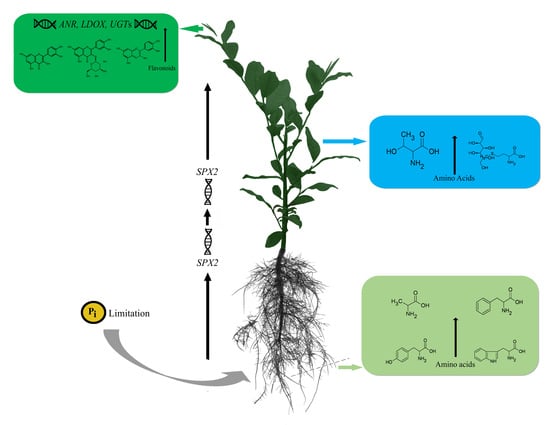
Metabolic Changes of Amino Acids and Flavonoids in Tea Plants in Response to Inorganic Phosphate Limitation
Abstract
:1. Introduction
2. Results
2.1. Biomass and Pi Concentrations in Plants
2.2. Overview of Metabolomics Analysis of Tea Plants under −P and +P Conditions
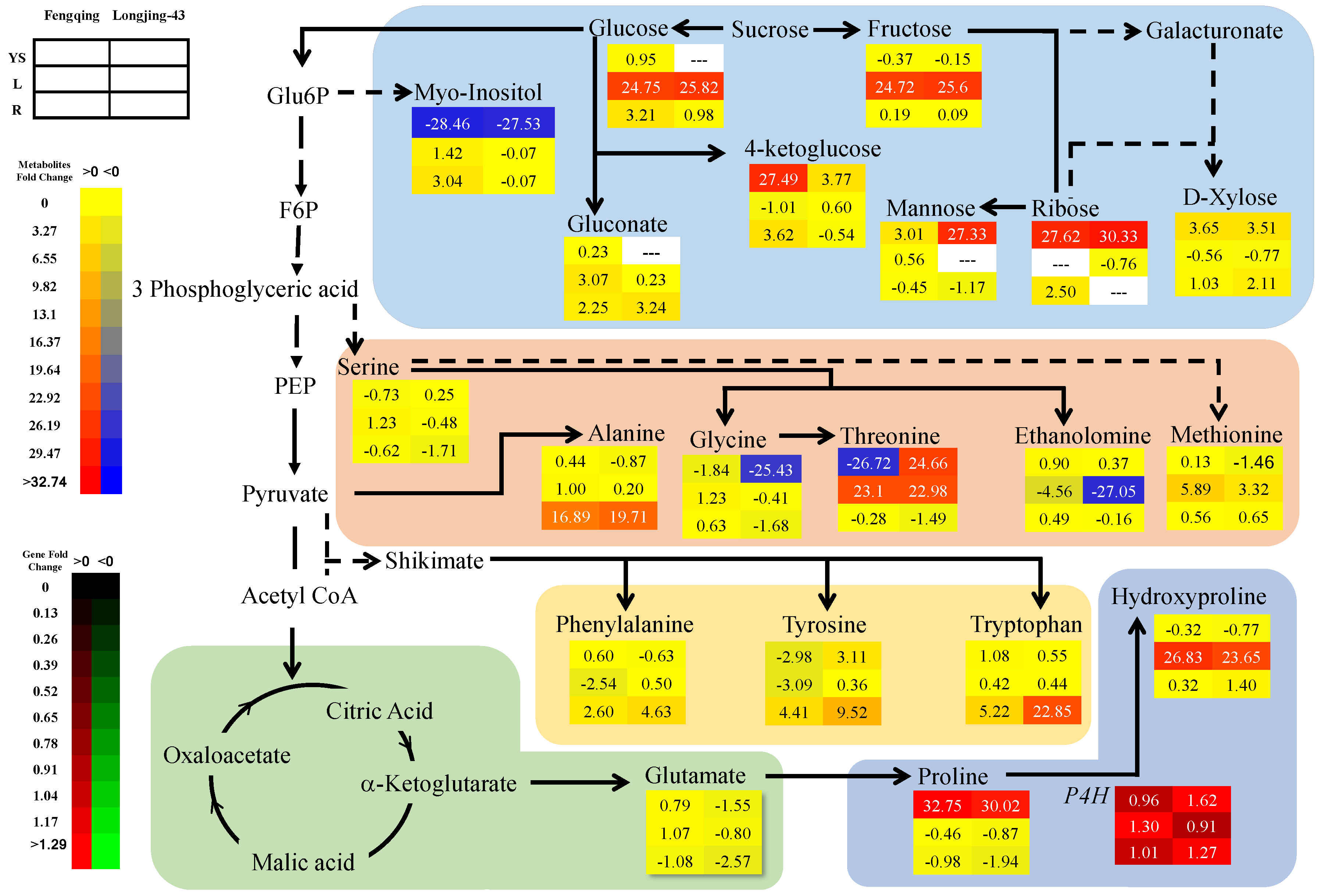
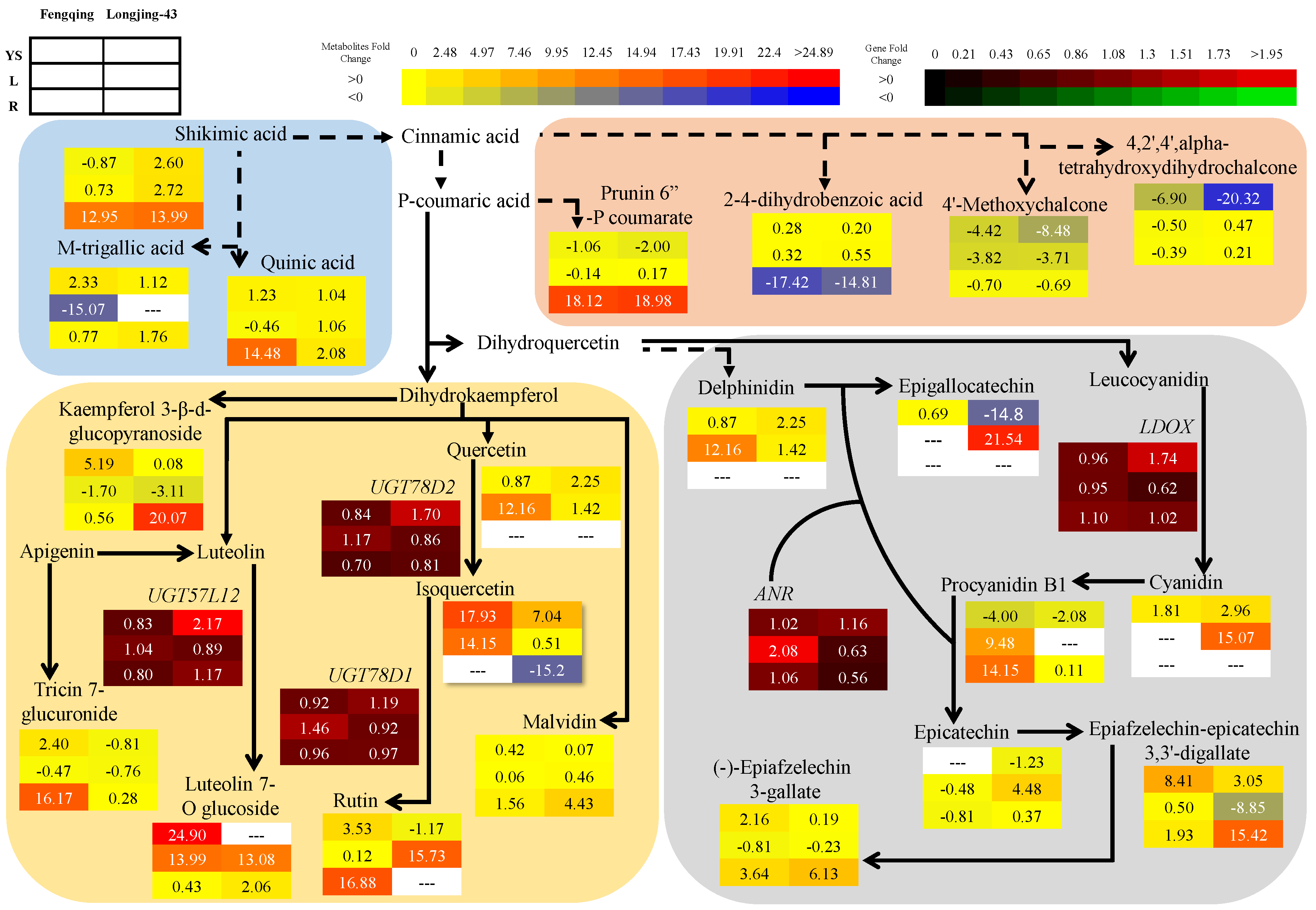
2.3. Metabolomics Changes in Primary and Secondary Metabolites
2.3.1. Leaves
2.3.2. Root
2.3.3. Young Shoots
2.4. Targeted Analysis of Amino Acids and Catechins in Young Shoots
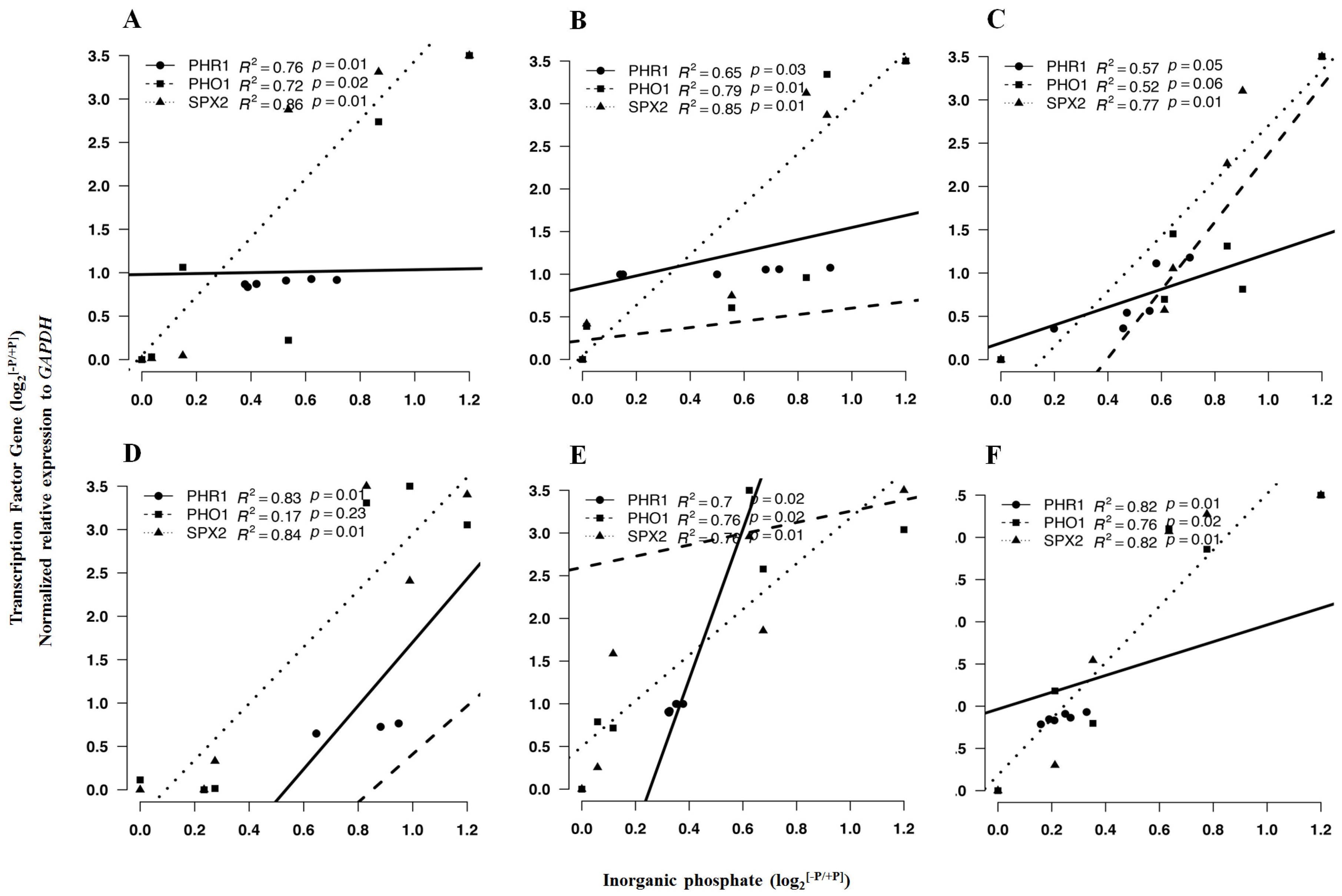
2.5. Response of Pi-Dependent Transcription Factors and Metabolic Genes to P Conditions
2.5.1. Pi-Dependent Gene Transcriptional Expression
2.5.2. Expression of Selected Genes in Response to P Limitation
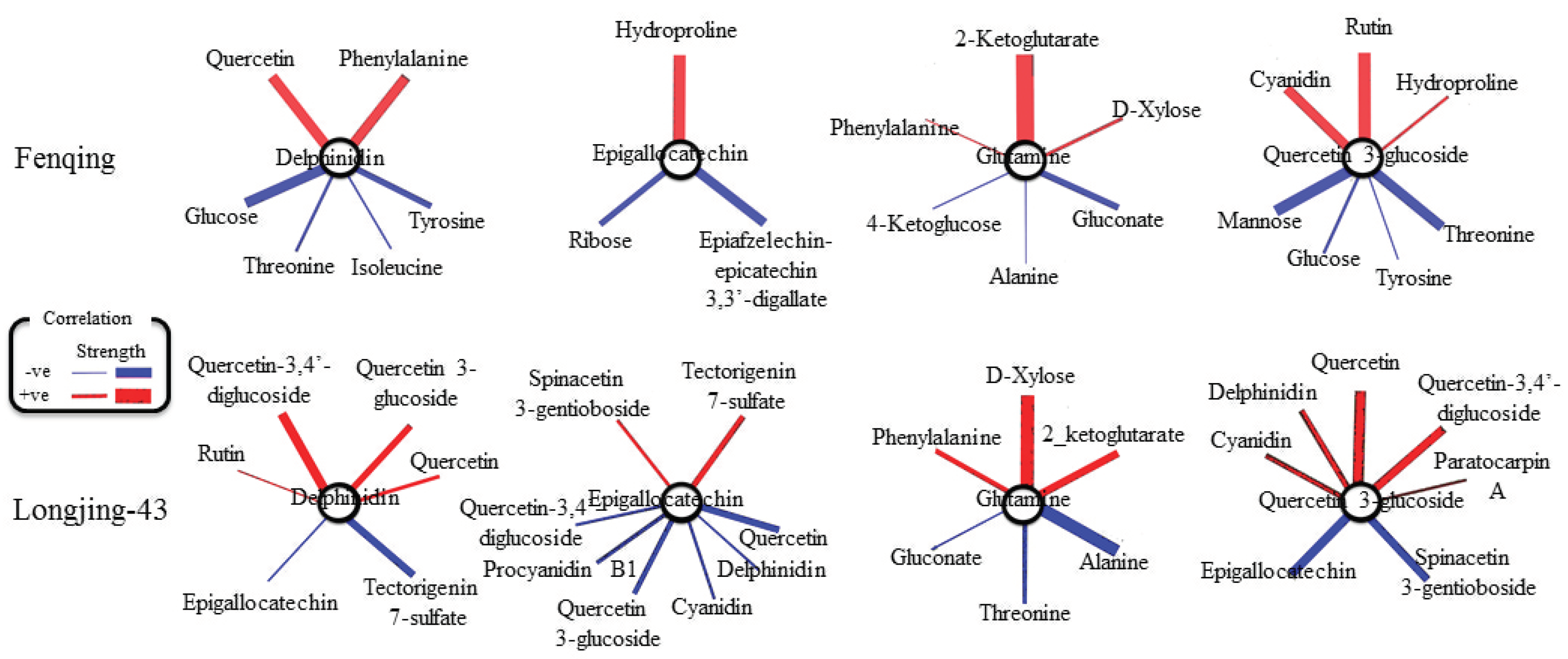
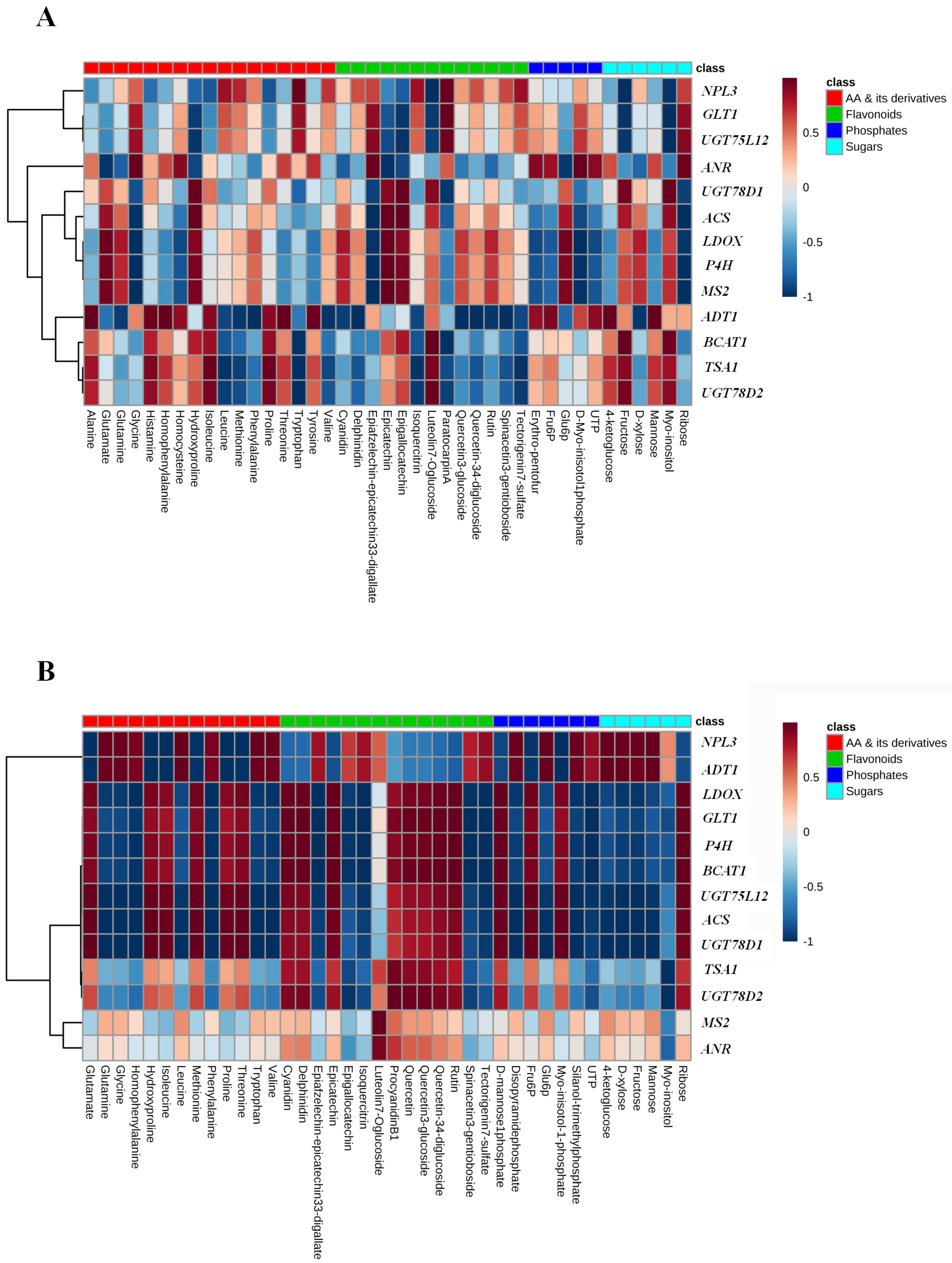
2.6. Correlation and Pathway Impact Analysis of Metabolites and Gene Expression with High-Fold Response Ratios
3. Discussion
4. Materials and Methods
4.1. Plant Cultivation, P Treatment and Sampling
4.2. Measurements of Root Growth Parameters and Concentrations of Pi
4.3. GC×GC-TOF/MS Analysis for Primary Metabolites
4.4. UPLC-Q-TOF/MS Analysis for Secondary Metabolites
4.5. Extraction and Determination of Targeted Catechin and Amino Acid by High Performance Liquid Chromatography (HPLC) Analysis
4.6. Quantitative Real-Time Polymerase Chain Reaction (QRT-PCR) Expression Analysis
4.7. Data Analysis, Visualization, and Cross Verification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus Uptake by Plants: From Soil to Cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirier, Y.; Bucher, M. Phosphate transport and homeostasis in Arabidopsis. In The Arabidopsis Book; The American Society of Plant Biologists: Rockville, MD, USA, 2002; Volume 1, p. e0024. [Google Scholar]
- Brembu, T.; Muhlroth, A.; Alipanah, L.; Bones, A.M. The effects of phosphorus limitation on carbon metabolism in diatoms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, J.P. Root phenes for enhanced soil exploration and phosphorus acquisition: Tools for future crops. Plant Physiol. 2011, 156, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Plaxton, W.C.; Tran, H.T. Metabolic adaptations of phosphate-starved plants. Plant Physiol. 2011, 156, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Alexova, R.; Nelson, C.J.; Millar, A.H. Temporal development of the barley leaf metabolic response to Pi limitation. Plant Cell Environ. 2017, 40, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Byrne, S.L.; Foito, A.; Hedley, P.E.; Morris, J.A.; Stewart, D.; Barth, S. Early response mechanisms of perennial ryegrass (Lolium perenne) to phosphorus deficiency. Ann. Bot. 2011, 107, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Ganie, A.H.; Ahmad, A.; Pandey, R.; Aref, I.M.; Yousuf, P.Y.; Ahmad, S.; Iqbal, M. Metabolite Profiling of Low-P Tolerant and Low-P Sensitive Maize Genotypes under Phosphorus Starvation and Restoration Conditions. PLoS ONE 2015, 10, e0129520. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Godde, V.; Niehaus, K.; Zorb, C. Metabolic Adaptations of White Lupin Roots and Shoots under Phosphorus Deficiency. Front. Plant Sci. 2015, 6, 1014. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.D.; Pant, P.; Erban, A.; Huhman, D.; Kopka, J.; Scheible, W.R. Identification of primary and secondary metabolites with phosphorus status-dependent abundance in Arabidopsis, and of the transcription factor PHR1 as a major regulator of metabolic changes during phosphorus limitation. Plant Cell Environ. 2015, 38, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Roessner, U.; Eickmeier, I.; Genc, Y.; Callahan, D.L.; Shirley, N.; Langridge, P.; Bacic, A. Metabolite profiling reveals distinct changes in carbon and nitrogen metabolism in phosphate-deficient barley plants (Hordeum vulgare L.). Plant Cell Physiol. 2008, 49, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Morcuende, R.; Bari, R.; Gibon, Y.; Zheng, W.; Pant, B.D.; Blasing, O.; Usadel, B.; Czechowski, T.; Udvardi, M.K.; Stitt, M.; et al. Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant Cell Environ. 2007, 30, 85–112. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.R. How does P affect photosynthesis and metabolite profiles of Eucalyptus globulus? Tree Physiol. 2011, 31, 727–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, R.; Morant, M.; Jarmer, H.; Nilsson, L.; Nielsen, T.H. Genome-wide analysis of the Arabidopsis leaf transcriptome reveals interaction of phosphate and sugar metabolism. Plant Physiol. 2007, 143, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Misson, J.; Raghothama, K.G.; Jain, A.; Jouhet, J.; Block, M.A.; Bligny, R.; Ortet, P.; Creff, A.; Somerville, S.; Rolland, N.; et al. A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc. Natl. Acad. Sci. USA 2005, 102, 11934–11939. [Google Scholar] [CrossRef] [PubMed]
- Arpat, A.B.; Magliano, P.; Wege, S.; Rouached, H.; Stefanovic, A.; Poirier, Y. Functional expression of PHO1 to the Golgi and trans-Golgi network and its role in export of inorganic phosphate. Plant J. 2012, 71, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Yi, K.; Dang, L.; Huang, H.; Wu, W.; Wu, P. Characterization of a sub-family of Arabidopsis genes with the SPX domain reveals their diverse functions in plant tolerance to phosphorus starvation. Plant J. 2008, 54, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ruan, W.; Shi, J.; Zhang, L.; Xiang, D.; Yang, C.; Li, C.; Wu, Z.; Liu, Y.; Yu, Y.; et al. Rice SPX1 and SPX2 inhibit phosphate starvation responses through interacting with PHR2 in a phosphate-dependent manner. Proc. Natl. Acad. Sci. USA 2014, 111, 14953–14958. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.P.; White, P.J. Sugar signalling in root responses to low P availability. Plant Physiol. 2011, 156, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Ma, L.; Hou, X.; Wang, M.; Wu, Y.; Liu, F.; Deng, X.W. Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiol. 2003, 132, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Li, C.F.; Zhu, Y.; Yu, Y.; Zhao, Q.Y.; Wang, S.J.; Wang, X.C.; Yao, M.Z.; Luo, D.; Li, X.; Chen, L.; et al. Global transcriptome and gene regulation network for secondary metabolite biosynthesis of tea plant (Camellia sinensis). BMC Genom. 2015, 16, 560. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.H.; Chen, L.S.; Chen, R.B.; Zhang, F.Z.; Jiang, H.X.; Tang, N. CO2 assimilation, ribulose-1,5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport probed by the JIP-test, of tea leaves in response to phosphorus supply. BMC Plant Biol. 2009, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.H.; Qi, Y.P.; Chen, R.B.; Zhang, F.Z.; Chen, L.S. Effects of phosphorus supply on the quality of green tea. Food Chem. 2012, 130, 908–914. [Google Scholar] [CrossRef]
- Ding, Z.; Jia, S.; Wang, Y.; Xiao, J.; Zhang, Y. Phosphate stresses affect ionome and metabolome in tea plants. Plant Physiol. Biochem. 2017, 120, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.H.; Chen, L.S.; Chen, R.B.; Zhang, F.Z.; Jiang, H.X.; Tang, N.; Smith, B.R. Root release and metabolism of organic acids in tea plants in response to phosphorus supply. J. Plant Physiol. 2011, 168, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Hajiboland, R. A high internal phosphorus use efficiency in tea (Camellia sinensis L.) plants. Asian J. Plant Sci. 2008, 7, 30–36. [Google Scholar] [CrossRef]
- Cheng, L.; Tang, X.; Vance, C.P.; White, P.J.; Zhang, F.; Shen, J. Interactions between light intensity and phosphorus nutrition affect the phosphate-mining capacity of white lupin (Lupinus albus L.). J. Exp. Bot. 2014, 65, 2995–3003. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Chapman, W.; Jenkins, G.; Graham, I.; Martin, T.; Crozier, A. The effect of nitrogen and phosphorus deficiency on flavonol accumulation in plant tissues. Plant Cell Environ. 2001, 24, 1189–1197. [Google Scholar] [CrossRef] [Green Version]
- Bates, T.R.; Lynch, J.P. Root hairs confer a competitive advantage under low phosphorus availability. Plant Soil 2001, 236, 243–250. [Google Scholar] [CrossRef]
- Lambers, H.; Finnegan, P.M.; Laliberté, E.; Pearse, S.J.; Ryan, M.H.; Shane, M.W.; Veneklaas, E.J. Phosphorus Nutrition of Proteaceae in Severely Phosphorus-Impoverished Soils: Are There Lessons To Be Learned for Future Crops? Plant Physiol. 2011, 156, 1058–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghothama, K.G.; Karthikeyan, A.S. Phosphate acquisition. Plant Soil 2005, 274, 37. [Google Scholar] [CrossRef]
- Williamson, L.C.; Ribrioux, S.P.; Fitter, A.H.; Leyser, H.M. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol. 2001, 126, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Puga, M.I.; Mateos, I.; Charukesi, R.; Wang, Z.; Franco-Zorrilla, J.M.; de Lorenzo, L.; Irigoyen, M.L.; Masiero, S.; Bustos, R.; Rodriguez, J.; et al. SPX1 is a phosphate-dependent inhibitor of Phosphate Starvation Response 1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 14947–14952. [Google Scholar] [CrossRef] [PubMed]
- Yong-Villalobos, L.; Gonzalez-Morales, S.I.; Wrobel, K.; Gutierrez-Alanis, D.; Cervantes-Perez, S.A.; Hayano-Kanashiro, C.; Oropeza-Aburto, A.; Cruz-Ramirez, A.; Martinez, O.; Herrera-Estrella, L. Methylome analysis reveals an important role for epigenetic changes in the regulation of the Arabidopsis response to phosphate starvation. Proc. Natl. Acad. Sci. USA 2015, 112, E7293–E7302. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.F.; Chai, R.S.; Jin, G.L.; Wang, H.; Tang, C.X.; Zhang, Y.S. Responses of root architecture development to low phosphorus availability: A review. Ann. Bot. 2013, 112, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Péret, B.; Clement, M.; Nussaume, L.; Desnos, T. Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci. 2011, 16, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Almvik, M.; Clarke, N.; Eich-Greatorex, S.; Ogaard, A.F.; Krogstad, T.; Lambers, H.; Clarke, J.L. Contrasting responses of root morphology and root-exuded organic acids to low phosphorus availability in three important food crops with divergent root traits. AoB Plants 2015, 7, plv097. [Google Scholar] [CrossRef] [PubMed]
- Bolouri-Moghaddam, M.R.; Le Roy, K.; Xiang, L.; Rolland, F.; Van den Ende, W. Sugar signalling and antioxidant network connections in plant cells. FEBS J. 2010, 277, 2022–2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, M.; Liu, Y.; Zhang, B.; Zhao, Y.; Wang, X.; Zhou, Y.; Raghothama, K.G.; Liu, D. Genetic and genomic evidence that sucrose is a global regulator of plant responses to phosphate starvation in Arabidopsis. Plant Physiol. 2011, 156, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- Secco, D.; Jabnoune, M.; Walker, H.; Shou, H.; Wu, P.; Poirier, Y.; Whelan, J. Spatio-temporal transcript profiling of rice roots and shoots in response to phosphate starvation and recovery. Plant Cell 2013, 25, 4285–4304. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.W.; Ogita, S.; Ashihara, H. Biosynthesis of theanine (gamma-ethylamino-l-glutamic acid) in seedlings of Camellia sinensis. Phytochem. Lett. 2008, 1, 115–119. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Bowyer, M.C.; Roach, P.D. L-Theanine: Properties, synthesis and isolation from tea. J. Sci. Food Agric. 2011, 91, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Burgos, A.; Ma, L.; Zhang, Q.; Tang, D.; Ruan, J. Lipidomics analysis unravels the effect of nitrogen fertilization on lipid metabolism in tea plant (Camellia sinensis L.). BMC Plant Biol. 2017, 17, 165. [Google Scholar] [CrossRef] [PubMed]
- Aranjuelo, I.; Tcherkez, G.; Molero, G.; Gilard, F.; Avice, J.C.; Nogues, S. Concerted changes in N and C primary metabolism in alfalfa (Medicago sativa) under water restriction. J. Exp. Bot. 2013, 64, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Vlad, F.; Spano, T.; Vlad, D.; Daher, F.B.; Ouelhadj, A.; Fragkostefanakis, S.; Kalaitzis, P. Involvement of Arabidopsis prolyl 4 hydroxylases in hypoxia, anoxia and mechanical wounding. Plant Signal Behav. 2007, 2, 368–369. [Google Scholar] [CrossRef] [PubMed]
- Tannert, M.; May, A.; Ditfe, D.; Berger, S.; Balcke, G.U.; Tissier, A.; Köck, M. Pi starvation-dependent regulation of ethanolamine metabolism by phosphoethanolamine phosphatase PECP1 in Arabidopsis roots. J. Exp. Bot. 2018, 69, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Messner, B.; Faus-Kessler, T.; Hoffmann, T.; Schwab, W.; Hajirezaei, M.R.; von Saint Paul, V.; Heller, W.; Schaffner, A.R. Feedback inhibition of the general phenylpropanoid and flavonol biosynthetic pathways upon a compromised flavonol-3-O-glycosylation. J. Exp. Bot. 2012, 63, 2465–2478. [Google Scholar] [CrossRef] [PubMed]
- Yonekura-Sakakibara, K.; Tohge, T.; Matsuda, F.; Nakabayashi, R.; Takayama, H.; Niida, R.; Watanabe-Takahashi, A.; Inoue, E.; Saito, K. Comprehensive flavonol profiling and transcriptome coexpression analysis leading to decoding gene-metabolite correlations in Arabidopsis. Plant Cell 2008, 20, 2160–2176. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhuang, J.; Wu, Y.; Wang, P.; Zhao, G.; Liu, Y.; Jiang, X.; Gao, L.; Xia, T. Identification of a Flavonoid Glucosyltransferase Involved in 7-OH Site Glycosylation in Tea plants (Camellia sinensis). Sci. Rep. 2017, 7, 5926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, Y.; Ma, L.; Yi, X.; Ruan, J. Metabolomic analysis using ultra-performance liquid chromatography-quadrupole-time of flight mass spectrometry (UPLC-Q-TOF MS) uncovers the effects of light intensity and temperature under shading treatments on the metabolites in tea. PLoS ONE 2014, 9, e112572. [Google Scholar] [CrossRef] [PubMed]
- Valluru, R.; Van den Ende, W. Myo-inositol and beyond–emerging networks under stress. Plant Sci. 2011, 181, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Donahue, J.L.; Alford, S.R.; Torabinejad, J.; Kerwin, R.E.; Nourbakhsh, A.; Ray, W.K.; Hernick, M.; Huang, X.; Lyons, B.M.; Hein, P.P.; et al. The Arabidopsis thaliana Myo-Inositol 1-Phosphate Synthase1 Gene Is Required for Myo-inositol Synthesis and Suppression of Cell Death. Plant Cell 2010, 22, 888–903. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.H.; Raynaud, C.; Tcherkez, G.; Blanchet, S.; Massoud, K.; Domenichini, S.; Henry, Y.; Soubigou-Taconnat, L.; Lelarge-Trouverie, C.; Saindrenan, P.; et al. Crosstalks between Myo-Inositol Metabolism, Programmed Cell Death and Basal Immunity in Arabidopsis. PLoS ONE 2009, 4, e7364. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Parry, M.; Noctor, G. Markers and signals associated with nitrogen assimilation in higher plants. J. Exp. Bot. 2003, 54, 585–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novitskaya, L.; Trevanion, S.J.; Driscoll, S.; Foyer, C.H.; Noctor, G. How does photorespiration modulate leaf amino acid contents? A dual approach through modelling and metabolite analysis. Plant Cell Environ. 2002, 25, 821–835. [Google Scholar] [CrossRef] [Green Version]
- Hernández, I.; Munné-Bosch, S. Linking phosphorus availability with photo-oxidative stress in plants. J. Exp. Bot. 2015, 66, 2889–2900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleksza, D.; Horvath, G.V.; Sandor, G.; Szabados, L. Proline Accumulation Is Regulated by Transcription Factors Associated with Phosphate Starvation. Plant Physiol. 2017, 175, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zeng, Z.; Connor, J.N.; Huang, C.Y.; Melino, V.; Kumar, P.; Miklavcic, S.J. RootGraph: A graphic optimization tool for automated image analysis of plant roots. J. Exp. Bot. 2015, 66, 6551–6562. [Google Scholar] [CrossRef] [PubMed]
- Salahinejad, M.; Aflaki, F. Toxic and essential mineral elements content of black tea leaves and their tea infusions consumed in Iran. Biol. Trace Elem. Res. 2010, 134, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Q.; Liu, M.; Ma, L.; Shi, Y.; Ruan, J. Metabolomic Analyses Reveal Distinct Change of Metabolites and Quality of Green Tea during the Short Duration of a Single Spring Season. J. Agric. Food Chem. 2016, 64, 3302–3309. [Google Scholar] [CrossRef] [PubMed]
- De Vos, R.C.; Moco, S.; Lommen, A.; Keurentjes, J.J.; Bino, R.J.; Hall, R.D. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2007, 2, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Xiong, L.G.; Deng, T.T.; Wu, Y.; Li, J.; Liu, S.Q.; Huang, J.A.; Liu, Z.H. Comparative profiling of gene expression in Camellia sinensis L. cultivar AnJiBaiCha leaves during periodic albinism. Gene 2015, 561, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Li, J.; Gu, X.; Wang, Y.; Xia, Q.; Qin, B.; Zhu, L. Quantitative Analysis of ATP Sulfurylase and Selenocysteine Methyltransferase Gene Expression in Different Organs of Tea Plant (Camellia sinensis). Am. J. Plant Sci. 2012, 3, 51–59. [Google Scholar] [CrossRef]
- Xiong, L.; Li, J.; Li, Y.; Yuan, L.; Liu, S.; Huang, J.; Liu, Z. Dynamic changes in catechin levels and catechin biosynthesis-related gene expression in albino tea plants (Camellia sinensis L.). Plant Physiol. Biochem. 2013, 71, 132–143. [Google Scholar] [CrossRef] [PubMed]
| Cultivar | P Level | Young Shoots | Leaves | Root | Whole Plant |
|---|---|---|---|---|---|
| Fengqing | −P | 2.12 ± 0.5b | 2.39 ± 1.31b | 2.87 ± 1.41b | 2.46 ± 1.04b |
| Fengqing | +P | 3.80 ± 0.8a | 3.94 ± 0.83a | 7.17 ± 2.66a | 4.97 ± 1.41a |
| Longjing-43 | −P | 2.19 ± 1.5b | 3.81 ± 1.00b | 2.15 ± 0.57b | 2.72 ± 0.93b |
| Longjing-43 | +P | 12.05 ± 5.63a | 7.15 ± 2.16a | 9.15 ± 2.44a | 9.45 ± 3.33a |
| Parameter | Fengqing | Longjing-43 |
|---|---|---|
| Volume | −1.54 * | −2.84 * |
| Surface area | −1.67 * | −1.99 * |
| Number of root tips | −1.96 * | −0.77 * |
| Length | 2.32 * | 0.49 * |
| Average thickness | 0.15 * | 0.01NS |
| Metabolites | Fengqing | Longjing-43 | ||
|---|---|---|---|---|
| −P | +P | −P | +P | |
| Amino acids | ||||
| Asp | 2.06 ± 0.16b | 3.13 ± 0.05a | 1.36 ± 0.05b | 2.47 ± 0.22a |
| Gly | 0.29 ± 0.01b | 1.55 ± 0.14a | 0.11 ± 0.01b | 0.47 ± 0.04a |
| Leu | 0.28 ± 0.01b | 0.45 ± 0.04a | 0.11 ± 0.01b | 0.20 ± 0.01a |
| Pro | 3.56 ± 0.13a | 1.95 ± 0.04b | 1.66 ± 0.13a | 0.84 ± 0.01b |
| Ile | 1.21 ± 0.05a | 0.86 ± 0.08b | 0.43 ± 0.02a | 0.22 ± 0.02b |
| Glu | 9.64 ± 0.77a | 7.78 ± 0.13b | 2.36 ± 0.19b | 3.41 ± 0.06a |
| Val | 0.42 ± 0.01a | 0.55 ± 0.05b | 0.46 ± 0.02b | 0.62 ± 0.06a |
| Met | 2.52 ± 0.10a | 1.97 ± 0.78b | 1.08 ± 0.04b | 1.44 ± 0.3a |
| Phe | 1.19 ± 0.05a | 0.93 ± 0.01b | 0.52 ± 0.02b | 1.18 ± 0.2a |
| Thea | 8.52 ± 0.68b | 20.81 ± 0.36a | 22.78 ± 1.83a | 17.02 ± 0.29b |
| Ser | 0.16 ± 0.02b | 0.31 ± 0.03a | 0.20 ± 0.02a | 0.12 ± 0b |
| Thr | 0.24 ± 0.01b | 0.38 ± 0.34a | 0.78 ± 0.03a | 0.55 ± 0.05b |
| Arg | 1.18 ± 0.05a | 0.78 ± 0.01b | 0.41 ± 0.35 NS | 0.24 ± 0.25 |
| Tyr | 0.21 ± 0.15 NS | 0.43 ± 0.01 | 0.45 ± 0.05a | 0.24 ± 0.03b |
| His | 0.59 ± 0.17 NS | 0.52 ± 0.13 | 0.55 ± 0.14 NS | 0.53 ± 0.18 |
| Ala | 0.11 ± 0.02 NS | 0.08 ± 0.01 | 0.08 ± 0.04 NS | 0.11 ± 0.03 |
| Cys | 0.40 ± 0.25 NS | 0.49 ± 0.36 | 0.29 ± 0.08 NS | 0.56 ± 0.37 |
| Lys | 0.81 ± 0.49 NS | 1.72 ± 1.02 | 0.61 ± 0.39 NS | 1.17 ± 0.51 |
| Catechins | ||||
| EGCG | 34.03 ± 1.36a | 25.77 ± 2.34b | 33.25 ± 6.5 NS | 36.44 ± 0.4 |
| EGC | 6.92 ± 0.56a | 4.25 ± 0.07b | 3.02 ± 0.24b | 10.93 ± 0.19a |
| ECG | 2.71 ± 0.22a | 2.24 ± 0.04b | 2.80 ± 0.22b | 3.40 ± 0.06a |
| GC | 0.14 ± 0.05a | 0.03 ± 0.02b | 0.05 ± 0 NS | 0.07 ± 0.03 |
| CG | 0.03 ± 0b | 0.05 ± 0.01a | 0.06 ± 0.01a | 0.02 ± 0b |
| C | 1.06 ± 0.04a | 0.66 ± 0.06b | 1.37 ± 0.04a | 0.96 ± 0.19b |
| GCG | 0.19 ± 0.04 NS | 0.20 ± 0.02 | 0.23 ± 0.01 NS | 0.16 ± 0.03 |
| Genes | Fengqing | Longjing-43 | ||||
|---|---|---|---|---|---|---|
| Young Shoots | Leaves | Root | Young Shoots | Leaves | Root | |
| PHR1 | 0.89 *** | 1.03 * | 0.69 * | 0.01 * | 0.95 * | 0.86 ** |
| PHO1 | 1.01 * | 1.21 NS | 0.70 * | 0.81 NS | 0.78 * | 1.20 *** |
| SPX2 | 1.70 ** | 1.21 * | 1.53 *** | 1.76 *** | 0.82 *** | 0.55 *** |
| Genes | Fengqing | Longjing-43 | ||||
|---|---|---|---|---|---|---|
| Young Shoots | Leaves | Root | Young Shoots | Leaves | Root | |
| BCAT1 | 0.88 ** | 1.15 * | 0.86 ** | 0.88 ** | 0.85 * | 0.92 ** |
| TSA1 | 1.19 * | 1.50 ** | 1.12 *** | 1.61 *** | 0.85 *** | 0.92 *** |
| ADT1 | 1.55 *** | 1.09 * | 0.87 * | 0.91 ** | 1.15 ** | 0.67 NS |
| MS2 | 0.92 ** | 1.03 NS | 0.86 * | 1.67 *** | 0.98 NS | 0.99 NS |
| NPL3 | 0.98 * | 0.93 NS | 0.85 ** | 0.58 *** | 1.05 * | 0.92 * |
| GLT1 | 0.88 * | 1.56 ** | 0.63 ** | 0.71 ** | 0.90 * | 1.01 NS |
| ACS | 0.85 ** | 1.34 ** | 0.87 *** | 1.62 *** | 0.92 * | 1.19 *** |
| P4H | 0.96 * | 1.30 ** | 1.01 NS | 1.62 ** | 0.91 * | 1.27 * |
| ANR | 1.02 NS | 2.08 NS | 1.06 * | 1.16 NS | 0.63 ** | 0.56 *** |
| LDOX | 0.96 NS | 0.95 * | 1.10 NS | 1.74 ** | 0.62 *** | 1.02 NS |
| UGT75L12 | 0.83 ** | 1.04 * | 0.80 ** | 2.17 *** | 0.89 ** | 1.17 * |
| UGT78D1 | 0.92 * | 1.46 * | 0.96 * | 1.19 * | 0.92 * | 0.97 NS |
| UGT78D2 | 0.84 *** | 1.17 * | 0.70 *** | 1.70 *** | 0.86 ** | 0.81 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
KC, S.; Liu, M.; Zhang, Q.; Fan, K.; Shi, Y.; Ruan, J. Metabolic Changes of Amino Acids and Flavonoids in Tea Plants in Response to Inorganic Phosphate Limitation. Int. J. Mol. Sci. 2018, 19, 3683. https://doi.org/10.3390/ijms19113683
KC S, Liu M, Zhang Q, Fan K, Shi Y, Ruan J. Metabolic Changes of Amino Acids and Flavonoids in Tea Plants in Response to Inorganic Phosphate Limitation. International Journal of Molecular Sciences. 2018; 19(11):3683. https://doi.org/10.3390/ijms19113683
Chicago/Turabian StyleKC, Santosh, Meiya Liu, Qunfeng Zhang, Kai Fan, Yuanzhi Shi, and Jianyun Ruan. 2018. "Metabolic Changes of Amino Acids and Flavonoids in Tea Plants in Response to Inorganic Phosphate Limitation" International Journal of Molecular Sciences 19, no. 11: 3683. https://doi.org/10.3390/ijms19113683
APA StyleKC, S., Liu, M., Zhang, Q., Fan, K., Shi, Y., & Ruan, J. (2018). Metabolic Changes of Amino Acids and Flavonoids in Tea Plants in Response to Inorganic Phosphate Limitation. International Journal of Molecular Sciences, 19(11), 3683. https://doi.org/10.3390/ijms19113683