Calretinin Functions in Malignant Mesothelioma Cells Cannot Be Replaced by the Closely Related Ca2+-Binding Proteins Calbindin-D28k and Parvalbumin
Abstract
1. Introduction
2. Results
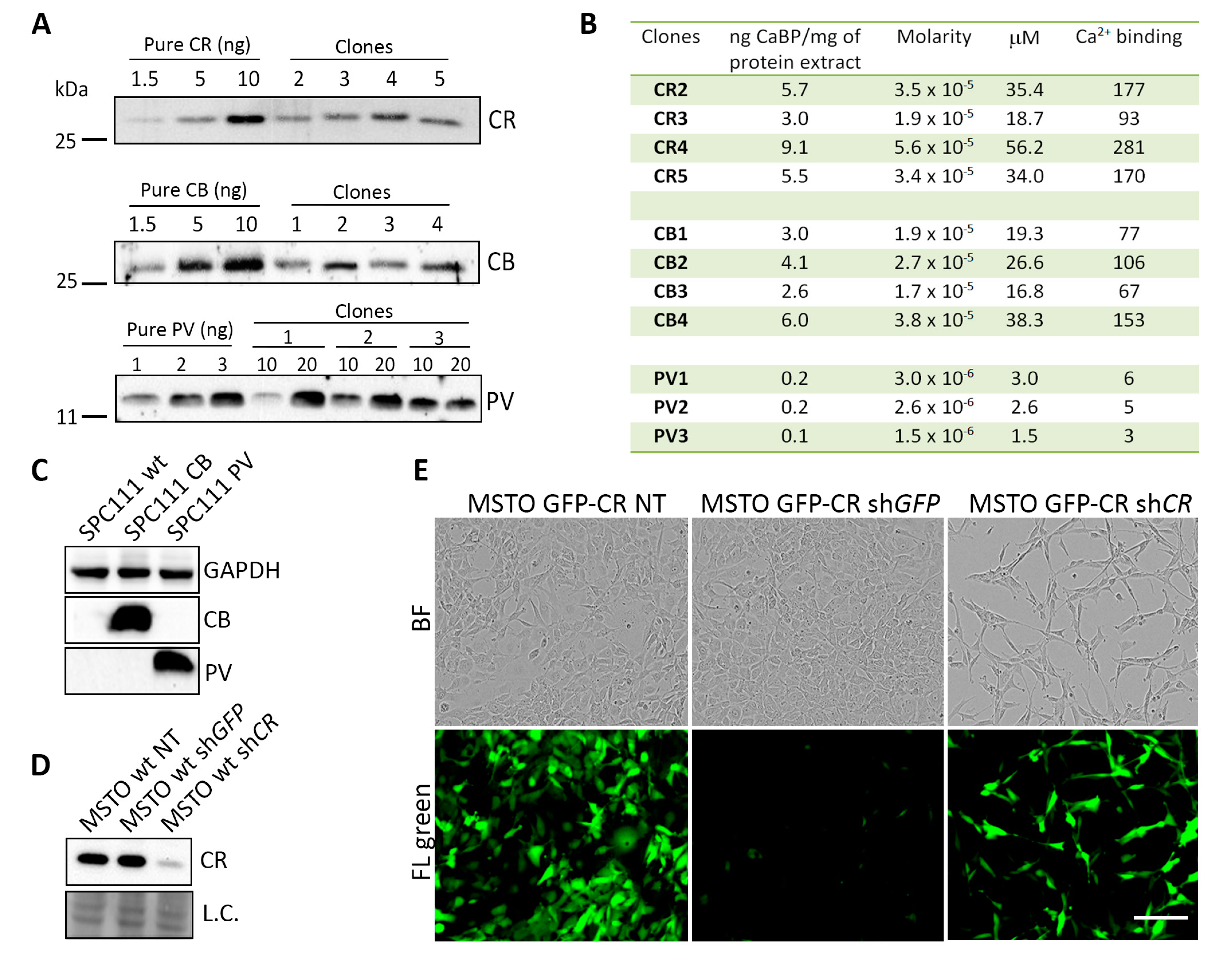
2.1. Generation and Characterization of MM Cell Clones Overexpressing the CaBPs CR, GFP-CR, CB and PV
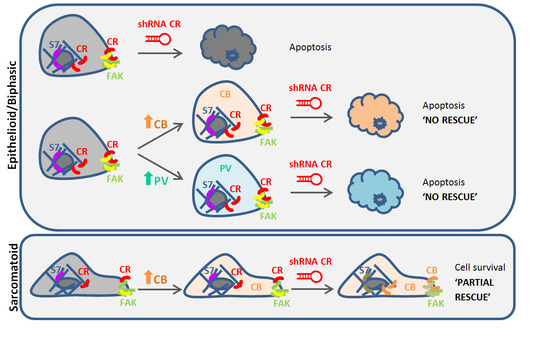
2.2. Calbindin-D28k Rescues the Viability Phenotype of SPC111 Cells Caused by LV-Mediated CR Downregulation
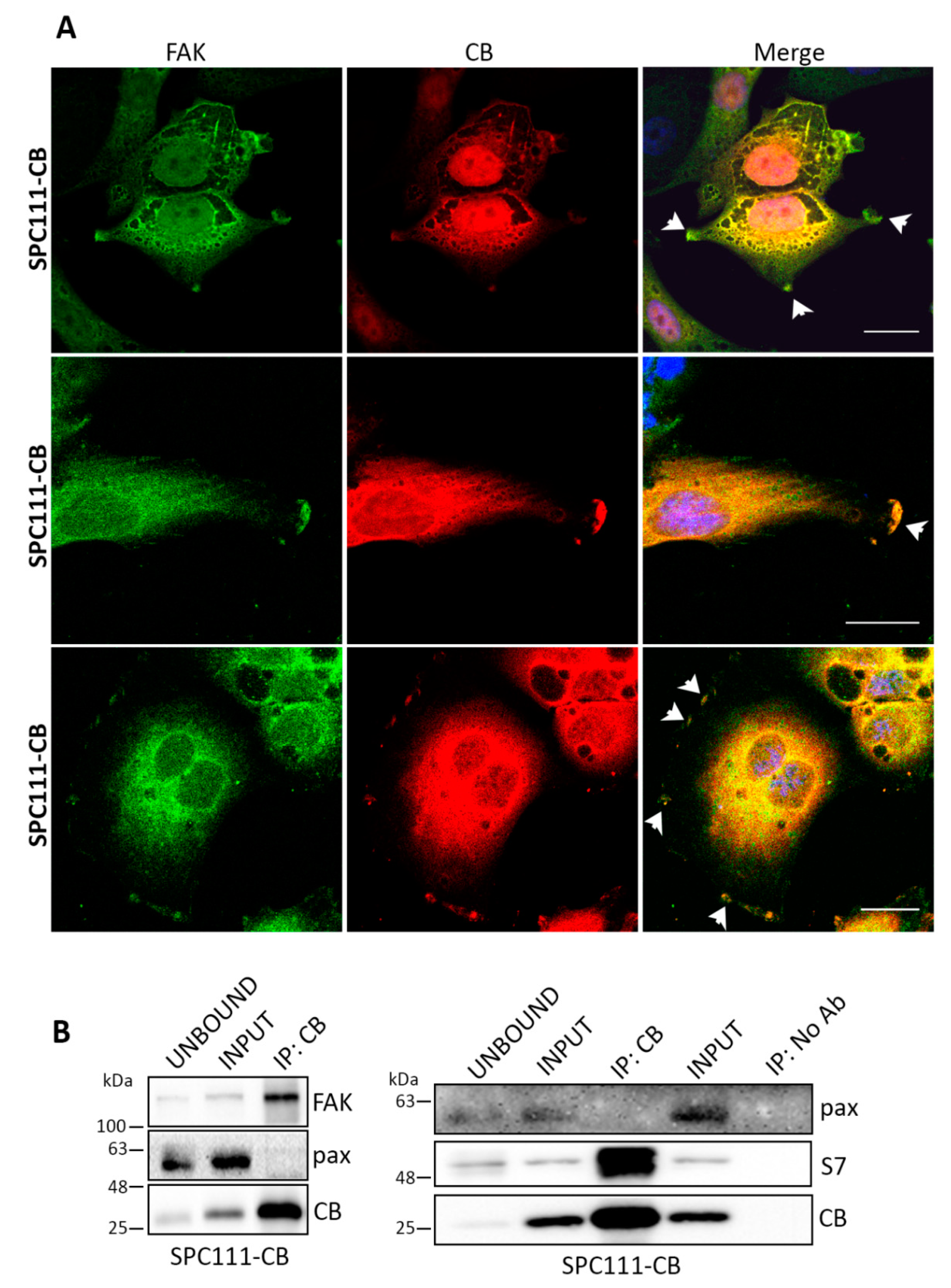
2.3. Calbindin-D28k Shares Common Targets with Calretinin in MM Cells
3. Discussion
4. Material and Methods
4.1. Cell Culture
4.2. Lentiviral (LV) Constructs, Vector Production, and Lentivirus Isolation
4.3. LV Titration by Limiting Dilution
4.4. Establishment of Stably Transduced Cell Lines and Western Blot Analysis
4.5. Clonal Selection
4.6. Semi-Quantification of the Different CaBPs-Expressing Clones
4.7. Downregulation of CR in the Different CaBPs-Expressing Clones Using LV-Mediated shRNA In Vitro
4.8. Immunofluorescence
4.9. Co-Immunoprecipitation
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CaBPs | Ca2+-binding proteins |
| CR | calretinin |
| PV | parvalbumin |
| CB | calbindin-D28k |
| MM | malignant mesothelioma |
| FAK | focal adhesion kinase |
References
- Schwaller, B. The Regulation of a Cell’s Ca2+ Signaling Toolkit: The Ca2+ Homeostasome. In Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2012; Volume 740, pp. 1–25. [Google Scholar]
- Schwaller, B. The continuing disappearance of “pure” Ca2+ buffers. Cell. Mol. Life Sci. 2009, 66, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Mojumder, D.K.; Wensel, T.G.; Frishman, L.J. Subcellular compartmentalization of two calcium binding proteins, calretinin and calbindin-28 kDa, in ganglion and amacrine cells of the rat retina. Mol. Vis. 2008, 14, 1600–1613. [Google Scholar] [PubMed]
- Schwaller, B. Cytosolic Ca2+ buffers. Cold Spring Harb. Perspect. Biol. 2010, 2, a004051. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H. Three functional facets of calbindin D-28k. Front. Mol. Neurosci. 2012, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Kuznicki, J.; Wang, T.L.; Martin, B.M.; Winsky, L.; Jacobowitz, D.M. Localization of Ca2+-dependent conformational changes of calretinin by limited tryptic proteolysis. Biochem. J. 1995, 308 Pt 2, 607–612. [Google Scholar] [CrossRef]
- Schwaller, B.; Durussel, I.; Jermann, D.; Herrmann, B.; Cox, J.A. Comparison of the Ca2+-binding properties of human recombinant calretinin-22k and calretinin. J. Biol. Chem. 1997, 272, 29663–29671. [Google Scholar] [CrossRef] [PubMed]
- Billing-Marczak, K.; Kuznicki, J. Calretinin—Sensor or buffer—Function still unclear. Pol. J. Pharmacol. 1999, 51, 173–178. [Google Scholar] [PubMed]
- Christel, C.J.; Schaer, R.; Wang, S.; Henzi, T.; Kreiner, L.; Grabs, D.; Schwaller, B.; Lee, A. Calretinin regulates Ca2+-dependent inactivation and facilitation of Cav2.1 Ca2+ channels through a direct interaction with the α12.1 subunit. J. Biol. Chem. 2012, 287, 39766–39775. [Google Scholar] [CrossRef]
- Dong, G.; Gross, K.; Qiao, F.; Ferguson, J.; Callegari, E.A.; Rezvani, K.; Zhang, D.; Gloeckner, C.J.; Ueffing, M.; Wang, H. Calretinin interacts with huntingtin and reduces mutant huntingtin-caused cytotoxicity. J. Neurochem. 2012, 123, 437–446. [Google Scholar] [CrossRef]
- Marilley, D.; Schwaller, B. Association between the calcium-binding protein calretinin and cytoskeletal components in the human colon adenocarcinoma cell line WiDr. Exp. Cell Res. 2000, 259, 12–22. [Google Scholar] [CrossRef]
- Blum, W.; Pecze, L.; Rodriguez, J.W.; Steinauer, M.; Schwaller, B. Regulation of calretinin in malignant mesothelioma is mediated by septin 7 binding to the CALB2 promoter. BMC Cancer 2018, 18, 475. [Google Scholar] [CrossRef] [PubMed]
- Wörthmüller, J.; Blum, W.; Pecze, L.; Salicio, V.; Schwaller, B. Calretinin promotes invasiveness and EMT in malignant mesothelioma cells involving the activation of the FAK signaling pathway. Oncotarget 2018, 9, 36256–36272. [Google Scholar] [CrossRef]
- Lee, B.Y.; Timpson, P.; Horvath, L.G.; Daly, R.J. FAK signaling in human cancer as a target for therapeutics. Pharmacol. Ther. 2015, 146, 132–149. [Google Scholar] [CrossRef]
- Gotzos, V.; Vogt, P.; Celio, M.R. The calcium binding protein calretinin is a selective marker for malignant pleural mesotheliomas of the epithelial type. Pathol. Res. Pract. 1996, 192, 137–147. [Google Scholar] [CrossRef]
- Doglioni, C.; Dei Tos, A.P.; Laurino, L.; Iuzzolino, P.; Chiarelli, C.; Celio, M.R.; Viale, G. Calretinin: A novel immunocytochemical marker for mesothelioma. Am. J. Surg. Pathol. 1996, 20, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Ly, B.H.; Dodson, R.F.; Pagano, I.; Morris, P.T.; Dogan, U.A.; Gazdar, A.F.; Pass, H.I.; Yang, H. Malignant mesothelioma: Facts, myths, and hypotheses. J. Cell Physiol. 2012, 227, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Henzi, T.; Blum, W.V.; Pfefferli, M.; Kawecki, T.J.; Salicio, V.; Schwaller, B. SV40-induced expression of calretinin protects mesothelial cells from asbestos cytotoxicity and may be a key factor contributing to mesothelioma pathogenesis. Am. J. Pathol. 2009, 174, 2324–2336. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gillis, A.; Zhao, C.; Lee, E.; Wu, J.; Zhang, F.; Ye, F.; Zhang, D.Y. Crocidolite asbestos-induced signal pathway dysregulation in mesothelial cells. Mutat. Res. 2011, 723, 171–176. [Google Scholar] [CrossRef]
- Blum, W.; Schwaller, B. Calretinin is essential for mesothelioma cell growth/survival in vitro: A potential new target for malignant mesothelioma therapy? Int. J. Cancer 2013, 133, 2077–2088. [Google Scholar] [CrossRef]
- Celio, M.R. (Ed.) Guidebook to the Calcium-Binding Proteins, 1st ed.; Oxford University Press: Oxford, UK, 1996; pp. 23–28. [Google Scholar]
- Rogers, J.H.; Resibois, A. Calretinin and calbindin-D28k in rat brain: Patterns of partial co-localization. Neuroscience 1992, 51, 843–865. [Google Scholar] [CrossRef]
- Schwaller, B.; Meyer, M.; Schiffmann, S. ‘New’ functions for ‘old’ proteins: The role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum 2002, 1, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Le Devedec, S.E.; Geverts, B.; de Bont, H.; Yan, K.; Verbeek, F.J.; Houtsmuller, A.B.; van de Water, B. The residence time of focal adhesion kinase (FAK) and paxillin at focal adhesions in renal epithelial cells is determined by adhesion size, strength and life cycle status. J. Cell Sci. 2012, 125, 4498–4506. [Google Scholar] [CrossRef] [PubMed]
- Mostowy, S.; Cossart, P. Septins: The fourth component of the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2012, 13, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Palczewska, M.; Groves, P.; Batta, G.; Heise, B.; Kuznicki, J. Calretinin and calbindin D28k have different domain organizations. Protein Sci. 2003, 12, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Palczewska, M.; Groves, P.; Ambrus, A.; Kaleta, A.; Kover, K.E.; Batta, G.; Kuznicki, J. Structural and biochemical characterization of neuronal calretinin domain I-II (residues 1–100). Comparison to homologous calbindin D28k domain I-II (residues 1–93). Eur. J. Biochem. 2001, 268, 6229–6237. [Google Scholar] [CrossRef]
- Arendt, O.; Schwaller, B.; Brown, E.B.; Eilers, J.; Schmidt, H. Restricted diffusion of calretinin in cerebellar granule cell dendrites implies Ca2+-dependent interactions via its EF-hand 5 domain. J. Physiol. 2013, 591, 3887–3899. [Google Scholar] [CrossRef]
- Kordys, D.R.; Bobay, B.G.; Thompson, R.J.; Venters, R.A.; Cavanagh, J. Peptide binding proclivities of calcium loaded calbindin-D28k. FEBS Lett. 2007, 581, 4778–4782. [Google Scholar] [CrossRef]
- Schmitter, D.; Lauber, B.; Fagg, B.; Stahel, R.A. Hematopoietic growth factors secreted by seven human pleural mesothelioma cell lines: Interleukin-6 production as a common feature. Int. J. Cancer 1992, 51, 296–301. [Google Scholar] [CrossRef]
- Blum, W.; Pecze, L.; Felley-Bosco, E.; Worthmuller-Rodriguez, J.; Wu, L.; Vrugt, B.; de Perrot, M.; Schwaller, B. Establishment of immortalized murine mesothelial cells and a novel mesothelioma cell line. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 714–721. [Google Scholar] [CrossRef]
- Henzi, T.; Schwaller, B. Antagonistic Regulation of Parvalbumin Expression and Mitochondrial Calcium Handling Capacity in Renal Epithelial Cells. PLoS ONE 2015, 10, e0142005. [Google Scholar] [CrossRef]
- Marilley, D.; Vonlanthen, S.; Gioria, A.; Schwaller, B. Calretinin and calretinin-22k increase resistance toward sodium butyrate-induced differentiation in CaCo-2 colon adenocarcinoma cells. Exp. Cell Res. 2001, 268, 93–103. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wörthmüller, J.; Oberson, A.; Salicio, V.; Blum, W.; Schwaller, B. Calretinin Functions in Malignant Mesothelioma Cells Cannot Be Replaced by the Closely Related Ca2+-Binding Proteins Calbindin-D28k and Parvalbumin. Int. J. Mol. Sci. 2018, 19, 4015. https://doi.org/10.3390/ijms19124015
Wörthmüller J, Oberson A, Salicio V, Blum W, Schwaller B. Calretinin Functions in Malignant Mesothelioma Cells Cannot Be Replaced by the Closely Related Ca2+-Binding Proteins Calbindin-D28k and Parvalbumin. International Journal of Molecular Sciences. 2018; 19(12):4015. https://doi.org/10.3390/ijms19124015
Chicago/Turabian StyleWörthmüller, Janine, Anne Oberson, Valérie Salicio, Walter Blum, and Beat Schwaller. 2018. "Calretinin Functions in Malignant Mesothelioma Cells Cannot Be Replaced by the Closely Related Ca2+-Binding Proteins Calbindin-D28k and Parvalbumin" International Journal of Molecular Sciences 19, no. 12: 4015. https://doi.org/10.3390/ijms19124015
APA StyleWörthmüller, J., Oberson, A., Salicio, V., Blum, W., & Schwaller, B. (2018). Calretinin Functions in Malignant Mesothelioma Cells Cannot Be Replaced by the Closely Related Ca2+-Binding Proteins Calbindin-D28k and Parvalbumin. International Journal of Molecular Sciences, 19(12), 4015. https://doi.org/10.3390/ijms19124015