Studies on the Curing Efficiency and Mechanical Properties of Bis-GMA and TEGDMA Nanocomposites Containing Silver Nanoparticles
Abstract
:1. Introduction
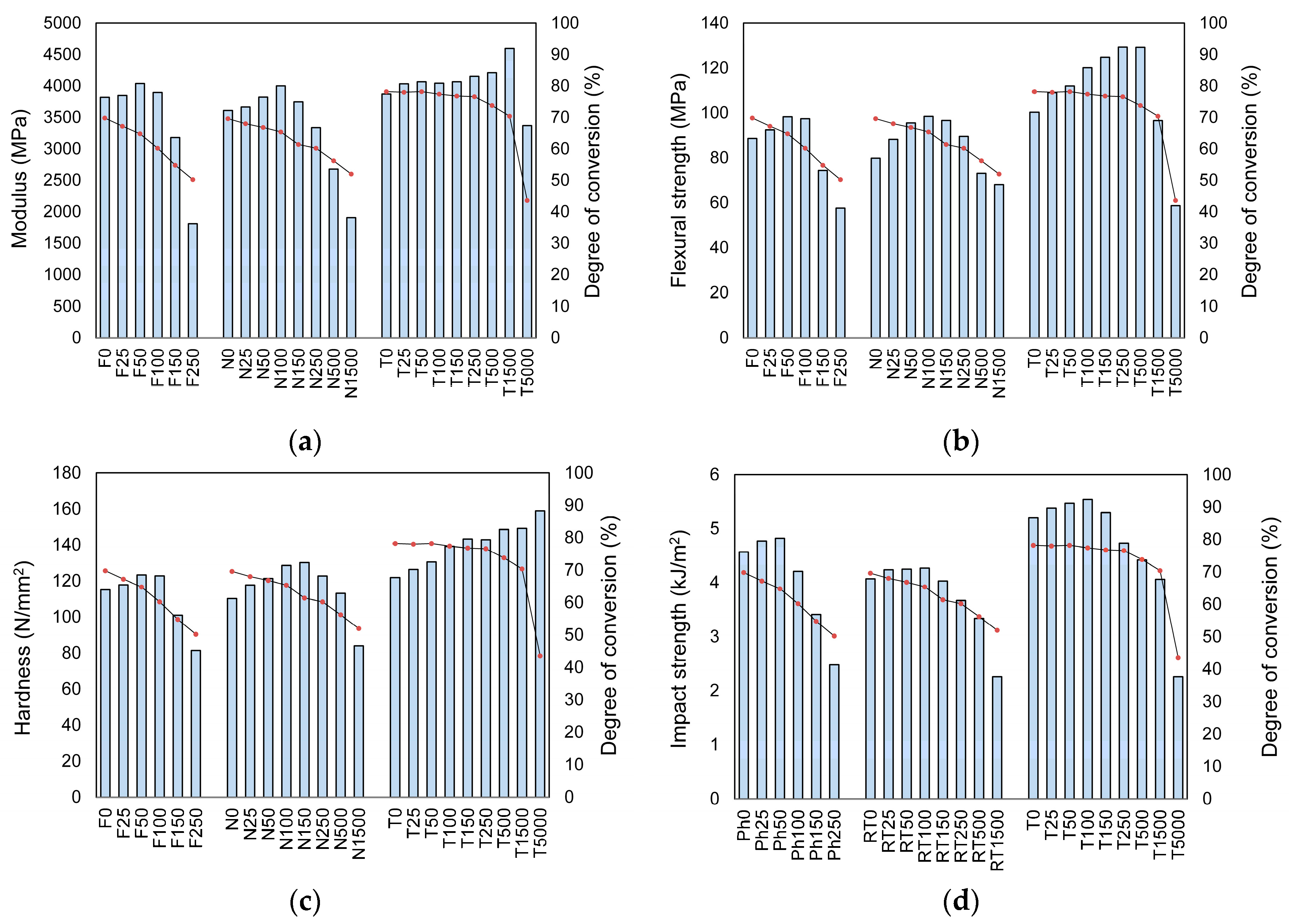
2. Results
- Photopolymerization—by the addition of CQ (camphorquinone) 0.4 wt.% and DMAEMA (N,N-dimethylaminoethyl methacrylate) 1 wt.%;
- Room temperature polymerization—by the addition of BPO (benzoyl peroxide) 0.5 wt% and DMPT (N,N-dimethyl-p-toluidine) 0.05 wt%;
- Thermal polymerization—by the addition of BPO 1 wt%.
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of AgNP-Loaded Resin Compositions
4.3. Curing Procedure
4.3.1. Photopolymerization
4.3.2. Room Temperature Polymerization (Chemical Polymerization)
4.3.3. Thermal Polymerization
4.4. UV/VIS Spectroscopy
4.5. Viscosity
4.6. Polymerization Shrinkage
4.7. Degree of Conversion
4.8. Mechanical Properties
4.8.1. Flexural Properties
4.8.2. Hardness
4.8.3. Impact Strength
4.9. Water Sorption
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AgNP | Silver nanoparticles |
| Bis-GMA | 2,2′-bis-[4-(2-hydroxy-3-methacryloyloxy propoxy)phenyl]propane |
| BPO | Benzoyl peroxide |
| RT | Room temperature polymerization |
| CQ | Camphorquinone |
| DC | Degree of conversion |
| DMPT | N,N-dimethyl-p-toluidine |
| DMAEMA | N,N-dimethylaminoethyl methacrylate |
| E | Flexural modulus |
| H | Hardness |
| TEGDMA | Triethylene glycol dimethacrylate |
| Ph | Photopolymerization |
| S | Polymerization shrinkage |
| T | Thermal polymerization |
| WS | Water sorption |
References
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and antimicrobial biomaterials: An overview. APMIS 2017, 125, 392–417. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.M.; Sakaguchi, R.L. Restorative materials—Composites and polymers. In Craig’s Restorative Dental Materials, 13th ed.; Mosby: St. Louis, MI, USA, 2013; ISBN 9780323081085. [Google Scholar]
- Khader, B.A.; Towler, M.R. Materials and techniques used in cranioplasty fixation: A review. Mater. Sci. Eng. C 2016, 66, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Vallo, C.I.; Schroeder, W.F. Properties of acrylic bone cements formulated with Bis-GMA. J. Biomed. Mater. Res. 2005, 74B, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Sawada, M.; Matsuda, Y.; Nakamura, T.; Kokubo, T. Antibiotic delivery system using bioactive bone cement consisting of Bis-GMA/TEGDMA resin and bioactive glass ceramics. Biomaterials 1997, 18, 1559–1564. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.; Jurczyk, S. Comparative study of structure-property relationships in polymer networks based on Bis-GMA, TEGDMA and various urethane-dimethacrylates. Materials 2015, 8, 1230–1248. [Google Scholar] [CrossRef] [PubMed]
- Barszczewska-Rybarek, I. Structure-property relationships in dimethacrylate networks based on Bis-GMA, UDMA and TEGDMA. Dent. Mater. 2009, 25, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.; Suzuki, K.; Watts, D.C. Marginal gap formation of light-activated restorative materials: Effects of immediate setting shrinkage and bond strength. Dent. Mater. 2002, 18, 203–210. [Google Scholar] [CrossRef]
- Mishra, S.K.; Chowdhary, R.; Kumari, S. Microleakage at the Different Implant Abutment Interface: A Systematic Review. J. Clin. Diagn. Res. 2017, 11, ZE10–ZE15. [Google Scholar] [CrossRef]
- Hamouda, I.M. Current perspectives of nanoparticles in medical and dental biomaterials. J. Biomed. Res. 2012, 26, 143–151. [Google Scholar] [CrossRef]
- Ástvaldsdóttir, Á.; Dagerhamn, J.; van Dijken, J.W.V.; Naimi-Akbar, A.; Sandborgh-Englund, G.; Tranæus, S.; Nilsson, M. Longevity of posterior resin composite restorations in adults—A systematic review. J. Dent. 2015, 43, 934–954. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Wagar, A. Nanotechnology and its applications in dentistry. In Emerging Nanotechnologies in Dentistry, 2nd ed.; Karthikeyan, S., Wagar, A., Eds.; William Andrew (Elsevier): Norwich, NY, USA, 2017; pp. 1–15. ISBN 9780128122914. [Google Scholar]
- Kasraei, S.; Sami, L.; Hendi, S.; AliKhani, M.-Y.; Rezaei-Soufi, L.; Khamverdi, Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor. Dent. Endod. 2014, 39, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Yudovin-Farber, I.; Bahir, R.; Domb, A.J.; Weiss, E.I. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials 2006, 27, 3995–4002. [Google Scholar] [CrossRef] [PubMed]
- Tunney, M.; Dunne, N.; Einarsson, G.; McDowell, A.; Kerr, A.; Patrick, S. Biofilm formation by bacteria isolated from retrieved failed prosthetic hip implants in an in vitro model of hip arthroplasty antibiotic prophylaxis. J. Orthop. Res. 2007, 25, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.; Cheng, L.; Zhang, K.; Weir, M.D.; Rodrigues, L.K.; Xu, H.H. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent. Mater. 2013, 29, 199–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.H.K.; Moreau, J.L.; Sun, L.; Chow, L.C. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent. Mater. 2011, 27, 762–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydin Sevinç, B.; Hanley, L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli Hojati, S.; Alaghemand, H.; Hamze, F.; Babaki, F.A.; Rajab-Nia, R.; Rezvani, M.B.; Kaviani, M.; Atai, M. Antibacterial, physical and mechanical properties of flowable resin composites containing zinc oxide nanoparticles. Dent. Mater. 2013, 29, 495–505. [Google Scholar] [CrossRef]
- Poosti, M.; Ramazanzadeh, B.A.; Zebarjad, M.; Javadzadeh, P.; Naderinasab, M.; Shakeri, M.T. Shear bond strength and antibacterial effects of orthodontic composite containing TiO2 nanoparticles. Eur. J. Orthod. 2013, 35, 676–679. [Google Scholar] [CrossRef]
- Russo, T.; Gloria, A.; De Santis, R.; Amora, U.D.; Balato, G.; Vollaro, A.; Oliviero, O.; Improta, G.; Triassi, M.; Ambrosio, L. Preliminary focus on the mechanical and antibacterial activity of a PMMA-based bone cement loaded with gold nanoparticles. Bioact. Mater. 2017, 2, 156–161. [Google Scholar] [CrossRef]
- Chamundeeswari, M.; Sobhana, S.S.; Jacob, J.P.; Kumar, M.G.; Devi, M.P.; Sastry, T.P.; Mandal, A.B. Preparation, characterization and evaluation of a biopolymeric gold nanocomposite with antimicrobial activity. Biotechnol. Appl. Biochem. 2010, 55, 29–35. [Google Scholar] [CrossRef]
- Prokopovich, P.; Köbrick, M.; Brousseau, E.; Perni, S. Potent antimicrobial activity of bone cement encapsulating silver nanoparticles capped with oleic acid. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Fatemeh, K.; Mohammad Javad, M.; Samaneh, K. The effect of silver nanoparticles on composite shear bond strength to dentin with different adhesion protocols. J. Appl. Oral Sci. 2017, 25, 367–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chladek, G.; Kasperski, J.; Barszczewska-Rybarek, I.; Żmudzki, J. Sorption, Solubility, Bond Strength and Hardness of Denture Soft Lining Incorporated with Silver Nanoparticles. Int. J. Mol. Sci. 2013, 14, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Kassaee, M.Z.; Akhavan, A.; Sheikh, N.; Sodagar, A. Antibacterial effects of a new dental acrylic resin containing silver nanoparticles. J. Appl. Polym. Sci. 2008, 110, 1699–1703. [Google Scholar] [CrossRef]
- Corrêa, J.M.; Mori, M.; Sanches, H.L.; da Cruz, A.D.; Poiate, I.A.V.P. Silver Nanoparticles in Dental Biomaterials. Int. J. Biomater. 2015, 485275. [Google Scholar] [CrossRef]
- Durner, J.; Stojanovic, M.; Urcan, E.; Hickel, R.; Reichl, F.X. Influence of silver nano-particles on monomer elution from light-cured composites. Dent. Mater. 2011, 27, 631–636. [Google Scholar] [CrossRef]
- Nam, K.-Y. In vitro antimicrobial effect of the tissue conditioner containing silver nanoparticles. J. Adv. Prosthodont. 2011, 3, 20–24. [Google Scholar] [CrossRef]
- Shubnikov, A.V. Nanomaterials. In Advances in Nanotechnology Research and Application, 2012 ed.; Acton, A.Q., Ed.; ScholarlyEditions™ eBook: Atlanta, GR, USA, 2012; Volume 86, pp. 4339–4579. ISBN 978-1-4649-046-5. [Google Scholar]
- Pfeifer, C.S.; Shelton, Z.R.; Braga, R.R.; Windmoller, D.; Machado, J.C.; Stansbury, J.W. Characterization of dimethacrylate polymeric networks: A study of the crosslinked structure formed by monomers used in dental composites. Eur. Polym. J. 2011, 47, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Kamoun, E.A.; Winkel, A.; Eisenburger, M.; Menzel, H. Carboxylated camphorquinone as visible-light photoinitiator for biomedical application: Synthesis, characterization, and application. Arab. J. Chem. 2016, 9, 745–754. [Google Scholar] [CrossRef] [Green Version]
- Abliz, D.; Duan, Y.; Steuernagel, L.; Xie, L.; Li, D.; Ziegmann, G. Curing Methods for Advanced Polymer Composites—A Review. Polym. Polym. Compos. 2018, 21, 341–348. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Stansbury, J.W. Dimethacrylate network formation and polymer property evolution as determined by the selection of monomers and curing conditions. Dent. Mater. 2012, 28, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomrink, G.J.; DiCicco, M.P.; Clineff, T.D.; Erbe, E.M. Evaluation of the reaction kinetics of CORTOSS, a thermoset cortical bone void filler. Biomaterials 2003, 24, 1023–1031. [Google Scholar] [CrossRef]
- Jancar, J.; Wang, W.; DiBenedetto, A.T. On the heterogeneous structure of thermally cured bis-GMA/TEGDMA resins. J. Mater. Sci. Mater. Med. 2000, 11, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, M. Small Radical Chemistry. In Handbook of Radical Polymerization, 1st ed.; Matyjaszewski, K., Davis, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2002; Volume 2, pp. 77–116. ISBN 978-0-471-39274-3. [Google Scholar]
- Barszczewska-Rybarek, I.; Korytkowska, A.; Gibas, M. Investigations on the structure of poly(dimethacrylate)s. Des. Monomers Polym. 2001, 4, 301–314. [Google Scholar] [CrossRef]
- Bercier, A.; Gonçalves, S.; Lignon, O.; Fitremann, J. Calcium Phosphate Bone Cements Including Sugar Surfactants: Part One—Porosity, Setting Times and Compressive Strength. Materials 2010, 3, 4695–4709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mau, H.; Schelling, K.; Heisel, C.; Wang, J.S.; Breusch, S.J. Comparison of various vacuum mixing systems and bone cements as regards reliability, porosity and bending strength. Acta Orthop. Scand. 2004, 75, 160–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, R.; Peng, L.; Lan, J.; Jiang, S.; Yan, W. Microstructure and hydrophobic properties of silver nanoparticles on amino-functionalised cotton fabric. Mater. Technol. 2016, 31, 139–144. [Google Scholar] [CrossRef]
- Chladek, G.; Mertas, A.; Barszczewska-Rybarek, I.; Nalewajek, T.; Żmudzki, J.; Król, W.; Łukaszczyk, J. Antifungal Activity of Denture Soft Lining Material Modified by Silver Nanoparticles—A Pilot Study. Int. J. Mol. Sci. 2011, 12, 4735–4744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barszczewska-Rybarek, I.M. Quantitative determination of degree of conversion in photocured poly(urethane-dimethacrylate)s by FTIR spectroscopy. J. Appl. Polym. Sci. 2012, 123, 1604–1611. [Google Scholar] [CrossRef]
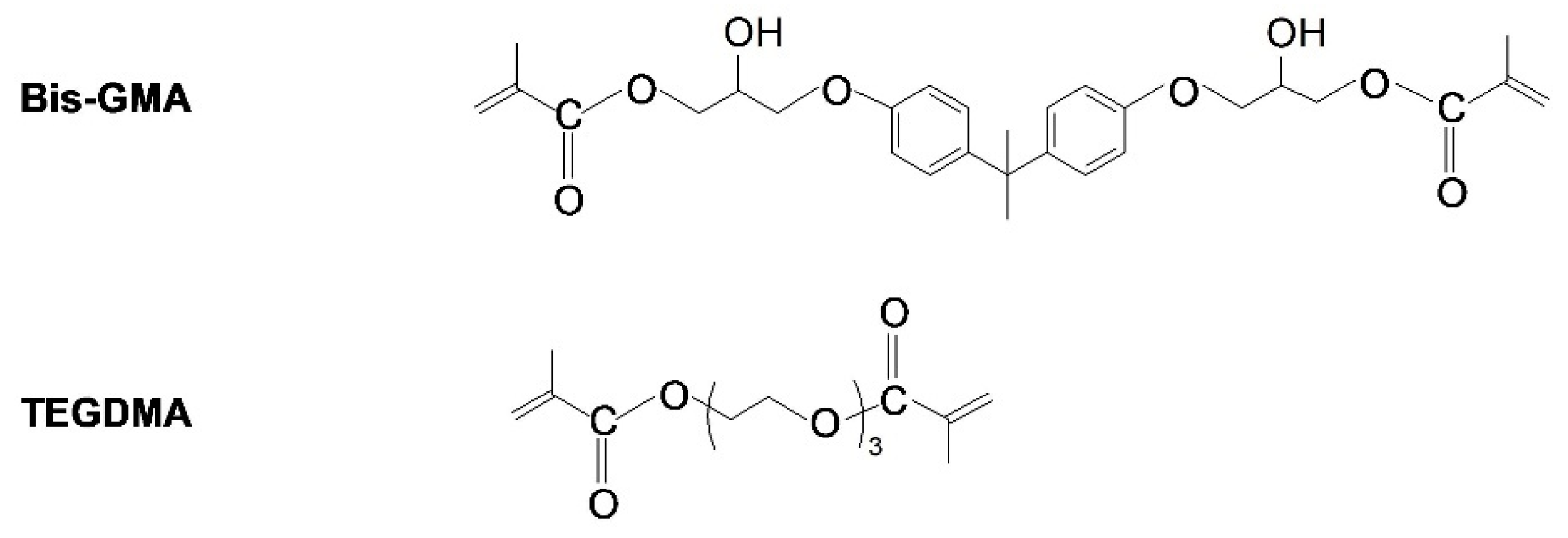



| AgNP Concentration in Bis-GMA/TEGDMA/AgNP Compositions (ppm) | AgNP Concentration in hexane Colloid (ppm) | The Amount of AgNP/hexane Colloid (g) |
|---|---|---|
| 25 | 100 | 25.0 |
| 50 | 100 | 50.0 |
| 100 | 100 | 100.0 |
| 150 | 100 | 150.0 |
| 250 | 100 | 250.0 |
| 500 | 100 | 500.0 |
| 1500 | 500 | 300.4 |
| 5000 | 1000 | 502.5 |
| AgNP Concentration in Nanocomposite (ppm) | Sample Name | ||
|---|---|---|---|
| Photopolymerization | Room Temperature Polymerization | Thermal Polymerization | |
| 0 | Ph0 | RT0 | T0 |
| 25 | Ph25 | RT25 | T25 |
| 50 | Ph50 | RT50 | T50 |
| 100 | Ph100 | RT100 | T100 |
| 150 | Ph150 | RT150 | T150 |
| 250 | Ph250 | RT250 | T250 |
| 500 | - | RT500 | T500 |
| 1500 | - | RT1500 | T1500 |
| 5000 | - | - | T5000 |
| Sample | Polymerization Shrinkage (%) | Degree of Conversion (%) |
|---|---|---|
| Ph0 | 8.37 ± 0.58 1 | 69.8 ± 6.0 31,32 |
| Ph25 | 8.22 ± 0.60 2,6 | 67.2 ± 7.0 33 |
| Ph50 | 8.01 ± 0.70 3,7 | 64.8 ± 4.6 34,35,f |
| Ph100 | 7.41 ± 0.62 4,6 | 60.2 ± 4.2 g |
| Ph150 | 6.95 ± 0.96 5,7,a,b | 54.8 ± 5.6 31,34,h |
| Ph250 | 5.44 ± 0.53 1–5,c,d | 50.2 ± 7.5 32,33,35,i,j |
| RT0 | 8.39 ± 0.60 8,9 | 69.6 ± 5.4 36,39,k |
| RT25 | 8.13 ± 0.70 10 | 68.0 ± 4.9 37,40 |
| RT50 | 7.99 ± 0.62 11 | 66.8 ± 4.7 41,l |
| RT100 | 7.84 ± 0.44 12 | 65.4 ± 7.2 38,42 |
| RT150 | 7.77 ± 0.78 13,a | 61.4 ± 7.2 m |
| RT250 | 7.66 ± 0.73 14,c | 60.2 ± 6.3 j,n |
| RT500 | 7.41 ± 0.62 8,15 | 56.2 ± 7.7 36–38,o |
| RT1500 | 5.34 ± 0,59 9–15,e | 52.0 ± 9.0 39–42,p |
| T0 | 8.50 ± 0.50 16,23 | 78.2 ± 5.5 43,k |
| T25 | 8.37 ± 0.43 17,24 | 78.0 ± 5.7 44 |
| T50 | 8.28 ± 0.57 18,25 | 78.2 ± 5.4 45,f,l |
| T100 | 8.19 ± 0.48 19,26 | 77.4 ± 7.0 46,g |
| T150 | 8.13 ± 0.69 20,27,b | 76.8 ± 7.0 47,h,m |
| T250 | 8.11 ± 0.51 21,28,d | 76.6 ± 7.2 48,i,n |
| T500 | 8.01 ± 0.70 22,29 | 73.8 ± 4.9 49,o |
| T1500 | 7.15 ± 0.74 16–22,30,e | 70.4 ± 7.4 50,p |
| T5000 | 5.01 ± 0.99 23–30 | 43.6 ± 7.8 43–50 |
| Sample | Flexural Strength (MPa) | Flexural Modulus (MPa) |
|---|---|---|
| Ph0 | 88.6 ± 9.1 1,5 | 3819.4 ± 255.8 40 |
| Ph25 | 92.4 ± 11.7 2,6,a | 3848.5 ± 210.5 41,45 |
| Ph50 | 98.3 ± 11.7 3,7,b | 4039.2 ± 361.9 42,46 |
| Ph100 | 97.4 ± 8.7 4,8,c | 3897.2 ± 314.3 43,47 |
| Ph150 | 74.4 ± 7.7 1–4,9,d,f | 3179.6 ± 343.6 44,45–47,p |
| Ph250 | 57.6 ± 6.8 5–9,e,g | 1808.2 ± 254.9 40–44,q,r |
| RT0 | 79.8 ± 10.2 10,h | 3610.5 ± 316.0 48,54 |
| RT25 | 88.2 ± 9.8 11,16,i | 3665.6 ± 248.6 49,55 |
| RT50 | 95.5 ± 9.0 12,17,j | 3822.3 ± 292.7 50,56,61 |
| RT100 | 98.5 ± 10.4 13,18,k | 4001.6 ± 442.3 51,57,62 |
| RT150 | 96.7 ± 10.8 10,14,19,a,f,l | 3746.8 ± 290.3 52,58 |
| RT250 | 89.6 ± 5.9 15, 20,c,g,m | 3336.5 ± 307.4 53,59,61,62,q,s |
| RT500 | 73.1 ± 7.8 11–15,n | 2676.8 ± 227.8 47–53,60,t |
| RT1500 | 68.1 ± 7.1 16–20,o | 1905.9 ± 289.8 54–60,u |
| T0 | 100.3 ± 6.8 21,25,h | 3872.5 ± 297.9 |
| T25 | 109.0 ± 11.0 26,27,33,a,i | 4034.0 ± 437.6 |
| T50 | 112.0 ± 12.5 28,34,b,j | 4066.4 ± 415.0 |
| T100 | 120.16 ± 8.4 21,29,35,c,k | 4044.4 ± 272.6 61 |
| T150 | 124.8 ± 6.3 22,30,36,d,l | 4066.5 ± 407.3 p |
| T250 | 129.3 ± 15.8 23,26,31,37,e,m | 4153.5 ± 303.4 62,r,s |
| T500 | 129.2 ± 11.2 24,32,38,n | 4210.6 ± 396.6 63,t |
| T1500 | 96.6 ± 7.2 27–32,39,o | 4596.1 ± 590.6 64,u |
| T5000 | 58.7 ± 6.0 25,33–39 | 3368.9 ± 479.4 61–64 |
| Sample | Hardness (N/mm2) | Impact Resistance (kJ/m2) |
|---|---|---|
| Ph0 | 115.1 ± 9.5 1 | 4.57 ± 0.35 26,30 |
| Ph25 | 117.9 ± 6.1 2 | 4.77 ± 0.36 27,31 |
| Ph50 | 123.3 ± 16.4 3,6 | 4.82 ± 0.47 28,32 |
| Ph100 | 122.8 ± 10.6 4,7 | 4.21 ± 0.45 29,33,g |
| Ph150 | 100.9 ± 12.1 5,6,7,a,b | 3.41 ± 0.35 26–29,34,h |
| Ph250 | 81.4 ± 7.3 1–5,c,d | 2.48 ± 0.31 30–34,i,j |
| RT0 | 110.2 ± 7.6 8,9 | 4.07 ± 0.37 35,40,k |
| RT25 | 117.7 ± 4.2 10 | 4.24 ± 0.44 36,41,l |
| RT50 | 121.3 ± 6.5 11 | 4.25 ± 0.42 37,42,m |
| RT100 | 128.5 ± 13.1 12 | 4.27 ± 0.49 38,43,n |
| RT150 | 130.2 ± 15.1 8,13,16,a | 4.03 ± 0.44 39,44,o |
| RT250 | 122.7 ± 14.5 14,c | 3.67 ± 0.36 45,j,p |
| RT500 | 113.1 ± 11.6 15,16,e | 3.34 ± 0.28 35–39,46,q |
| RT1500 | 83.94 ± 6.7 9–15,f | 2.26 ± 0.15 40–46,r |
| T0 | 121.8 ± 11.6 17,19,22 | 5.20 ± 0.28 47,50,56,k |
| T25 | 126.4 ± 12.1 18,20,23 | 5.38 ± 0.51 51,57,l |
| T50 | 130.5 ± 15.5 21,24 | 5.47 ± 0.56 48,52,58,m |
| T100 | 139.2 ± 18.3 | 5.54 ± 0.44 49,53,59,64,f,n |
| T150 | 143.2 ± 21.7 b | 5.30 ± 0.45 54,60,h,o |
| T250 | 142.8 ± 20.8 25,d | 4.73 ± 0.45 55,61,64,i,p |
| T500 | 148.5 ± 13.0 17,18,e | 4.42 ± 0.50 47–49,62,q |
| T1500 | 149.1 ± 7.1 19–21,f | 4.06 ± 0.41 50–55,63,r |
| T5000 | 158.9 ± 13.5 22–25 | 2.26 ± 0.15 56–63 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barszczewska-Rybarek, I.; Chladek, G. Studies on the Curing Efficiency and Mechanical Properties of Bis-GMA and TEGDMA Nanocomposites Containing Silver Nanoparticles. Int. J. Mol. Sci. 2018, 19, 3937. https://doi.org/10.3390/ijms19123937
Barszczewska-Rybarek I, Chladek G. Studies on the Curing Efficiency and Mechanical Properties of Bis-GMA and TEGDMA Nanocomposites Containing Silver Nanoparticles. International Journal of Molecular Sciences. 2018; 19(12):3937. https://doi.org/10.3390/ijms19123937
Chicago/Turabian StyleBarszczewska-Rybarek, Izabela, and Grzegorz Chladek. 2018. "Studies on the Curing Efficiency and Mechanical Properties of Bis-GMA and TEGDMA Nanocomposites Containing Silver Nanoparticles" International Journal of Molecular Sciences 19, no. 12: 3937. https://doi.org/10.3390/ijms19123937
APA StyleBarszczewska-Rybarek, I., & Chladek, G. (2018). Studies on the Curing Efficiency and Mechanical Properties of Bis-GMA and TEGDMA Nanocomposites Containing Silver Nanoparticles. International Journal of Molecular Sciences, 19(12), 3937. https://doi.org/10.3390/ijms19123937






