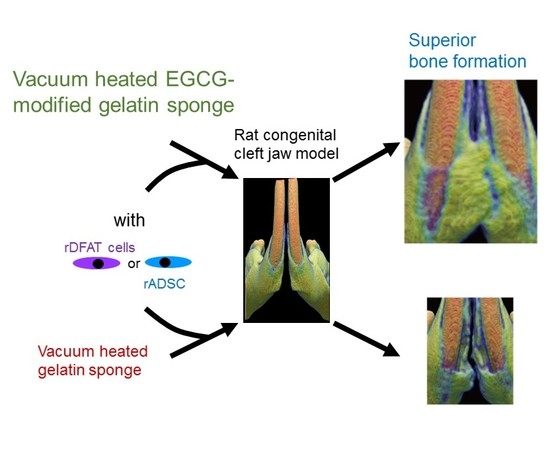
Osteogenesis of Multipotent Progenitor Cells using the Epigallocatechin Gallate-Modified Gelatin Sponge Scaffold in the Rat Congenital Cleft-Jaw Model
Abstract
:1. Introduction
2. Results
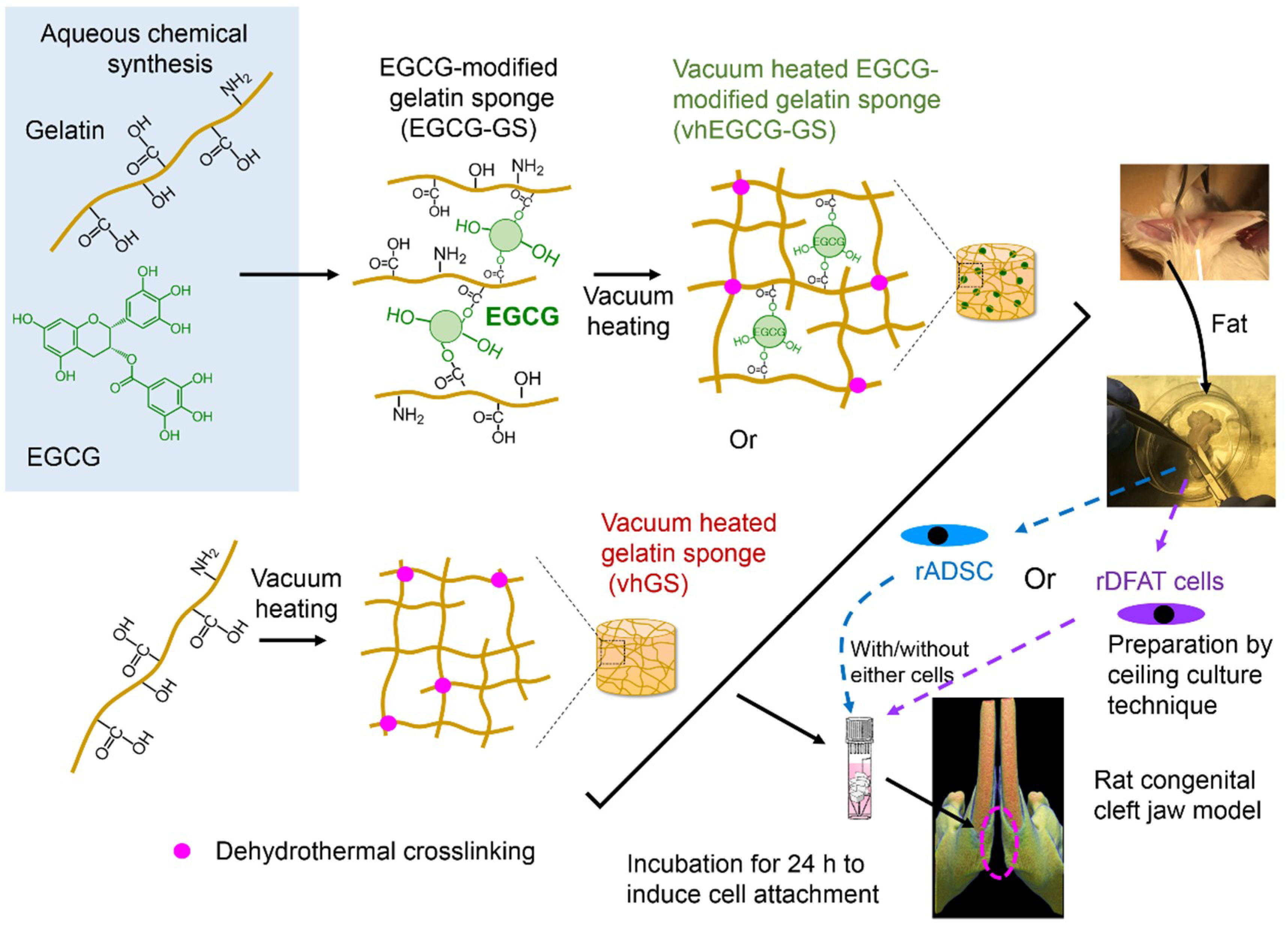
2.1. Work Flow of Cell Implantation
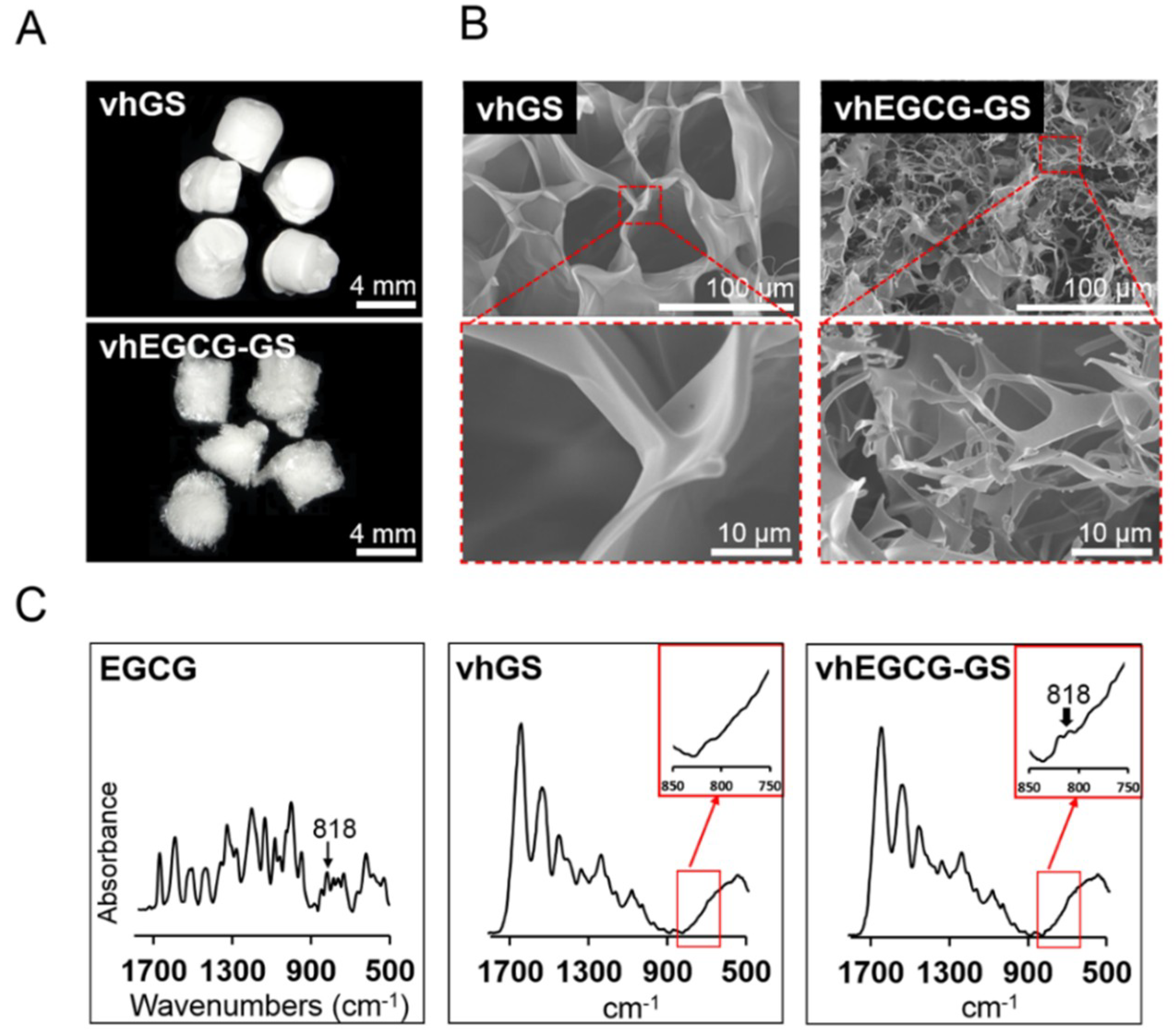
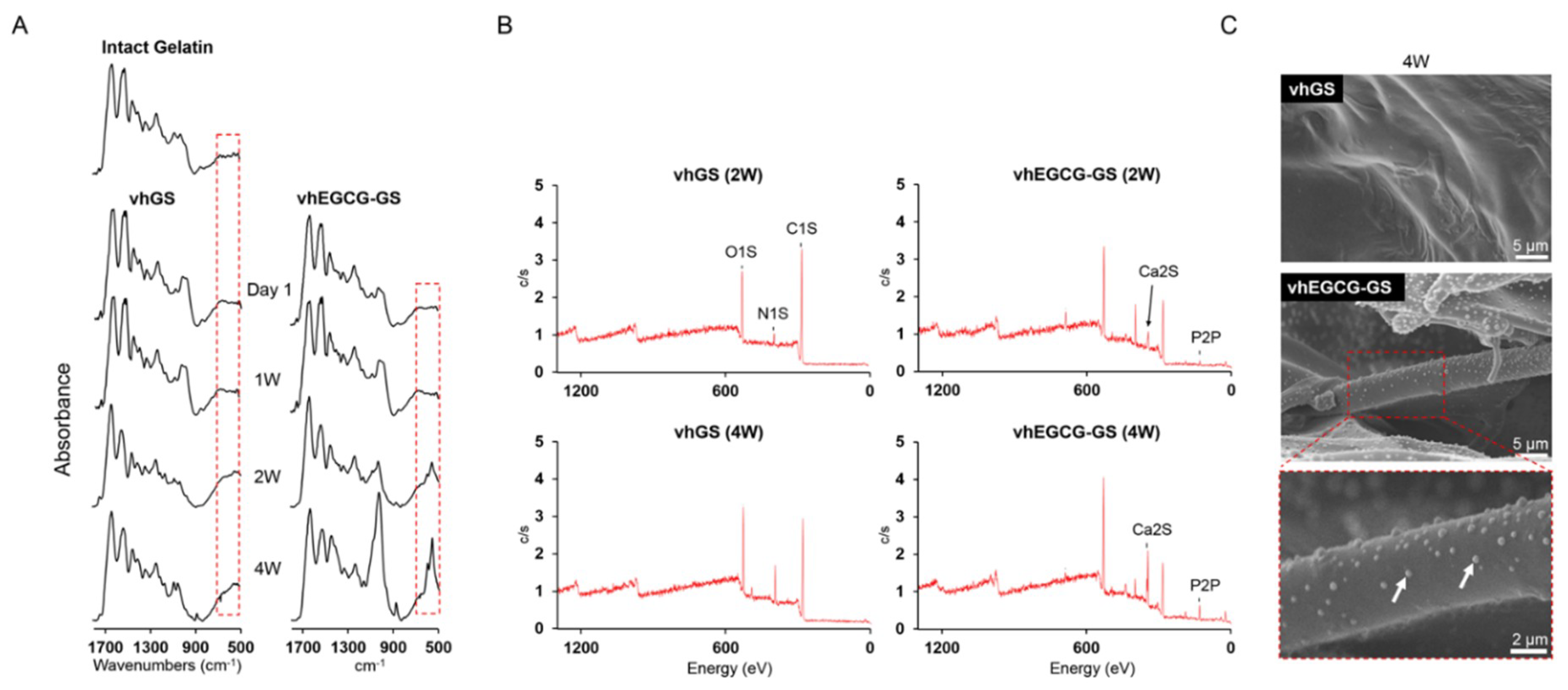
2.2. Characteristic of Sponges
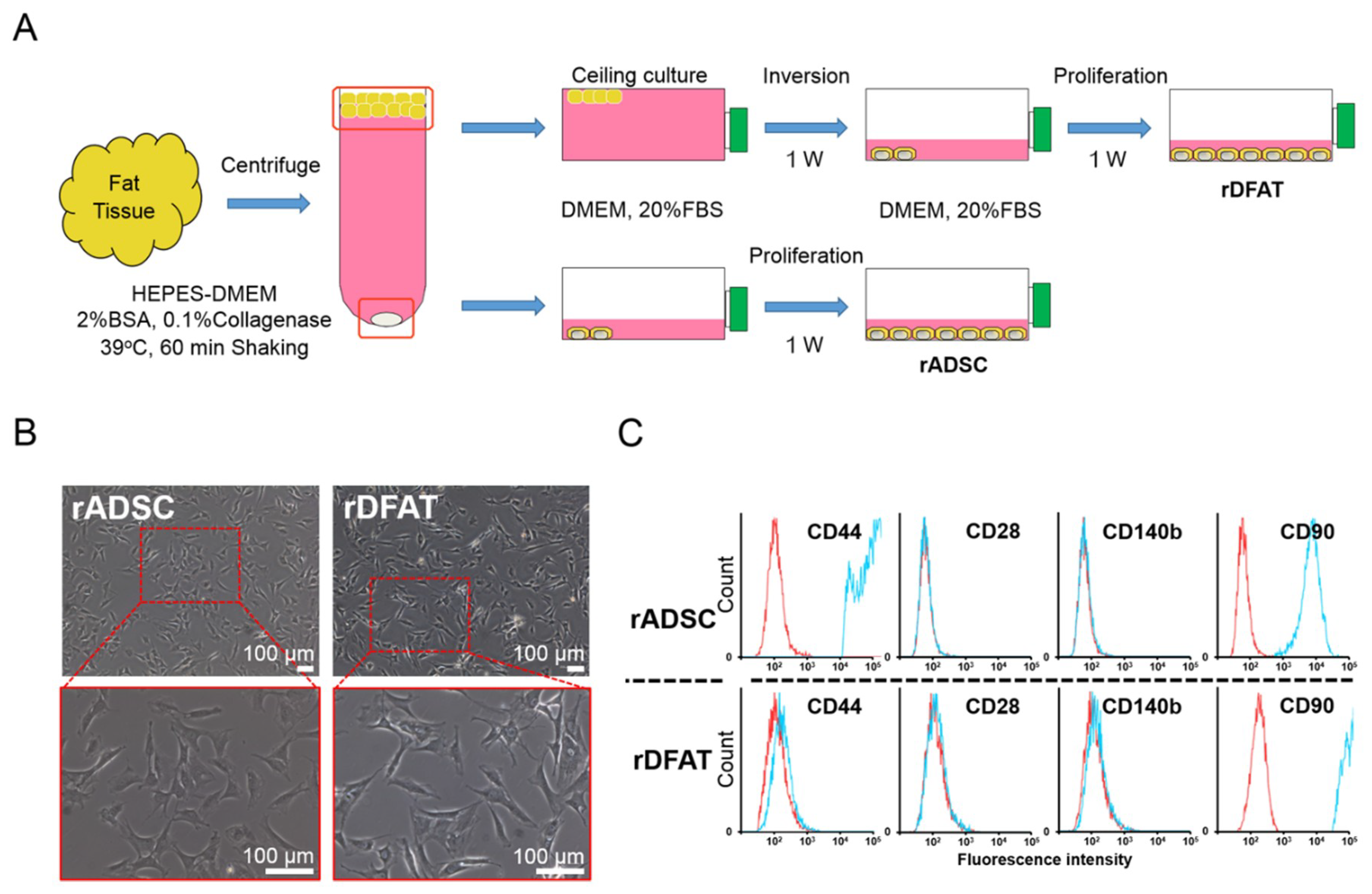
2.3. Identification of Adipose-Derived Stem Cells and Dedifferentiated Fat Cells
2.4. Bone Formation in Rat Congenital Cleft-Jaw Model
2.5. In Vitro Cell Attachment Assay
2.6. Evaluation of Surface Property on Sponges
3. Discussion
4. Materials and Methods
4.1. Preparation of EGCG-GS and vhEGCG-GS
4.2. Characterization of Sponges
4.3. Cell Preparation of Adipose-Derived Stem Cells and Dedifferentiated Fat Cells
4.4. Cell-Seeding and Cell Attachment Assay
4.5. Preparation of Congenital Cleft-Jaw Model and Implantation of Samples
4.6. Micro-Computed Tomographical and Histological Analysis
4.7. Calcium Phosphate Precipitation
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EGCG | Epigallocatechin gallate |
| GS | Gelatin sponge |
| vh | Vacuum heated |
| EGCG-GS | Epigallocatechin gallate-modified gelatin sponge |
| vhEGCG-GS | Vacuum heated EGCG-GS |
| DFAT cell | Dedifferentiated fat cell |
| ADSC | Adipose-derived stem cell |
| SEM | Scanning election microscopy |
| XPS | X-ray photoelectron spectroscopy |
| FTIR | Fourier transform infrared |
| BV | Bone volume |
| TV | Total volume |
| BMC | Bone mineral content |
| DAPI | 4′,6-diamidino-2-phenylindole |
| H-E | Hematoxylin and eosin |
| DMEM | Dulbecco’s modified Eagle’s media |
References
- Szpalski, C.; Barr, J.; Wetterau, M.; Saadeh, P.B.; Warren, S.M. Cranial bone defects: Current and future strategies. Neurosurg. Focus. 2010, 29, E8. [Google Scholar] [CrossRef] [PubMed]
- Im, G.I. Clinical use of stem cells in orthopaedics. Eur. Cell Mater. 2017, 33, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Mazzetti, M.P.V.; Alonso, N.; Brock, R.S.; Ayoub, A.; Massumoto, S.M.; Eca, L.P. Importance of stem cell transplantation in cleft lip and palate surgical treatment protocol. J. Craniofac. Surg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gregor, A.; Filova, E.; Novak, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnova, V.; Lukasova, V.; Bartos, M.; Necas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.C.; Xu, Z.; Lin, L.; Koo, A.; Ndon, S.; Berthiaume, F.; Dardik, A.; Hsia, H. Stem cells and engineered scaffolds for regenerative wound healing. Bioengineering 2018, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Anada, T.; Masuda, T.; Honda, Y.; Sakai, Y.; Kato, Y.; Kamakura, S.; Echigo, S.; Suzuki, O. The effect of synthetic octacalcium phosphate in a collagen scaffold on the osteogenicity of mesenchymal stem cells. Eur. Cell Mater. 2011, 22, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Akita, D.; Kano, K.; Saito-Tamura, Y.; Mashimo, T.; Sato-Shionome, M.; Tsurumachi, N.; Yamanaka, K.; Kaneko, T.; Toriumi, T.; Arai, Y.; et al. Use of rat mature adipocyte-derived dedifferentiated fat cells as a cell source for periodontal tissue regeneration. Front. Physiol. 2016, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Keshav Narayan, A.; Logith Kumar, R.; Viji Chandran, S.; Vairamani, M.; Selvamurugan, N. Proliferation and differentiation of mesenchymal stem cells on scaffolds containing chitosan, calcium polyphosphate and pigeonite for bone tissue engineering. Cell. Prolif. 2018, 51, e12408. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; D’Aurora, M.; Gugliandolo, A.; Merciaro, I.; Orsini, T.; Gatta, V.; Piattelli, A.; Trubiani, O.; Mazzon, E. Biofunctionalized scaffold in bone tissue repair. Int. J. Mol. Sci. 2018, 19, 1022. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, Z. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids. Surf. B 2018, 164, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Honda, Y.; Kakinoki, S.; Yamaoka, T.; Baba, S. Surface modification of porous alpha-tricalcium phosphate granules with heparin enhanced their early osteogenic capability in a rat calvarial defect model. Dent. Mater. J. 2018, 37, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Song, J.; Zhang, Y.; Xu, X.; Zhang, S.; Ji, P.; Wei, S. Bioinspired design of polycaprolactone composite nanofibers as artificial bone extracellular matrix for bone regeneration application. ACS Appl. Mater. Interfaces 2016, 8, 27594–27610. [Google Scholar] [CrossRef] [PubMed]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Fontana, G.; Gershlak, J.; Adamski, M.; Lee, J.S.; Matsumoto, S.; Le, H.D.; Binder, B.; Wirth, J.; Gaudette, G.; Murphy, W.L. Biofunctionalized plants as diverse biomaterials for human cell culture. Adv. Healthcare Mater. 2017, 6, 1601225. [Google Scholar] [CrossRef] [PubMed]
- Hedge, P.; Agrawal, P.; Gupta, P. k. Polyphenols—A useful biomaterial: A review. J. Environ. Res. Develop. 2016, 10, 547–554. [Google Scholar]
- Granja, A.; Pinheiro, M.; Reis, S. Epigallocatechin gallate nanodelivery systems for cancer therapy. Nutrients 2016, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Sun, J.; Chen, Y.; Zong, M.; Li, S.; Wang, Y. EGCG attenuates uric acid-induced inflammatory and oxidative stress responses by medicating the notch pathway. Oxid. Med. Cell Longev. 2015, 2015, 214836. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Ochiai, K. Exploring the possible applications of catechin (gel) for oral care of the elderly and disabled individuals. Jpn. Dent. Sci. Rev. 2012, 48, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, H.H.; Cai, Y.; Hakim, I.A.; Crowell, J.A.; Shahi, F.; Brooks, C.A.; Dorr, R.T.; Hara, Y.; Alberts, D.S. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenone in healthy individuals. Clin. Cancer Res. 2003, 9, 3312–3319. [Google Scholar] [PubMed]
- Min, K.J.; Kwon, T.K. Anticancer effects and molecular mechanisms of epigallocatechin-3-gallate. Integr. Med. Res. 2014, 3, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.H.; Hiipakka, R.A.; Liao, S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology 2000, 141, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Kuzuya, M.; Cheng, X.W.; Asai, T.; Kanda, S.; Tamaya-Mori, N.; Sasaki, T.; Shibata, T.; Iguchi, A. Green tea catechins inhibit the cultured smooth muscle cell invasion through the basement barrier. Atherosclerosis 2003, 166, 23–30. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Shytle, D.; Sun, N.; Mori, T.; Hou, H.; Jeanniton, D.; Ehrhart, J.; Townsend, K.; Zeng, J.; Morgan, D.; et al. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in alzheimer transgenic mice. J. Neurosci. 2005, 25, 8807–8814. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Ho, M.L.; Chang, J.K.; Hung, S.H.; Wang, G.J. Green tea catechin enhances osteogenesis in a bone marrow mesenchymal stem cell line. Osteoporos Int. 2005, 16, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Kondo, H.; Nyan, M.; Hao, J.; Miyahara, T.; Ohya, K.; Kasugai, S. Implantation of green tea catechin alpha-tricalcium phosphate combination enhances bone repair in rat skull defects. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Tanaka, T.; Tokuda, T.; Kashiwagi, T.; Kaida, K.; Hieda, A.; Umezaki, Y.; Hashimoto, Y.; Imai, K.; Matsumoto, N.; et al. Local controlled release of polyphenol conjugated with gelatin facilitates bone formation. Int. J. Mol. Sci. 2015, 16, 14143–14157. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Evaluation of nanohydroxyapaptite (nano-HA) coated epigallocatechin-3-gallate (EGCG) cross-linked collagen membranes. Mater. Sci. Eng. C 2017, 78, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lee, J.S.; Lee, M.S.; An, S.; Yang, K.; Lee, K.; Yang, H.S.; Lee, H.; Cho, S.-W. Plant flavonoid-mediated multifunctional surface modification chemistry: Catechin coating for enhanced osteogenesis of human stem cells. Chem. Mater. 2017, 29, 4375–4384. [Google Scholar] [CrossRef]
- Honda, Y.; Takeda, Y.; Li, P.; Huang, A.; Sasayama, S.; Hara, E.; Uemura, N.; Ueda, M.; Hashimoto, M.; Arita, K.; et al. Epigallocatechin gallate-modified gelatin sponges treated by vacuum heating as a novel scaffold for bone tissue engineering. Molecules. 2018, 23, 876. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Honda, Y.; Arima, Y.; Yasui, K.; Inami, K.; Nishiura, A.; Hashimoto, Y.; Matsumoto, N. Interferon-gamma enhances the efficacy of autogenous bone grafts by inhibiting postoperative bone resorption in rat calvarial defects. J. Prosthodont. Res. 2016, 60, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, N.; Honda, Y.; Momota, Y.; Tran, S.D. Dedifferentiated fat (DFAT) cells: A cell source for oral and maxillofacial tissue engineering. Oral. Dis. 2018, 24, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; George, R.L.; Evancho-Chapman, M.M.; Zhang, G. Current challenges in dedifferentiated fat cells research. Organogenesis. 2016, 12, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kano, K.; Kondo, D.; Fukuda, N.; Iribe, Y.; Tanaka, N.; Matsubara, Y.; Sakuma, T.; Satomi, A.; Otaki, M.; et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J. Cell Physiol. 2008, 215, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Wu, J.Y.; Kong, S.C.; Chiang, M.H.; Ho, M.L.; Yeh, M.L.; Chen, C.H. Low power laser irradiation and human adipose-derived stem cell treatments promote bone regeneration in critical-sized calvarial defects in rats. PLoS ONE 2018, 13, e0195337. [Google Scholar] [CrossRef] [PubMed]
- Fennema, E.M.; Tchang, L.A.H.; Yuan, H.; van Blitterswijk, C.A.; Martin, I.; Scherberich, A.; de Boer, J. Ectopic bone formation by aggregated mesenchymal stem cells from bone marrow and adipose tissue: A comparative study. J. Tissue Eng. Regen. Med. 2018, 12, e150–e158. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, D.; Akita, D.; Tsurumachi, N.; Kano, K.; Yamanaka, K.; Kaneko, T.; Kawano, E.; Iguchi, S.; Toriumi, T.; Arai, Y.; et al. Transplantation of mature adipocyte-derived dedifferentiated fat cells into three-wall defects in the rat periodontium induces tissue regeneration. J. Oral Sci. 2017, 59, 611–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaida, K.; Honda, Y.; Hashimoto, Y.; Tanaka, M.; Baba, S. Application of green tea catechin for inducing the osteogenic differentiation of human dedifferentiated fat cells in vitro. Int. J. Mol. Sci. 2015, 16, 27988–28000. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, F.; Hashimoto, Y.; Kishimoto, N.; Honda, Y.; Matsumoto, N. The utility of human dedifferentiated fat cells in bone tissue engineering in vitro. Cytotechnology 2013, 67, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishimoto, N.; Momota, Y.; Hashimoto, Y.; Tatsumi, S.; Ando, K.; Omasa, T.; Kotani, J. The osteoblastic differentiation ability of human dedifferentiated fat cells is higher than that of adipose stem cells from the buccal fat pad. Clin. Oral Investig. 2014, 18, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Yagyuu, T.; Kirita, T.; Hattori, K.; Tadokoro, M.; Ohgushi, H. Unique and reliable rat model for the assessment of cell therapy: Bone union in the rat mandibular symphysis using bone marrow stromal cells. J. Tissue Eng. Regen. Med. 2015, 9, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, Y.; Yagyuu, T.; Maeda, M.; Imada, M.; Akahane, M.; Kawate, K.; Tanaka, Y.; Kirita, T. Maxillofacial bone regeneration with osteogenic matrix cell sheets: An experimental study in rats. Arch. Oral Biol. 2016, 72, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Ribot, E.J.; Tournier, C.; Aid-Launais, R.; Koonjoo, N.; Oliveira, H.; Trotier, A.J.; Rey, S.; Wecker, D.; Letourneur, D.; Amedee Vilamitjana, J.; et al. 3D anatomical and perfusion MRI for longitudinal evaluation of biomaterials for bone regeneration of femoral bone defect in rats. Sci. Rep. 2017, 7, 6100. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Wu, H.; Xu, G.; Zheng, L.; Zhao, J. Epigallocatechin-3-gallate (EGCG) as a pro-osteogenic agent to enhance osteogenic differentiation of mesenchymal stem cells from human bone marrow: An in vitro study. Cell. Tissue. Res. 2014, 356, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Gao, Y.; Li, H.; Meng, S.; Li, L.; Francisco, J.S.; Zeng, X.C. Characterizing hydrophobicity of amino acid side chains in a protein environment via measuring contact angle of a water nanodroplet on planar peptide network. Proc. Natl. Acad. Sci. USA 2016, 113, 12946–12951. [Google Scholar] [CrossRef] [PubMed]
- Chiono, V.; Ciardelli, G.; Vozzi, G.; Bertoni, F.; Barbani, N.; Giusti, P. Gellan/gelain-based biomaterials for tissue engineering. In Tissue Engineering: Roles, Materials and Appllications; Barnes, S.J., Harris, L.P., Eds.; Nova Science Pulishers, Inc.: Hauppauge, NY, USA, 2008; pp. 243–257. [Google Scholar]
- Prasertsung, I.; Mongkolnavin, R.; Damrongsakkul, S.; Wong, C.S. Surface modification of dehydrothermal crosslinked gelatin film using a 50hz oxygen glow discharge. Surf. Coat. Tech. 2010, 205, S133–S138. [Google Scholar] [CrossRef]
- Ding, Z.; Han, H.; Fan, Z.; Lu, H.; Sang, Y.; Yao, Y.; Cheng, Q.; Lu, Q.; Kaplan, D.L. Nanoscale silk-hydroxyapatite hydrogels for injectable bone biomaterials. ACS Appl. Mater. Interfaces 2017, 9, 16913–16921. [Google Scholar] [CrossRef] [PubMed]
- Wahaj, A.; Hafeez, K.; Zafar, M.S. Role of bone graft materials for cleft lip and palate patients: A systematic review. Saudi. J. Dent. Res. 2016, 7, 57–63. [Google Scholar] [CrossRef]
- Hung, K.-Y.; Lai, H.-C.; Feng, H.-P. Characteristics of RF-sputtered thin films of calcium phosphate on titanium dental implants. Coatings 2017, 7, 126. [Google Scholar] [CrossRef]





| No EGCG | EGCG-Modification | ||||||
|---|---|---|---|---|---|---|---|
| No Implant | vhGS | vhGS with rADSC | vhGS with rDFAT Cells | vhEGCG-GS | vhEGCG-GS with rADSC | vhEGCG-GS with rDFAT Cells | |
| 4 weeks | 0/5 | 0/5 | 1/5 | 1/5 | 0/5 | 0/5 | 0/5 |
| 8 weeks | 0/5 | 0/5 | 0/5 | 2/5 | 3/5 | 1/5 | 3/5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasayama, S.; Hara, T.; Tanaka, T.; Honda, Y.; Baba, S. Osteogenesis of Multipotent Progenitor Cells using the Epigallocatechin Gallate-Modified Gelatin Sponge Scaffold in the Rat Congenital Cleft-Jaw Model. Int. J. Mol. Sci. 2018, 19, 3803. https://doi.org/10.3390/ijms19123803
Sasayama S, Hara T, Tanaka T, Honda Y, Baba S. Osteogenesis of Multipotent Progenitor Cells using the Epigallocatechin Gallate-Modified Gelatin Sponge Scaffold in the Rat Congenital Cleft-Jaw Model. International Journal of Molecular Sciences. 2018; 19(12):3803. https://doi.org/10.3390/ijms19123803
Chicago/Turabian StyleSasayama, Satoshi, Tomoya Hara, Tomonari Tanaka, Yoshitomo Honda, and Shunsuke Baba. 2018. "Osteogenesis of Multipotent Progenitor Cells using the Epigallocatechin Gallate-Modified Gelatin Sponge Scaffold in the Rat Congenital Cleft-Jaw Model" International Journal of Molecular Sciences 19, no. 12: 3803. https://doi.org/10.3390/ijms19123803
APA StyleSasayama, S., Hara, T., Tanaka, T., Honda, Y., & Baba, S. (2018). Osteogenesis of Multipotent Progenitor Cells using the Epigallocatechin Gallate-Modified Gelatin Sponge Scaffold in the Rat Congenital Cleft-Jaw Model. International Journal of Molecular Sciences, 19(12), 3803. https://doi.org/10.3390/ijms19123803