.B.; Tracey, K.J.; Voelter, W.; Manogue, K.R.; Cerami, A.; Bucala, R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature 1993, 365, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Bernhagen, J.; Bacher, M.; Calandra, T.; Metz, C.N.; Doty, S.B.; Donnelly, T.; Bucala, R. An essential role for macrophage migration inhibitory factor in the tuberculin delayed-type hypersensitivity reaction. J. Exp. Med. 1996, 183, 277–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoi, A.Y.; Morand, E.F.; Leech, M. Is macrophage migration inhibitory factor a therapeutic target in systemic lupus erythematosus? Immunol. Cell Biol. 2003, 81, 367–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishibori, M.; Nakaya, N.; Tahara, A.; Kawabata, M.; Mori, S.; Saeki, K. Presence of macrophage migration inhibitory factor (MIF) in ependyma, astrocytes and neurons in the bovine brain. Neurosci. Lett. 1996, 213, 193–196. [Google Scholar] [CrossRef]
- Bacher, M.; Meinhardt, A.; Lan, H.Y.; Dhabhar, F.S.; Mu, W.; Metz, C.N.; Chesney, J.A.; Gemsa, D.; Donnelly, T.; Atkins, R.C.; et al. MIF expression in the rat brain: Implications for neuronal function. Mol. Med. Camb. Mass 1998, 4, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Fagone, P.; Mazzon, E.; Cavalli, E.; Bramanti, A.; Petralia, M.C.; Mangano, K.; Al-Abed, Y.; Bramati, P.; Nicoletti, F. Contribution of the macrophage migration inhibitory factor superfamily of cytokines in the pathogenesis of preclinical and human multiple sclerosis: In silico and in vivo evidences. J. Neuroimmunol. 2018, 322, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Créange, A.; Orlikowski, D.; Bolgert, F.; Mangano, K.; Metz, C.; Di Marco, R.; Al Abed, Y. Macrophage migration inhibitory factor (MIF) seems crucially involved in Guillain-Barré syndrome and experimental allergic neuritis. J. Neuroimmunol. 2005, 168, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Benedek, G.; Meza-Romero, R.; Jordan, K.; Zhang, Y.; Nguyen, H.; Kent, G.; Li, J.; Siu, E.; Frazer, J.; Piecychna, M.; et al. MIF and D-DT are potential disease severity modifiers in male MS subjects. Proc. Natl. Acad. Sci. USA 2017, 114, E8421–E8429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bay-Richter, C.; Janelidze, S.; Sauro, A.; Bucala, R.; Lipton, J.; Deierborg, T.; Brundin, L. Behavioural and neurobiological consequences of macrophage migration inhibitory factor gene deletion in mice. J. Neuroinflammation 2015, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.; Al-Abed, Y. MIF: Mood improving/inhibiting factor? J. Neuroinflammation 2014, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Foote, A.; Lee, J.P.W.; Morand, E.F.; Harris, J. MIF: Implications in the pathoetiology of systemic lupus erythematosus. Front. Immunol. 2015, 6, 577. [Google Scholar] [CrossRef] [PubMed]
- Hoi, A.Y.; Hickey, M.J.; Hall, P.; Yamana, J.; O’Sullivan, K.M.; Santos, L.L.; James, W.G.; Kitching, A.R.; Morand, E.F. Macrophage migration inhibitory factor deficiency attenuates macrophage recruitment, glomerulonephritis, and lethality in MRL/lpr mice. J. Immunol. Baltim. Md 1950 2006, 177, 5687–5696. [Google Scholar] [CrossRef]
- Bucala, R. Role of MIF gene polymorphisms in systemic lupus erythematosus and prospects for therapeutic intervention. Arthritis Res. Ther. 2012, 14, A33. [Google Scholar] [CrossRef]
- Feng, X.; Chen, W.; Xiao, L.; Gu, F.; Huang, J.; Tsao, B.P.; Sun, L. Artesunate inhibits type I interferon induced production of macrophage migration inhibitory factor in patients with systemic lupus erythematosus. Lupus 2017, 26, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.C.; Lee, Y.H. Circulating macrophage migration inhibitory factor levels and its polymorphisms in systemic lupus erythematosus: A meta-analysis. Cell. Mol. Biol. 2017, 63, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Lapter, S.; Marom, A.; Meshorer, A.; Elmann, A.; Sharabi, A.; Vadai, E.; Neufeld, A.; Sztainberg, Y.; Gil, S.; Getselter, D.; et al. Amelioration of brain pathology and behavioral dysfunction in mice with lupus following treatment with a tolerogenic peptide. Arthritis Rheum. 2009, 60, 3744–3754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapter, S.; Ben-David, H.; Sharabi, A.; Zinger, H.; Telerman, A.; Gordin, M.; Leng, L.; Bucala, R.; Shachar, I.; Mozes, E. A role for the B-cell CD74/macrophage migration inhibitory factor pathway in the immunomodulation of systemic lupus erythematosus by a therapeutic tolerogenic peptide. Immunology 2011, 132, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louveau, A.; Harris, T.H.; Kipnis, J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 2015, 36, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, B.; Vajkoczy, P.; Weller, R.O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 2017, 18, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Foster, J.; Sakić, B. Distribution and prevalence of leukocyte phenotypes in brains of lupus-prone mice. J. Neuroimmunol. 2006, 179, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Morawski, P.A.; Qi, C.-F.; Bolland, S. Non-pathogenic tissue-resident CD8+ T cells uniquely accumulate in the brains of lupus-prone mice. Sci. Rep. 2017, 7, 40838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Lee, K.; Diamond, B. Follicular helper T cells in systemic lupus erythematosus. Front. Immunol. 2018, 9, 1793. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, F.X.; Vogelweid, C.M.; Besch-Williford, C.L.; Walker, S.E. Differential effects of CD4+ T cell depletion on inflammatory central nervous system disease, arthritis and sialadenitis in MRL/lpr mice. J. Autoimmun. 1995, 8, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Ulivieri, C.; Baldari, C.T. Regulation of T cell activation and differentiation by extracellular vesicles and their pathogenic role in systemic lupus erythematosus and multiple sclerosis. Molecules 2017, 22, 225. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Doerner, J.; Weidenheim, K.; Xia, Y.; Stock, A.; Michaelson, J.S.; Baruch, K.; Deczkowska, A.; Gulinello, M.; Schwartz, M.; et al. TNF-like weak inducer of apoptosis promotes blood brain barrier disruption and increases neuronal cell death in MRL/lpr mice. J. Autoimmun. 2015, 60, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bódi, N.; Polgár, A.; Kiss, E.; Mester, Á.; Poór, G.; Kéri, S. Reduced volumes of the CA1 and CA4-dentate gyrus hippocampal subfields in systemic lupus erythematosus. Lupus 2017, 26, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Barcellini, W.; Rizzardi, G.P.; Borghi, M.O.; Nicoletti, F.; Fain, C.; Del Papa, N.; Meroni, P.L. In vitro type-1 and type-2 cytokine production in systemic lupus erythematosus: Lack of relationship with clinical disease activity. Lupus 1996, 5, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Song, H.; Gage, F.H. Neurogenesis in the adult hippocampus. Cold Spring Harb. Perspect. Biol. 2015, 7, a018812. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Jinnou, H.; Sawamoto, K.; Hitoshi, S. Adult neurogenesis and its role in brain injury and psychiatric diseases. J. Neurochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, M.E.; Dombeck, D.A. Dendritic mechanisms of hippocampal place field formation. Curr. Opin. Neurobiol. 2018, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stanojcic, M.; Burstyn-Cohen, T.; Nashi, N.; Lemke, G.; Sakić, B. Disturbed distribution of proliferative brain cells during lupus-like disease. Brain. Behav. Immun. 2009, 23, 1003–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakić, B.; Kirkham, D.L.; Ballok, D.A.; Mwanjewe, J.; Fearon, I.M.; Macri, J.; Yu, G.; Sidor, M.M.; Denburg, J.A.; Szechtman, H.; et al. Proliferating brain cells are a target of neurotoxic CSF in systemic autoimmune disease. J. Neuroimmunol. 2005, 169, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, A.; Sandler, V.M.; Toni, N.; Zhao, C.; Gage, F.H. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature 2006, 442, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Faust, T.W.; Chang, E.H.; Kowal, C.; Berlin, R.; Gazaryan, I.G.; Bertini, E.; Zhang, J.; Sanchez-Guerrero, J.; Fragoso-Loyo, H.E.; Volpe, B.T.; et al. Neurotoxic lupus autoantibodies alter brain function through two distinct mechanisms. Proc. Natl. Acad. Sci. USA 2010, 107, 18569–18574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alawieh, A.; Elvington, A.; Tomlinson, S. Complement in the homeostatic and ischemic brain. Front. Immunol. 2015, 6, 417. [Google Scholar] [CrossRef] [PubMed]
- Presumey, J.; Bialas, A.R.; Carroll, M.C. Complement system in neural synapse elimination in development and disease. Adv. Immunol. 2017, 135, 53–79. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.J.; Jacob, A.; Bao, L.; Macdonald, R.L.; Quigg, R.J. Complement-dependent apoptosis and inflammatory gene changes in murine lupus cerebritis. J. Immunol. 2005, 175, 8312–8319. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Dissing-Olesen, L.; Stevens, B. New insights on the role of microglia in synaptic pruning in health and disease. Curr. Opin. Neurobiol. 2016, 36, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Olsen, R.H.J.; Sun, J.; Ming, G.-L.; Song, H. Neuronal circuitry mechanisms regulating adult mammalian neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018937. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, O.J.; McGuirt, A.F.; Tang, G.; Sulzer, D. Roles for neuronal and glial autophagy in synaptic pruning during development. Neurobiol. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.J.; Bao, L.; Jacob, A.; Kraus, D.M.; Holers, V.M.; Quigg, R.J. Administration of the soluble complement inhibitor, Crry-Ig, reduces inflammation and aquaporin 4 expression in lupus cerebritis. Biochim. Biophys. Acta 2003, 1639, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telerman, A.; Lapter, S.; Sharabi, A.; Zinger, H.; Mozes, E. Induction of hippocampal neurogenesis by a tolerogenic peptide that ameliorates lupus manifestations. J. Neuroimmunol. 2011, 232, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Huerta, P.T.; Zhang, J.; Kowal, C.; Bertini, E.; Volpe, B.T.; Diamond, B. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat. Med. 2009, 15, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Loheswaran, G.; Kapadia, M.; Gladman, M.; Pulapaka, S.; Xu, L.; Stanojcic, M.; Sakić, B. Altered neuroendocrine status at the onset of CNS lupus-like disease. Brain. Behav. Immun. 2013, 32, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Ballok, D.A.; Millward, J.M.; Sakić, B. Neurodegeneration in autoimmune MRL-lpr mice as revealed by Fluoro Jade B staining. Brain Res. 2003, 964, 200–210. [Google Scholar] [CrossRef]
- Silverman, M.N.; Pearce, B.D.; Biron, C.A.; Miller, A.H. Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol. 2005, 18, 41–78. [Google Scholar] [CrossRef] [PubMed]
- Sakić, B.; Maric, I.; Koeberle, P.D.; Millward, J.M.; Szechtman, H.; Maric, D.; Denburg, J.A. Increased TUNEL staining in brains of autoimmune Fas-deficient mice. J. Neuroimmunol. 2000, 104, 147–154. [Google Scholar] [CrossRef]
- Sakić, B.; Szechtman, H.; Denburg, J.A.; Gorny, G.; Kolb, B.; Whishaw, I.Q. Progressive atrophy of pyramidal neuron dendrites in autoimmune MRL-lpr mice. J. Neuroimmunol. 1998, 87, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Verma, M.; Callio, J.; Otero, P.A.; Sekler, I.; Wills, Z.P.; Chu, C.T. Mitochondrial calcium dysregulation contributes to dendrite degeneration mediated by PD/LBD-associated LRRK2 mutants. J. Neurosci. 2017, 37, 11151–11165. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Wills, Z.; Chu, C.T. Excitatory dendritic mitochondrial calcium toxicity: Implications for Parkinson’s and other neurodegenerative diseases. Front. Neurosci. 2018, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Arcuri, C.; Mecca, C.; Bianchi, R.; Giambanco, I.; Donato, R. The pathophysiological role of microglia in dynamic surveillance, phagocytosis and structural remodeling of the developing CNS. Front. Mol. Neurosci. 2017, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.J.; Jacob, A.; Vezina, P.; Sekine, H.; Gilkeson, G.S.; Quigg, R.J. Absence of functional alternative complement pathway alleviates lupus cerebritis. Eur. J. Immunol. 2007, 37, 1691–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, A.; Hack, B.; Chiang, E.; Garcia, J.G.N.; Quigg, R.J.; Alexander, J.J. C5a alters blood-brain barrier integrity in experimental lupus. FASEB J. 2010, 24, 1682–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogelweid, C.M.; Johnson, G.C.; Besch-Williford, C.L.; Basler, J.; Walker, S.E. Inflammatory central nervous system disease in lupus-prone MRL/lpr mice: Comparative histologic and immunohistochemical findings. J. Neuroimmunol. 1991, 35, 89–99. [Google Scholar] [CrossRef]
- Mahajan, S.D.; Tutino, V.M.; Redae, Y.; Meng, H.; Siddiqui, A.; Woodruff, T.M.; Jarvis, J.N.; Hennon, T.; Schwartz, S.; Quigg, R.J.; et al. C5a induces caspase-dependent apoptosis in brain vascular endothelial cells in experimental lupus. Immunology 2016, 148, 407–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, J.; Chen, C.H.; Stock, A.; Doerner, J.; Gulinello, M.; Putterman, C. Intracerebroventricular administration of TNF-like weak inducer of apoptosis induces depression-like behavior and cognitive dysfunction in non-autoimmune mice. Brain. Behav. Immun. 2016, 54, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, N. Role of T follicular helper cells in multiple sclerosis. J. Nat. Sci. 2015, 1, e139. [Google Scholar] [PubMed]
- Stern, J.N.H.; Yaari, G.; Vander Heiden, J.A.; Church, G.; Donahue, W.F.; Hintzen, R.Q.; Huttner, A.J.; Laman, J.D.; Nagra, R.M.; Nylander, A.; et al. B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Sci. Transl. Med. 2014, 6, 248ra107. [Google Scholar] [CrossRef] [PubMed]
- Stock, A.D.; Wen, J.; Doerner, J.; Herlitz, L.C.; Gulinello, M.; Putterman, C. Neuropsychiatric systemic lupus erythematosus persists despite attenuation of systemic disease in MRL/lpr mice. J. Neuroinflammation 2015, 12, 205. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Doerner, J.; Chalmers, S.; Stock, A.; Wang, H.; Gullinello, M.; Shlomchik, M.J.; Putterman, C. B cell and/or autoantibody deficiency do not prevent neuropsychiatric disease in murine systemic lupus erythematosus. J. Neuroinflammation 2016, 13, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marmont du Haut Champ, A.M. Hematopoietic stem cell transplantation for systemic lupus erythematosus. Clin. Dev. Immunol. 2012, 2012, 380391. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Radin, M.; Almarzooqi, A.M.; Al-Saleh, J.; Roccatello, D.; Sciascia, S.; Khamashta, M. Autologous hematopoietic stem cell transplantation in systemic lupus erythematosus and antiphospholipid syndrome: A systematic review. Autoimmun. Rev. 2017, 16, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Lisukov, I.A.; Sizikova, S.A.; Kulagin, A.D.; Kruchkova, I.V.; Gilevich, A.V.; Konenkova, L.P.; Zonova, E.V.; Chernykh, E.R.; Leplina, O.Y.; Sentyakova, T.N.; et al. High-dose immunosuppression with autologous stem cell transplantation in severe refractory systemic lupus erythematosus. Lupus 2004, 13, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Lehnhardt, F.G.; Scheid, C.; Holtik, U.; Burghaus, L.; Neveling, M.; Impekoven, P.; Rüger, A.; Hallek, M.; Jacobs, A.H.; Rubbert, A. Autologous blood stem cell transplantation in refractory systemic lupus erythematodes with recurrent longitudinal myelitis and cerebral infarction. Lupus 2006, 15, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Sakata, K.; Nakayamada, S.; Miyazaki, Y.; Kubo, S.; Ishii, A.; Nakano, K.; Tanaka, Y. Up-regulation of TLR7-mediated IFN-α production by plasmacytoid dendritic cells in patients with systemic lupus erythematosus. Front. Immunol. 2018, 9, 1957. [Google Scholar] [CrossRef] [PubMed]
- Pérez de Lema, G.; Maier, H.; Nieto, E.; Vielhauer, V.; Luckow, B.; Mampaso, F.; Schlöndorff, D. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J. Am. Soc. Nephrol. 2001, 12, 1369–1382. [Google Scholar] [PubMed]
- Sakić, B.; Kolb, B.; Whishaw, I.Q.; Gorny, G.; Szechtman, H.; Denburg, J.A. Immunosuppression prevents neuronal atrophy in lupus-prone mice: Evidence for brain damage induced by autoimmune disease? J. Neuroimmunol. 2000, 111, 93–101. [Google Scholar] [CrossRef]
- Chalmers, S.A.; Wen, J.; Doerner, J.; Stock, A.; Cuda, C.M.; Makinde, H.M.; Perlman, H.; Bosanac, T.; Webb, D.; Nabozny, G.; et al. Highly selective inhibition of Bruton’s tyrosine kinase attenuates skin and brain disease in murine lupus. Arthritis Res. Ther. 2018, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Magro-Checa, C.; Zirkzee, E.J.; Huizinga, T.W.; Steup-Beekman, G.M. Management of Neuropsychiatric Systemic Lupus Erythematosus: Current Approaches and Future Perspectives. Drugs 2016, 76, 459–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertsias, G.K.; Ioannidis, J.P.A.; Aringer, M.; Bollen, E.; Bombardieri, S.; Bruce, I.N.; Cervera, R.; Dalakas, M.; Doria, A.; Hanly, J.G.; et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: Report of a task force of the EULAR standing committee for clinical affairs. Ann. Rheum. Dis. 2010, 69, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
- Fanouriakis, A.; Boumpas, D.T.; Bertsias, G.K. Pathogenesis and treatment of CNS lupus. Curr. Opin. Rheumatol. 2013, 25, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Pamfil, C.; Fanouriakis, A.; Damian, L.; Rinzis, M.; Sidiropoulos, P.; Tsivgoulis, G.; Rednic, S.; Bertsias, G.; Boumpas, D.T. EULAR recommendations for neuropsychiatric systemic lupus erythematosus vs. usual care: Results from two European centres. Rheumatology 2015, 54, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, S.A.; Wen, J.; Shum, J.; Doerner, J.; Herlitz, L.; Putterman, C. CSF-1R inhibition attenuates renal and neuropsychiatric disease in murine lupus. Clin. Immunol. 2017, 185, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Tian, T.; Yao, S.; Cao, K.; Zhu, X.; Zhang, M.; Wen, S.; Li, L.; Shi, M.; Zhou, H. FTY720 attenuates behavioral deficits in a murine model of systemic lupus erythematosus. Brain. Behav. Immun. 2018, 70, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Mike, E.V.; Makinde, H.M.; Der, E.; Stock, A.; Gulinello, M.; Gadhvi, G.T.; Winter, D.R.; Cuda, C.M.; Putterman, C. Neuropsychiatric systemic lupus erythematosus is dependent on sphingosine-1-phosphate signaling. Front. Immunol. 2018, 9, 2189. [Google Scholar] [CrossRef] [PubMed]
- Ravanan, P.; Srikumar, I.F.; Talwar, P. Autophagy: The spotlight for cellular stress responses. Life Sci. 2017, 188, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Anding, A.L.; Baehrecke, E.H. Cleaning house: Selective autophagy of organelles. Dev. Cell 2017, 41, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Cuervo, A.M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Mijaljica, D.; Prescott, M.; Devenish, R.J. Microautophagy in mammalian cells: Revisiting a 40-year-old conundrum. Autophagy 2011, 7, 673–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuervo, A.M.; Wong, E. Chaperone-mediated autophagy: Roles in disease and aging. Cell Res. 2014, 24, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Sanchez-Lopez, E.; Karin, M. Autophagy, NLRP3 inflammasome and auto-inflammatory/immune diseases. Clin. Exp. Rheumatol. 2016, 34, 12–16. [Google Scholar] [PubMed]
- Qian, M.; Fang, X.; Wang, X. Autophagy and inflammation. Clin. Transl. Med. 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Huang, M.; Yao, Y. Autophagy and proinflammatory cytokines: Interactions and clinical implications. Cytokine Growth Factor Rev. 2018, 43, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Cunha, L.D.; Park, S.; Yang, M.; Lu, Q.; Orchard, R.; Li, Q.-Z.; Yan, M.; Janke, L.; Guy, C.; et al. Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature 2016, 533, 115–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alirezaei, M.; Fox, H.S.; Flynn, C.T.; Moore, C.S.; Hebb, A.L.O.; Frausto, R.F.; Bhan, V.; Kiosses, W.B.; Whitton, J.L.; Robertson, G.S.; et al. Elevated ATG5 expression in autoimmune demyelination and multiple sclerosis. Autophagy 2009, 5, 152–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, A.; Parillon, X.; Zeng, S.; Han, S.; Eissa, N.T. Deficiency of autophagy in dendritic cells protects against experimental autoimmune encephalomyelitis. J. Biol. Chem. 2014, 289, 26525–26532. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Le, W. Role of autophagy in the pathogenesis of multiple sclerosis. Neurosci. Bull. 2015, 31, 435–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patergnani, S.; Castellazzi, M.; Bonora, M.; Marchi, S.; Casetta, I.; Pugliatti, M.; Giorgi, C.; Granieri, E.; Pinton, P. Autophagy and mitophagy elements are increased in body fluids of multiple sclerosis-affected individuals. J. Neurol. Neurosurg. Psychiatry 2018, 89, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Fagone, P.; Meroni, P.; McCubrey, J.; Bendtzen, K. mTOR as a multifunctional therapeutic target in HIV infection. Drug Discov. Today 2011, 16, 715–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donia, M.; Mangano, K.; Amoroso, A.; Mazzarino, M.C.; Imbesi, R.; Castrogiovanni, P.; Coco, M.; Meroni, P.; Nicoletti, F. Treatment with rapamycin ameliorates clinical and histological signs of protracted relapsing experimental allergic encephalomyelitis in Dark Agouti rats and induces expansion of peripheral CD4+CD25+Foxp3+ regulatory T cells. J. Autoimmun. 2009, 33, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Mammana, S.; Bramanti, P.; Mazzon, E.; Cavalli, E.; Basile, M.S.; Fagone, P.; Petralia, M.C.; McCubrey, J.A.; Nicoletti, F.; Mangano, K. Preclinical evaluation of the PI3K/Akt/mTOR pathway in animal models of multiple sclerosis. Oncotarget 2018, 9, 8263–8277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, L.M.; Adams, L.M.; Sehgal, S.N. Rapamycin prolongs survival and arrests pathophysiologic changes in murine systemic lupus erythematosus. Arthritis Rheum. 1994, 37, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D.; Bonilla, E.; Mirza, N.; Niland, B.; Perl, A. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 2006, 54, 2983–2988. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.-W.; Kelly, R.; Winans, T.; Marchena, I.; Shadakshari, A.; Yu, J.; Dawood, M.; Garcia, R.; Tily, H.; Francis, L.; et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: A single-arm, open-label, phase 1/2 trial. Lancet 2018, 391, 1186–1196. [Google Scholar] [CrossRef]
- Su, P.; Zhang, J.; Wang, D.; Zhao, F.; Cao, Z.; Aschner, M.; Luo, W. The role of autophagy in modulation of neuroinflammation in microglia. Neuroscience 2016, 319, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Gudsnuk, K.; Kuo, S.-H.; Cotrina, M.L.; Rosoklija, G.; Sosunov, A.; Sonders, M.S.; Kanter, E.; Castagna, C.; Yamamoto, A.; et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 2014, 83, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Cho, M.-H.; Shim, W.H.; Kim, J.K.; Jeon, E.-Y.; Kim, D.-H.; Yoon, S.-Y. Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol. Psychiatry 2017, 22, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, B.L.; Boada-Romero, E.; Cunha, L.D.; Magne, J.; Green, D.R. LC3-associated phagocytosis and inflammation. J. Mol. Biol. 2017, 429, 3561–3576. [Google Scholar] [CrossRef] [PubMed]
- Monaco, A.; Ferrandino, I.; Boscaino, F.; Cocca, E.; Cigliano, L.; Maurano, F.; Luongo, D.; Spagnuolo, M.S.; Rossi, M.; Bergamo, P. Conjugated linoleic acid prevents age-dependent neurodegeneration in a mouse model of neuropsychiatric lupus via the activation of an adaptive response. J. Lipid Res. 2018, 59, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, C.; Jiang, N.; He, D.; Bai, Y.; Xin, Y. Rapamycin combined with MCC950 to treat multiple sclerosis in experimental autoimmune encephalomyelitis. J. Cell. Biochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, S.; Pan, R.; Li, G.; Tang, H.; Jiang, M.; Xing, Y.; Jin, F.; Lin, L.; Dong, J. Curcumin Attenuates gp120-Induced Microglial Inflammation by Inhibiting Autophagy via the PI3K Pathway. Cell. Mol. Neurobiol. 2018, 38, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, F.; Schall, N.; Muller, S. Rescue of autophagy and lysosome defects in salivary glands of MRL/lpr mice by a therapeutic phosphopeptide. J. Autoimmun. 2018, 90, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Wang, F.; Schall, N.; Kleinmann, J.-F.; Faludi, M.; Nashi, E.P.; Sibilia, J.; Martin, T.; Schaeffer, E.; Muller, S. Lupus regulator peptide P140 represses B-cell differentiation by reducing HLA class II molecule overexpression. Arthritis Rheumatol. 2018, 70, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Macri, C.; Wang, F.; Tasset, I.; Schall, N.; Page, N.; Briand, J.-P.; Cuervo, A.M.; Muller, S. Modulation of deregulated chaperone-mediated autophagy by a phosphopeptide. Autophagy 2015, 11, 472–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monneaux, F.; Lozano, J.M.; Patarroyo, M.E.; Briand, J.-P.; Muller, S. T cell recognition and therapeutic effect of a phosphorylated synthetic peptide of the 70K snRNP protein administered in MR/lpr mice. Eur. J. Immunol. 2003, 33, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, R.; Scherbarth, H.R.; Rillo, O.L.; Gomez-Reino, J.J.; Muller, S. Lupuzor/P140 peptide in patients with systemic lupus erythematosus: A randomised, double-blind, placebo-controlled phase IIb clinical trial. Ann. Rheum. Dis. 2013, 72, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Deczkowska, A.; Schwartz, M. Targeting neuro–immune communication in neurodegeneration: Challenges and opportunities. J. Exp. Med. 2018, 215, 2702–2704. [Google Scholar] [CrossRef] [PubMed]
- Garbuzova-Davis, S.; Ehrhart, J.; Sanberg, P.R.; Borlongan, C.V. Potential role of humoral IL-6 cytokine in mediating pro-inflammatory endothelial cell response in amyotrophic lateral sclerosis. Int. J. Mol. Sci. 2018, 19, 423. [Google Scholar] [CrossRef] [PubMed]
- Sankowski, R.; Mader, S.; Valdés-Ferrer, S.I. Systemic inflammation and the brain: Novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front. Cell. Neurosci. 2015, 9, 28. [Google Scholar] [CrossRef] [PubMed]




| Central Nervous System | Peripheral Nervous System | |
|---|---|---|
| Focal manifestations | Diffuse manifestations | |
| Cerebrovascular disease | Depression | Cranial neuropathy |
| Seizures | Cognitive dysfunction | Autonomic neuropathy |
| Aseptic meningitis | Mood and anxiety disorders 1 | Mononeuropathy (single/multiplex) |
| Movement disorder | Psychosis | Polyneuropathy |
| Myelopathy | Acute confusional state | Plexopathy |
| Demyelinating syndrome | Headaches 1 | Myasthenia gravis |
| Acute inflammatory demyelinating polyradiculoneuropathy (Guillain-Barré syndrome) | ||
| Hallmarks | Location | Levels/Expression | References |
|---|---|---|---|
| AutoAbs | |||
| aPL (e.g., anticardiolipin) | Serum | Increased levels | [122] |
| Anti-dsDNA | Serum | Increased levels | [123] |
| Anti-nucleosome * | Serum | Increased levels | [124] |
| Anti-ribosomal P protein | Serum | Increased levels | [125] |
| Anti-Sm | Serum | Increased levels | [125] |
| Anti-ribosomal S10 | Serum | Increased levels | [126] |
| Anti-NMDAR | Serum | Increased levels | [57,66] |
| Cytokines | |||
| IL-1β | Serum CNS | Increased levels | [127,128,129,130] |
| IL-2 | T cells | Decreased expression | [131,132] |
| IL-6 | Serum CSF | Increased levels | [127,133,134,135] |
| IL-9 | Serum | Increased levels | [128,130,136] |
| IL-10 | B cells CNS | Dysregulation | [128,130,137,138] |
| IL-12 | Serum | Increased levels | [139] |
| IL-17 | Serum | Increased levels | [140] |
| IL-18 | Serum | Increased levels | [141,142] |
| IL-21 | Serum | Dysregulation | [143] |
| IL-22 | Serum | Increased levels | [144] |
| M-CSF | Serum | Increased levels | [145] |
| MIF | Serum | Increased levels | [146] |
| IFNγ | Splenocytes CNS | Dysregulation | [128,147,148] |
| TNFα | Serum | Increased levels | [127,129,149] |
| TWEAK | CNS | Tendency to increase | [150] |
| Receptors | |||
| sIL-6R | Serum | Increased levels | [133] |
| Fn14 (TWEAK receptor) | CNS | Increased levels | [150] |
| Therapeutic Molecule | Molecular Target | Neuroinflammatory Process | Reference |
|---|---|---|---|
| Crry-Ig | C3 convertase | Complement deposition Apoptosis Neurodegeneration Cytokine production Adhesion molecule expression | [193,197] |
| Anti-ICAM-1 Ab | ICAM-1 | Sciatic nerve conductivity | [155] |
| Anti-CD4 Ab | CD4+ T cells | Immune cell infiltration Inflammation of the CP | [179] |
| Cyclophosphamide | Immune cell dsDNA | Synaptic pruning | [223] |
| BI-BTK-1 | BTK | Immune cell infiltration | [224] |
| GW2580 | CSF-1R | Cytokine expression | [229] |
| PLX5622 | CSF-1R | Synaptic pruning | [44] |
| Captopril | ACE | Microglial activation Synaptic pruning | [44] |
| hCDR1 peptide/Edratide | Anti-dsDNA Ab | Inhibition of neurogenesis Complement deposition Immune cell infiltration Neurodegeneration | [171,198] |
| FTY720/Fingolimod (Gilenya) | S1P receptor | BBB leakage Immune cell infiltration Cytokine production Neurodegeneration | [230,231] |
| P140 peptide/Lupuzor | HSPA8 | CMA regulation | [85] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendorius, M.; Po, C.; Muller, S.; Jeltsch-David, H. From Systemic Inflammation to Neuroinflammation: The Case of Neurolupus. Int. J. Mol. Sci. 2018, 19, 3588. https://doi.org/10.3390/ijms19113588
Bendorius M, Po C, Muller S, Jeltsch-David H. From Systemic Inflammation to Neuroinflammation: The Case of Neurolupus. International Journal of Molecular Sciences. 2018; 19(11):3588. https://doi.org/10.3390/ijms19113588
Chicago/Turabian StyleBendorius, Mykolas, Chrystelle Po, Sylviane Muller, and Hélène Jeltsch-David. 2018. "From Systemic Inflammation to Neuroinflammation: The Case of Neurolupus" International Journal of Molecular Sciences 19, no. 11: 3588. https://doi.org/10.3390/ijms19113588
APA StyleBendorius, M., Po, C., Muller, S., & Jeltsch-David, H. (2018). From Systemic Inflammation to Neuroinflammation: The Case of Neurolupus. International Journal of Molecular Sciences, 19(11), 3588. https://doi.org/10.3390/ijms19113588