Modulation of Chemokine- and Adhesion-Molecule Gene Expression and Recruitment of Neutrophil Granulocytes in Rat and Mouse Liver after a Single Gadolinium Chloride or Zymosan Treatment
Abstract
1. Introduction
2. Results
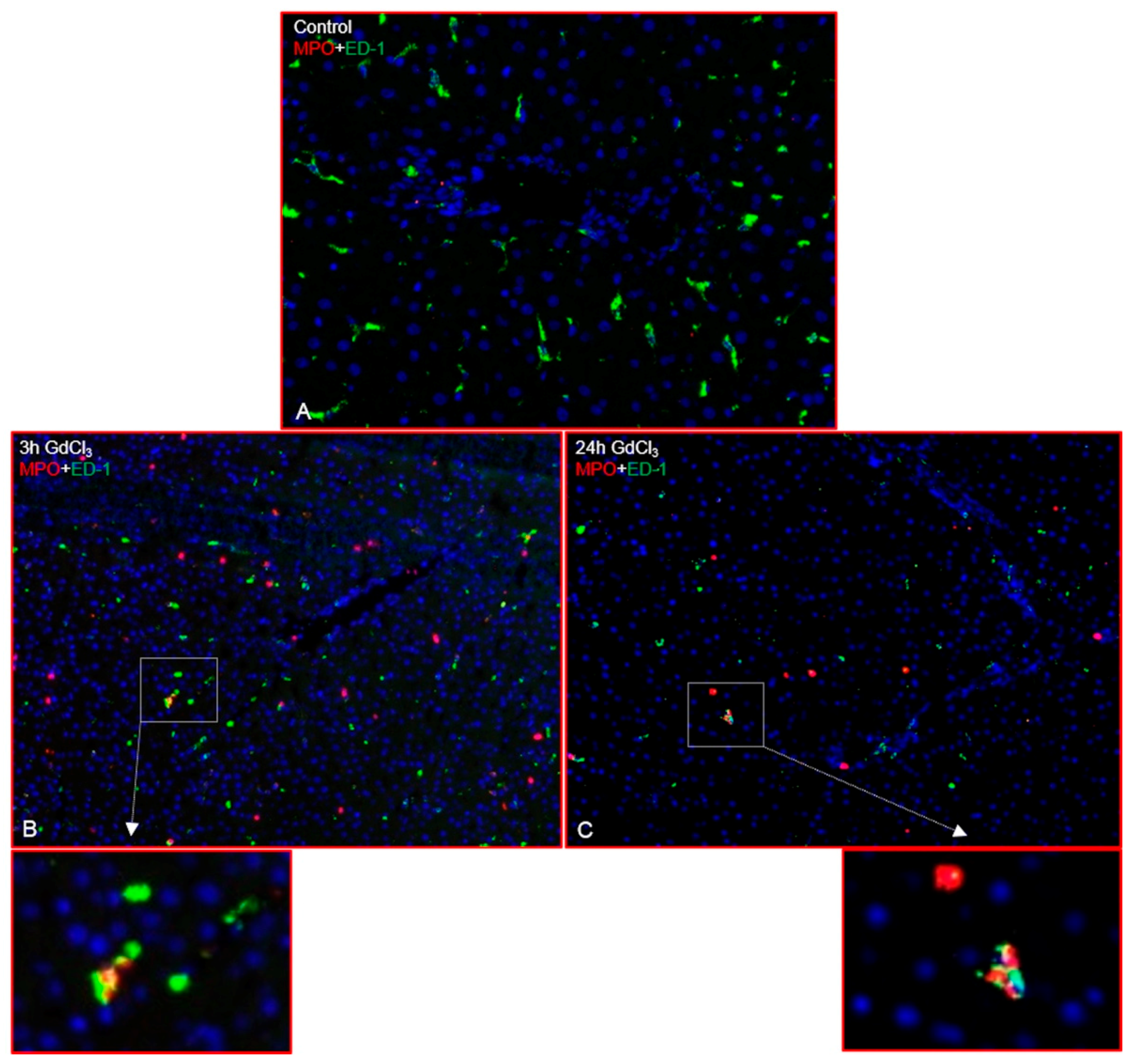
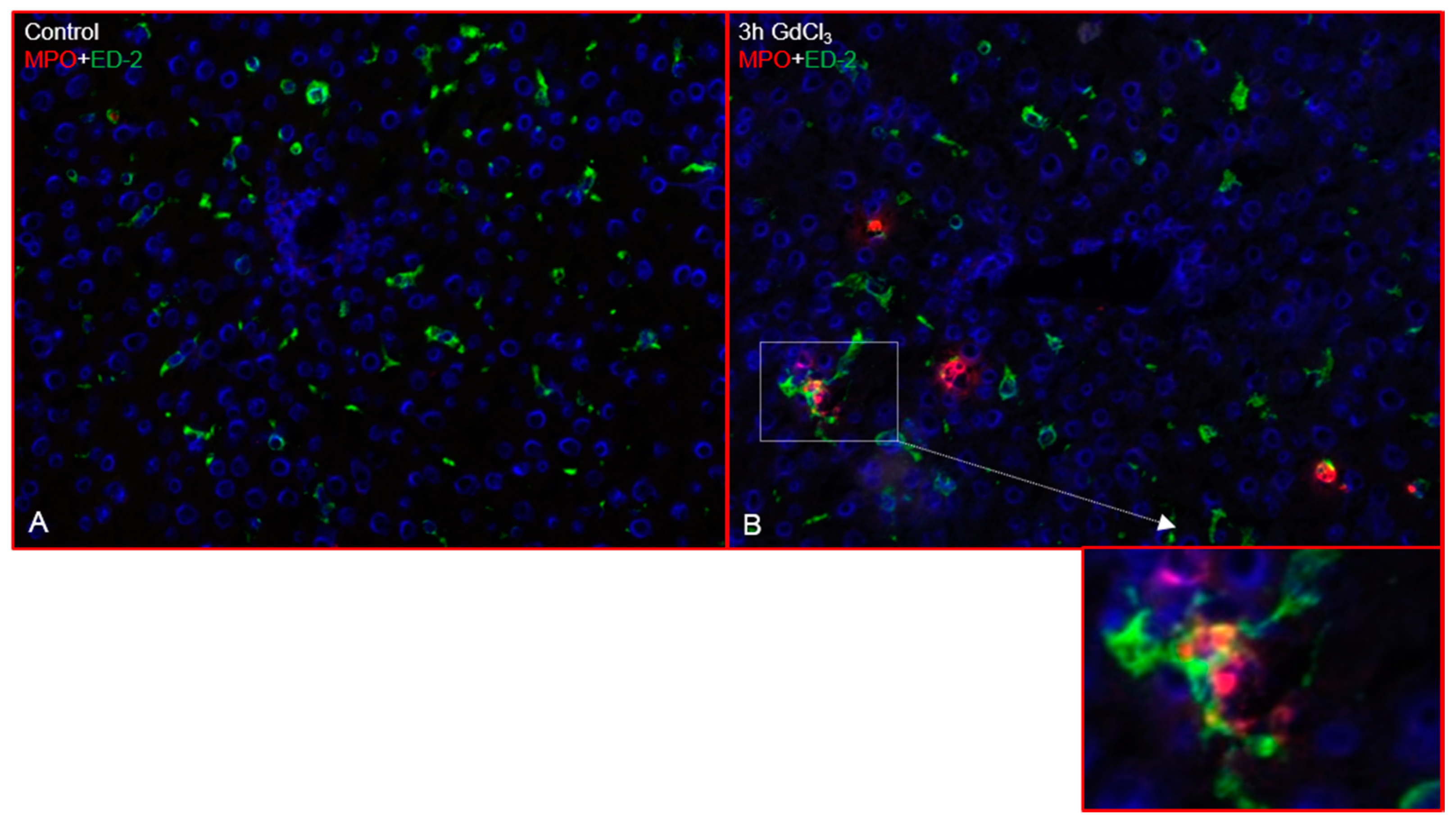
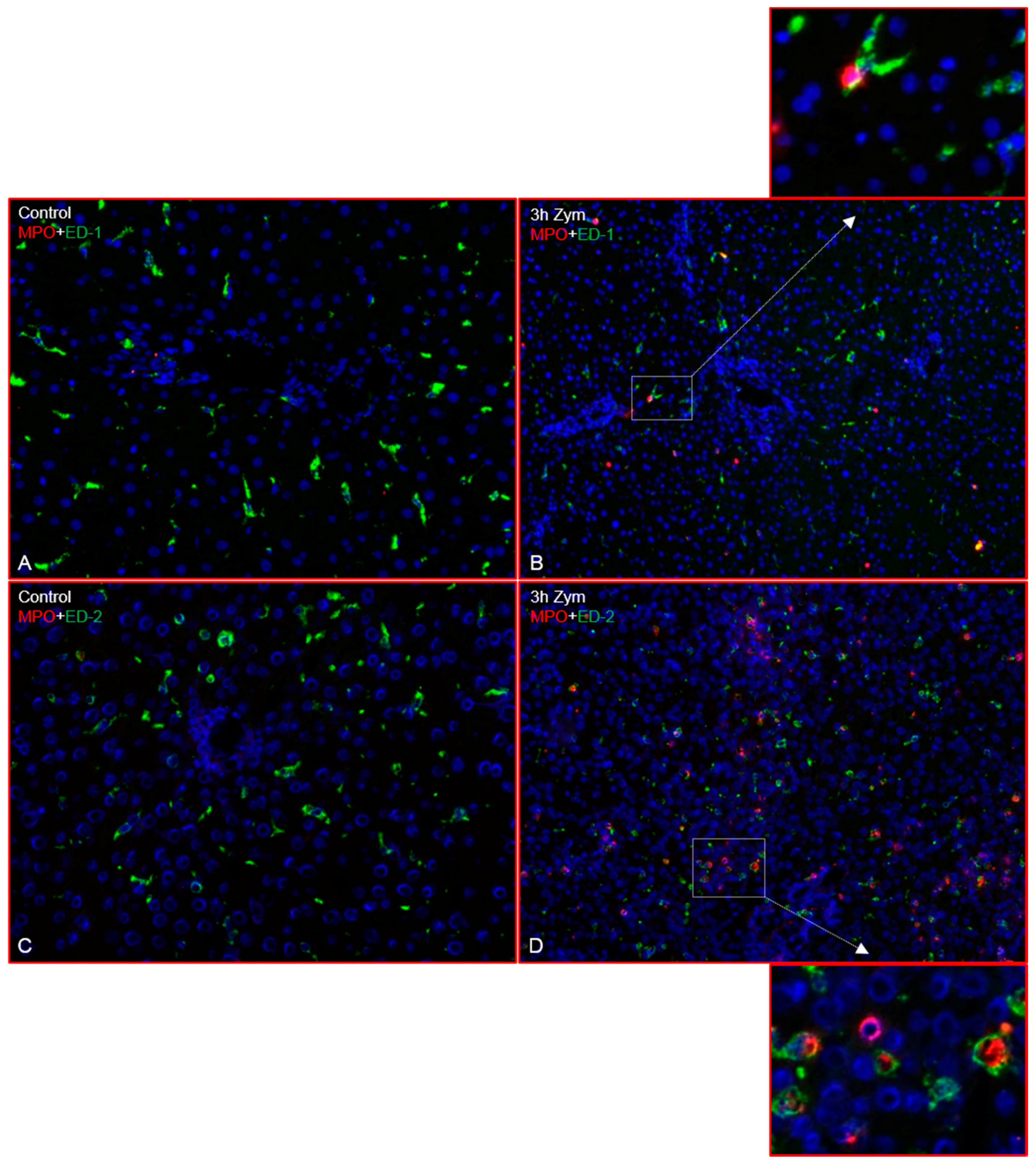
2.1. Immunofluorescence Analysis of the Rat Liver Following Intraperitoneal Injection of GdCl3 or Zymosan
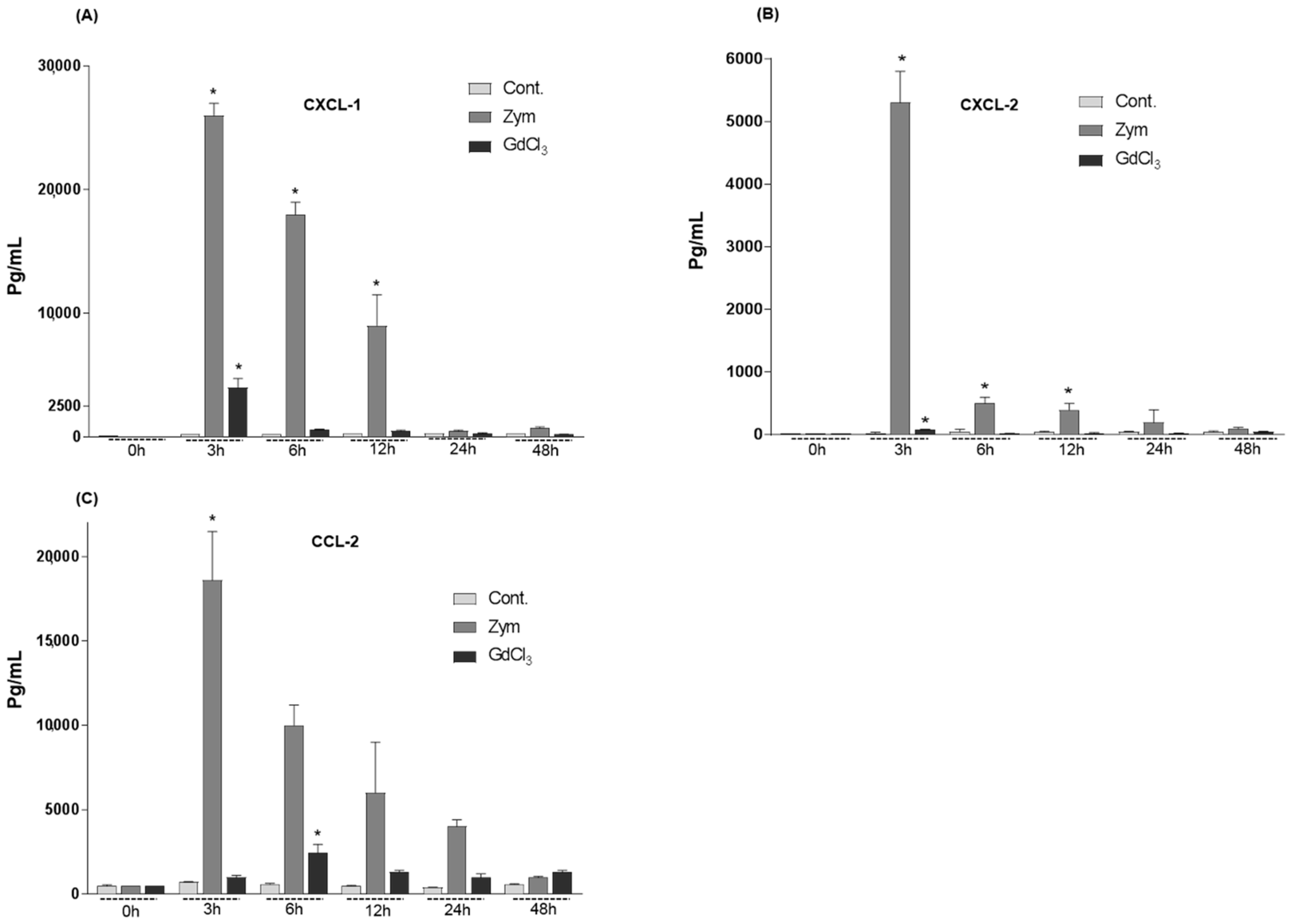
2.2. Changes in the Serum Levels of CXCL-1, CXCL-2 and CCL-2 of Rat after GdCl3 or Zymosan Administration
2.3. Expression of CXC- and CC-Chemokines in Rat Livers after GdCl3 Administration
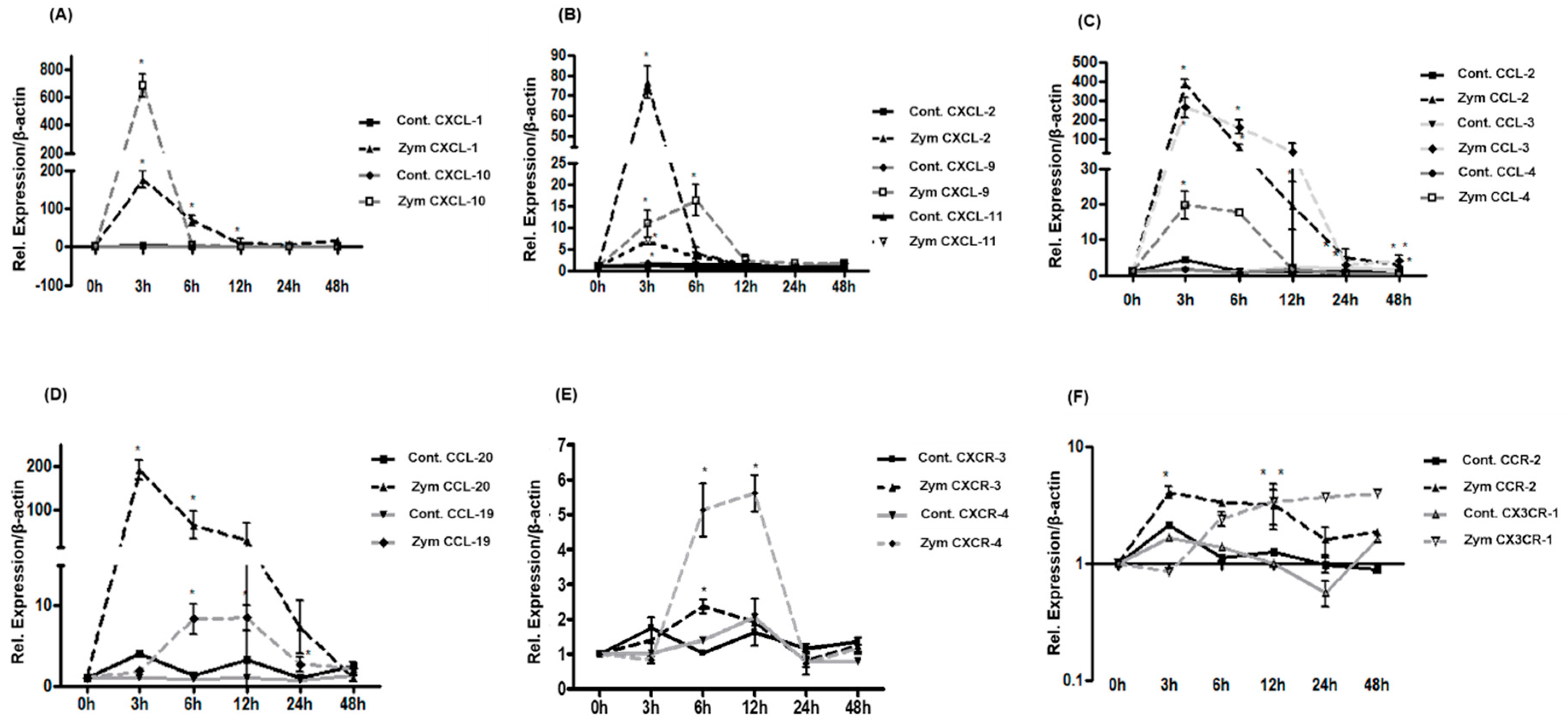
2.4. Expression of CXC-, CC-Chemokines and Chemokine Receptors in Rat Liver after Zymosan Administration
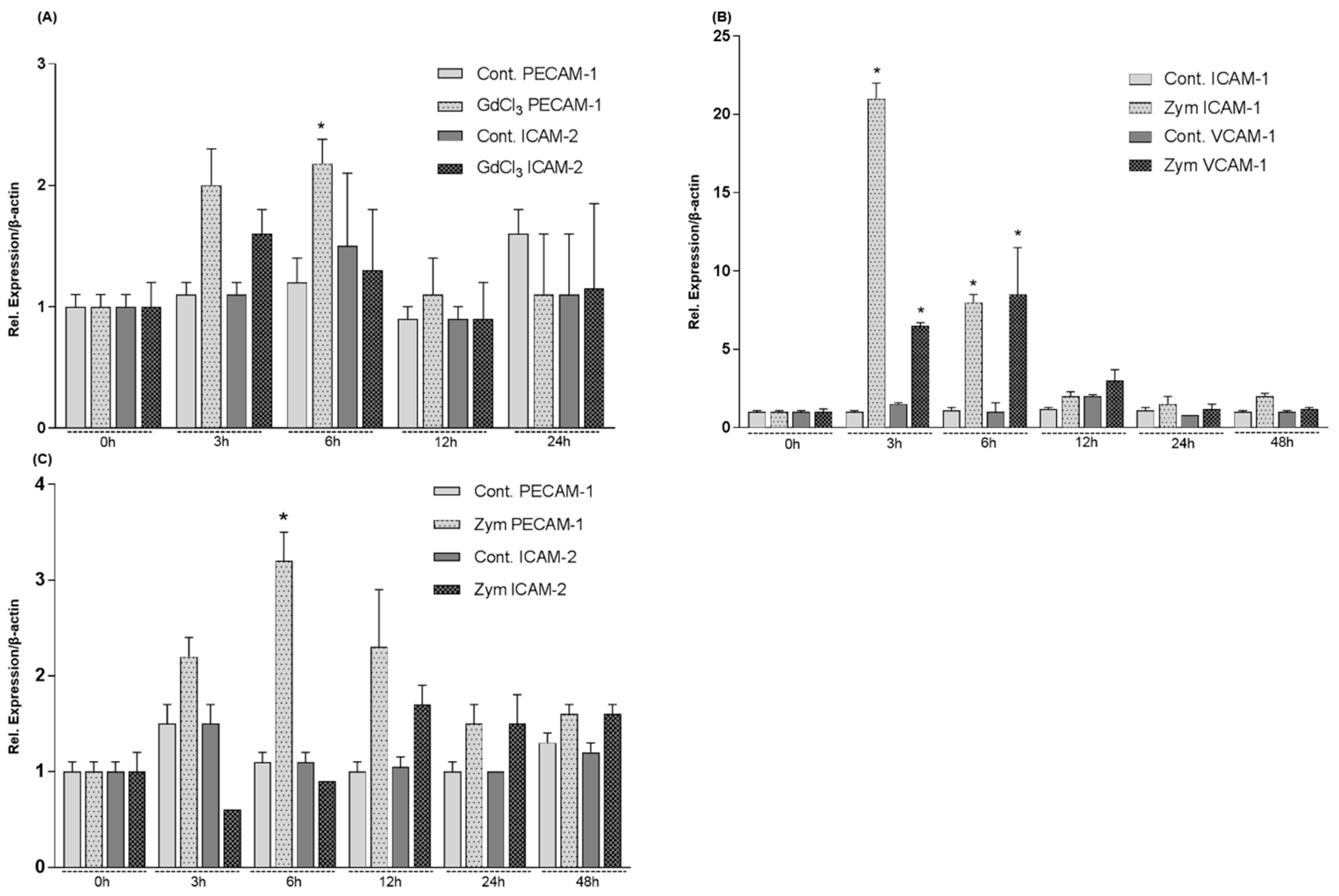
2.5. Expression of the Genes of Adhesion Molecules in Rat Livers after GdCl3 or Zymosan Administration
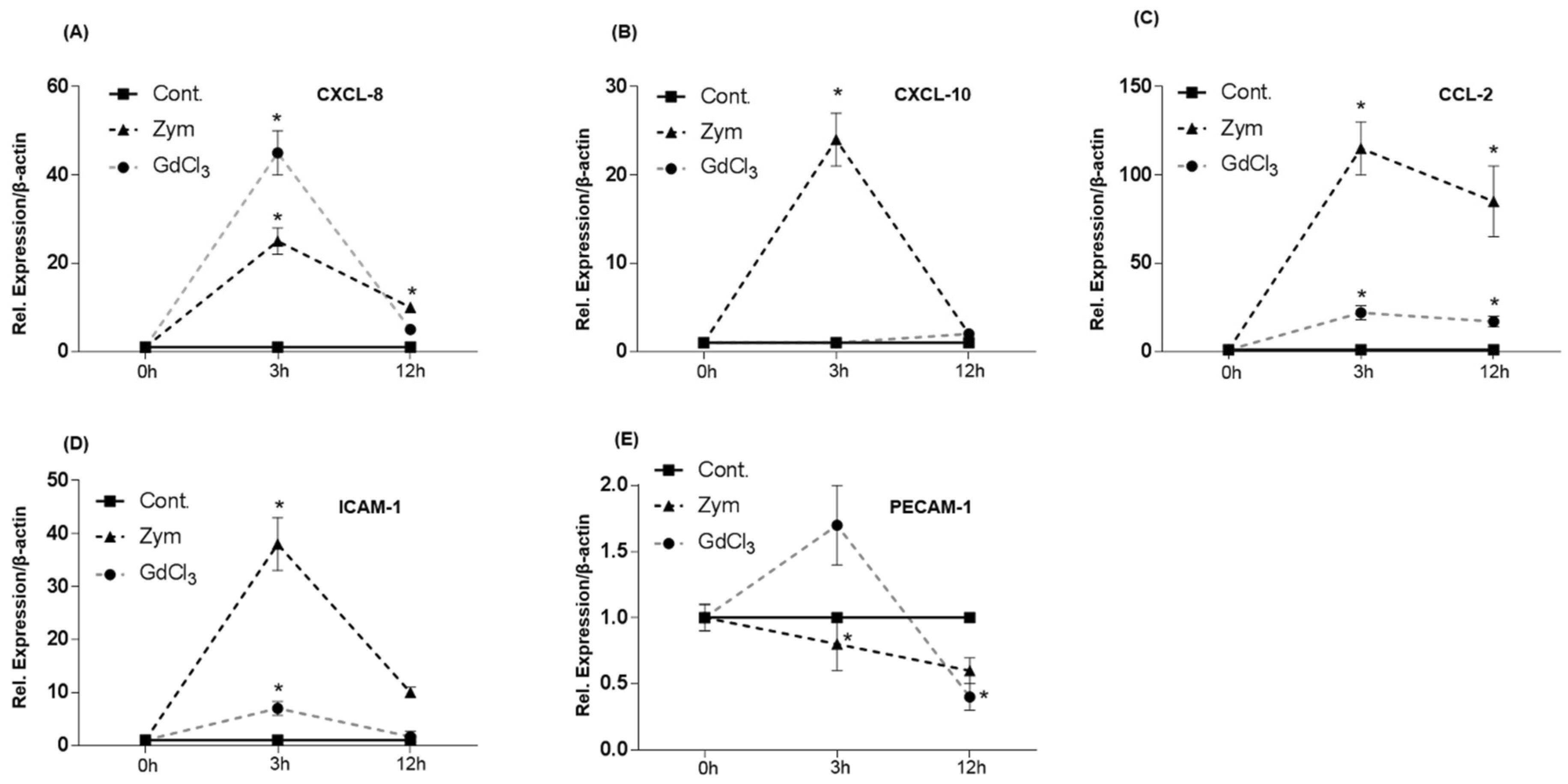
2.6. Expression of CC- and CXC-Chemokine Receptors in C3H/HeJ Mice after GdCl3 or Zymosan Administration
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Reagents
4.3. Antibodies
4.4. GdCl3 and Zymosan Administration to Rats and C3H/HeJ Endotoxin-Resistant Mice
4.5. Liver Immunohistochemistry
4.6. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (PCR)
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amanzada, A.; Malik, I.A.; Nischwitz, M.; Sultan, S.; Naz, N.; Ramadori, G. Myeloperoxidase and elastase are only expressed by neutrophils in normal and in inflamed liver. Histochem. Cell Biol. 2011, 135, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Amanzada, A.; Moriconi, F.; Mansuroglu, T.; Cameron, S.; Ramadori, G.; Malik, I.A. Induction of chemokines and cytokines before neutrophils and macrophage recruitment in different regions of rat liver after TAA administration. Lab. Investig. 2014, 94, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G.; Moriconi, F.; Malik, I.; Dudas, J. Physiology and pathophysiology of liver inflammation, damage and repair. J. Physiol. Pharmacol. 2008, 59 (Suppl. 1), 107–117. [Google Scholar] [PubMed]
- Malik, I.A.; Moriconi, F.; Sheikh, N.; Naz, N.; Khan, S.; Dudas, J.; Mansuroglu, T.; Hess, C.F.; Rave-Frank, M.; Christiansen, H.; et al. Single-dose gamma-irradiation induces up-regulation of chemokine gene expression and recruitment of granulocytes into the portal area but not into other regions of rat hepatic tissue. Am. J. Pathol. 2010, 176, 1801–1815. [Google Scholar] [CrossRef] [PubMed]
- Bajt, M.L.; Farhood, A.; Jaeschke, H. Effects of CXC chemokines on neutrophil activation and sequestration in hepatic vasculature. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G1188–G1195. [Google Scholar] [CrossRef] [PubMed]
- Dorman, R.B.; Gujral, J.S.; Bajt, M.L.; Farhood, A.; Jaeschke, H. Generation and functional significance of CXC chemokines for neutrophil-induced liver injury during endotoxemia. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G880–G886. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G.; Christ, B. Cytokines and the hepatic acute-phase response. Semin. Liver Dis. 1999, 19, 141–155. [Google Scholar] [CrossRef]
- Schlayer, H.J.; Laaff, H.; Peters, T.; Woort-Menker, M.; Estler, H.C.; Karck, U.; Schaefer, H.E.; Decker, K. Involvement of tumor necrosis factor in endotoxin-triggered neutrophil adherence to sinusoidal endothelial cells of mouse liver and its modulation in acute phase. J. Hepatol. 1988, 7, 239–249. [Google Scholar] [CrossRef]
- Simonet, W.S.; Hughes, T.M.; Nguyen, H.Q.; Trebasky, L.D.; Danilenko, D.M.; Medlock, E.S. Long-term impaired neutrophil migration in mice overexpressing human interleukin-8. J. Clin. Investig. 1994, 94, 1310–1319. [Google Scholar] [CrossRef]
- Ruttinger, D.; Vollmar, B.; Wanner, G.A.; Messmer, K. In vivo assessment of hepatic alterations following gadolinium chloride-induced Kupffer cell blockade. J. Hepatol. 1996, 25, 960–967. [Google Scholar] [CrossRef]
- Michael, S.L.; Pumford, N.R.; Mayeux, P.R.; Niesman, M.R.; Hinson, J.A. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology 1999, 30, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Kinoshita, M.; Nakashima, M.; Habu, Y.; Shono, S.; Uchida, T.; Shinomiya, N.; Seki, S. Superoxide produced by Kupffer cells is an essential effector in concanavalin A-induced hepatitis in mice. Hepatology 2008, 48, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Gehring, S.; Dickson, E.M.; San Martin, M.E.; van Rooijen, N.; Papa, E.F.; Harty, M.W.; Tracy, T.F., Jr.; Gregory, S.H. Kupffer cells abrogate cholestatic liver injury in mice. Gastroenterology 2006, 130, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Blazka, M.E.; Germolec, D.R.; Simeonova, P.; Bruccoleri, A.; Pennypacker, K.R.; Luster, M.I. Acetaminophen-induced hepatotoxicity is associated with early changes in NF-kB and NF-IL6 DNA binding activity. J. Inflamm. 1995, 47, 138–150. [Google Scholar] [PubMed]
- Casanova-Acebes, M.; Pitaval, C.; Weiss, L.A.; Nombela-Arrieta, C.; Chevre, R.; Gonzalez, N.; Kunisaki, Y.; Zhang, D.; van Rooijen, N.; Silberstein, L.E.; et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 2013, 153, 1025–1035. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Stark, M.A.; Huo, Y.; Burcin, T.L.; Morris, M.A.; Olson, T.S.; Ley, K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 2005, 22, 285–294. [Google Scholar] [CrossRef]
- Bonder, C.S.; Ajuebor, M.N.; Zbytnuik, L.D.; Kubes, P.; Swain, M.G. Essential role for neutrophil recruitment to the liver in concanavalin A-induced hepatitis. J. Immunol. 2004, 172, 45–53. [Google Scholar] [CrossRef]
- Jaeschke, H. Chemokines, neutrophils, and inflammatory liver injury. Shock 1996, 6, 403–404. [Google Scholar] [CrossRef]
- Ohta, Y.; Imai, Y.; Matsura, T.; Kitagawa, A.; Yamada, K. Preventive effect of neutropenia on carbon tetrachloride-induced hepatotoxicity in rats. J. Appl. Toxicol. 2006, 26, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Moriconi, F.; Ahmad, G.; Ramadori, P.; Malik, I.; Sheikh, N.; Merli, M.; Riggio, O.; Dudas, J.; Ramadori, G. Phagocytosis of gadolinium chloride or zymosan induces simultaneous upregulation of hepcidin- and downregulation of hemojuvelin- and Fpn-1-gene expression in murine liver. Lab. Investig. 2009, 89, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Underhill, D.M. Macrophage recognition of zymosan particles. J. Endotoxin Res. 2003, 9, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Friedland, J.S.; Remick, D.G.; Shattock, R.; Griffin, G.E. Secretion of interleukin-8 following phagocytosis of Mycobacterium tuberculosis by human monocyte cell lines. Eur J. Immunol. 1992, 22, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Sanguedolce, M.V.; Capo, C.; Bongrand, P.; Mege, J.L. Zymosan-stimulated tumor necrosis factor-alpha production by human monocytes. Down-modulation by phorbol ester. J. Immunol. 1992, 148, 2229–2236. [Google Scholar] [PubMed]
- Petit, F.; Bagby, G.J.; Lang, C.H. Tumor necrosis factor mediates zymosan-induced increase in glucose flux and insulin resistance. Am. J. Physiol. 1995, 268 Pt 1, E219–E228. [Google Scholar] [CrossRef]
- Hardonk, M.J.; Dijkhuis, F.W.; Hulstaert, C.E.; Koudstaal, J. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. J. Leukoc. Biol. 1992, 52, 296–302. [Google Scholar] [CrossRef]
- Zimmermann, H.W.; Trautwein, C.; Tacke, F. Functional role of monocytes and macrophages for the inflammatory response in acute liver injury. Front. Physiol. 2012, 3, 56. [Google Scholar] [CrossRef]
- Zaldivar, M.M.; Berres, M.L.; Sahin, H.; Nellen, A.; Heinrichs, D.; Schmitz, P.; Gassler, N.; Streetz, K.L.; Trautwein, C.; Wasmuth, H.E. The chemokine receptor CXCR3 limits injury after acute toxic liver damage. Lab. Investig. 2012, 92, 724–734. [Google Scholar] [CrossRef]
- Moriconi, F.; Christiansen, H.; Raddatz, D.; Dudas, J.; Hermann, R.M.; Rave-Frank, M.; Sheikh, N.; Saile, B.; Hess, C.F.; Ramadori, G. Effect of radiation on gene expression of rat liver chemokines: In vivo and in vitro studies. Radiat. Res. 2008, 169, 162–169. [Google Scholar] [CrossRef]
- Christiansen, H.; Batusic, D.; Saile, B.; Hermann, R.M.; Dudas, J.; Rave-Frank, M.; Hess, C.F.; Schmidberger, H.; Ramadori, G. Identification of genes responsive to gamma radiation in rat hepatocytes and rat liver by cDNA array gene expression analysis. Radiat. Res. 2006, 165, 318–325. [Google Scholar] [CrossRef]
- Neubauer, K.; Lindhorst, A.; Tron, K.; Ramadori, G.; Saile, B. Decrease of PECAM-1-gene-expression induced by proinflammatory cytokines IFN-gamma and IFN-alpha is reversed by TGF-beta in sinusoidal endothelial cells and hepatic mononuclear phagocytes. BMC Physiol. 2008, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Moriconi, F.; Malik, I.A.; Amanzada, A.; Blaschke, M.; Raddatz, D.; Khan, S.; Ramadori, G. The anti-TNF-alpha antibody infliximab indirectly regulates PECAM-1 gene expression in two models of in vitro blood cell activation. Lab. Investig. 2012, 92, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, Y.; Morishima, T.; Kimura, H.; Nishikawa, K.; Tsurumi, T.; Kuzushima, K. Antigen-driven expansion and contraction of CD8+-activated T cells in primary EBV infection. J. Immunol. 1999, 163, 5735–5740. [Google Scholar] [PubMed]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; Van, H.C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018. [Google Scholar] [CrossRef]
- Saile, B.; Ramadori, G. Inflammation, damage repair and liver fibrosis—Role of cytokines and different cell types. Z. Gastroenterol. 2007, 45, 77–86. [Google Scholar] [CrossRef]
- Wang, J.; Hossain, M.; Thanabalasuriar, A.; Gunzer, M.; Meininger, C.; Kubes, P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 2017, 358, 111–116. [Google Scholar] [CrossRef]
- Sultan, S.; Ahmad, S.; Rave-Frank, M.; Malik, I.A.; Hess, C.F.; Christiansen, H.; Cameron, S. Induction of Lipocalin2 in a Rat Model of Lung Irradiation. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef]
- Ramadori, P.; Ahmad, G.; Ramadori, G. Cellular and molecular mechanisms regulating the hepatic erythropoietin expression during acute-phase response: A role for IL-6. Lab. Investig. 2010, 90, 1306–1324. [Google Scholar] [CrossRef]

| Primer | Forward 5′ → 3′ | Reverse 5′ → 3′ |
|---|---|---|
| CXCL-1 | GGC AGG GAT TCA CTT CAA GA | GCC ATC GGT GCA ATC TAT CT |
| CXCL-2 | ATC CAG AGC TTG ACG GTG AC | AGG TAC GAT CCA GGC TTC CT |
| CXCL-5 | CTC AAG CTG CTC CTT TCT CG | GCG ATC ATT TTG GGG TTA AT |
| CXCL-9 | GCC TTG ACT CCA GCA CGG T | GAC TTC ATG GCA GAG CCG AG |
| CXCL-10 | CTG TCG TTC TCT GCC TCG TG | GGA TCC CTT CTT GAG TCC CAC TCA |
| CXCL-11 | AGA ACA TGT GAT GGG CCC TC | GGG TCA GCT TCT TGG CAC AG |
| CCL-2 | AGG CAG ATG CAG TTA ATG CCC | ACA CCT GCT GCT GGT GAT TCT C |
| CCL-3 | TTT TGA GAC CAG CAG CAG CCT TT | CTC AAG CCC CTG CTC TAC AC |
| CCL-4 | TCC CGG AAG ATT CAT CGG | GCA CAG ATT TGC CTG CCT TTT |
| CCL-19 | AGA ACG CAT CAT CCG AAG AC | TGC TCA CAC TCA CGT TCA CA |
| CCL-20 | CAA CTT TGA CTG CTG CCT CA | TTC CAT CCC AGA AAA GCA TC |
| CCR-2 | CTT GTG GCC CTT ATT TTC CA | AGA TGA GCC TCA CAG CCC TA |
| CCR-4 | GAA TGC CAC AGA TGT CAC AG | GCA CAA ACA GTA AAT CCG AG |
| CXCR-3 | TAC CTT GAG GTC AGT GAA CG | AAA GAG GAG GCT GTA GAG GA |
| CXCR-4 | GCT GAG GAG CAT GAC AGA CA | GAT GAA GGC CAG GAT GAG AA |
| CX3CR-1 | TGA CTG GCA GAT CCA GAG GTT | GTA GAA TAT GGA CAG GAA CAC |
| ICAM-1 | TGC ACG TCC CTG GTG ATA CTC | TGT CAA ACG GGA GAT GAA TGG |
| ICAM-2 | AGC AGC AGG CAG AGA GTT TC | TCT GCC ACA GAG CAG AGA GA |
| PECAM-1 | TCA GCT GCC AGT CAG TAA ATG G | TCT GGA AGT TGC TCT TTG CTC TT |
| VCAM-1 | ACA TGT GCT GCT GTT GGC TGT | GCT CAG CGT CAG TGT GGA TGT A |
| β-actin | ACC ACC ATG TAG CCA GGC ATT | CCA CAC AGA GTA CTT GCC CTC A |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, S.; Ramadori, G.; Moriconi, F. Modulation of Chemokine- and Adhesion-Molecule Gene Expression and Recruitment of Neutrophil Granulocytes in Rat and Mouse Liver after a Single Gadolinium Chloride or Zymosan Treatment. Int. J. Mol. Sci. 2018, 19, 3891. https://doi.org/10.3390/ijms19123891
Ahmad S, Ramadori G, Moriconi F. Modulation of Chemokine- and Adhesion-Molecule Gene Expression and Recruitment of Neutrophil Granulocytes in Rat and Mouse Liver after a Single Gadolinium Chloride or Zymosan Treatment. International Journal of Molecular Sciences. 2018; 19(12):3891. https://doi.org/10.3390/ijms19123891
Chicago/Turabian StyleAhmad, Shakil, Giuliano Ramadori, and Federico Moriconi. 2018. "Modulation of Chemokine- and Adhesion-Molecule Gene Expression and Recruitment of Neutrophil Granulocytes in Rat and Mouse Liver after a Single Gadolinium Chloride or Zymosan Treatment" International Journal of Molecular Sciences 19, no. 12: 3891. https://doi.org/10.3390/ijms19123891
APA StyleAhmad, S., Ramadori, G., & Moriconi, F. (2018). Modulation of Chemokine- and Adhesion-Molecule Gene Expression and Recruitment of Neutrophil Granulocytes in Rat and Mouse Liver after a Single Gadolinium Chloride or Zymosan Treatment. International Journal of Molecular Sciences, 19(12), 3891. https://doi.org/10.3390/ijms19123891





