Histone H3 Lysine 9 Acetylation is Downregulated in GDM Placentas and Calcitriol Supplementation Enhanced This Effect
Abstract
:1. Introduction
2. Results
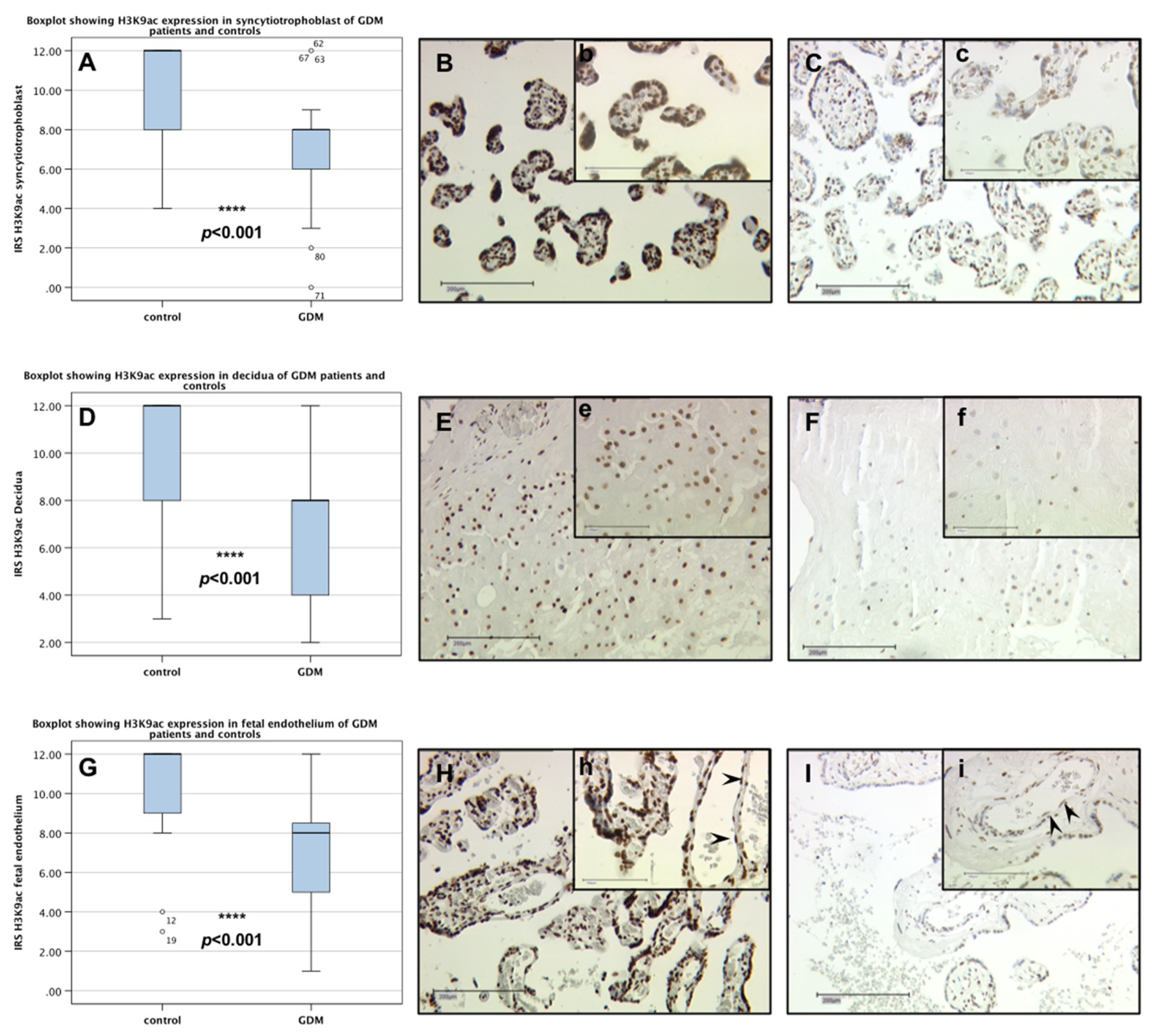
2.1. H3K9ac Expression is Downregulated in GDM Placentas
2.2. No Difference between H3K4me3 Expression in GDM and Control Placentas
2.3. Identification of H3K9ac Expressing Cells by Immunofluorescence Double Staining
2.4. Downregulation of H3K9ac in Trophoblast Tumour Cells BeWo by Human Calcitriol (Vitamin D)
2.5. Upregulation of FOXO1 in Trophoblast Tumour Cells BeWo by Human Calcitriol (Vitamin D)
2.6. Downregulation of H3K9ac in Primary Human Villous Trophoblast Cells HVT by Human Calcitriol (Vitamin D)
3. Discussion
4. Materials and Methods
4.1. Tissue Samples
4.2. Immunohistochemistry
4.2.1. Staining
4.2.2. Evaluation
4.3. Double Immunofluorescence
4.4. Cell Culture and Stimulation
4.5. Westernblotting of Stimulated BeWo Cells and HVT Cell
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CD31 | Cluster of differentiation 31 |
| CK7 | Cytokeratin 7 |
| CREB/CRTC2 | cAMP responsive element binding protein 1/CREB regulated transcriptional coactivator 2 |
| DM2 | Diabetes Mellitus Type 2 |
| DMEM | Dulbecco’s modified Eagle’s medium |
| EVT | Extra-villous trophoblast cells |
| FBS | Fetal bovine serum |
| FOXO1 | Forkhead box protein O1 |
| g | Gramm |
| GDM | Gestational Diabetes Mellitus |
| h | Hours |
| H3K4me3 | Trimethylation of Histone 3 at lysine 4 |
| H3K9ac | Acetylation of Histone 3 at lysine 9 |
| HAT | Histone acetyltransferase |
| HDAC | Histone deacetylase |
| HOMA-IR | Homeostatic model assessment—insulin resistance |
| HVT | Human villous trophoblast cells |
| IRS | Immunoreactivity score |
| LGA | Large for gestational age |
| M | Molar |
| mRNA | Messanger ribonuclease |
| ncRNA | Non-coding ribonuclease |
| oGTT | Oral glucose tolerance test |
| OR | Odds ratio |
| PTM | Post-translational modifications |
| RR | Relative Risk |
| SCT | Syncytiotrophoblast |
| TFIID | Transcription factor II D |
| TSS | Transcriptional start site |
| VDR | Vitamin D receptor |
| VEGF-A | Vascular endothelial growth factor A |
| Vit. D | Vitamin D |
| WB | Western Blot |
References
- Metzger, B.E.; Coustan, D.R. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care 1998, 21 (Suppl. 2), B161–B167. [Google Scholar]
- International Diabetes Federation; Han Cho, N.; Whiting, D.; Forouhi, N.; Guariguata, L.; Hambleton, I.; Li, R.; Majeed, A.; Mbanya, J.C.; Aschner Montoya, P.; et al. IDF Diabetes Atlas, 7th ed.; David Cavan, J.D.R.F., Makaroff, L., Ogurtsova, K., Webber, S., Eds.; International Diabetes Federation: Brussel, Belgium, 2015. [Google Scholar]
- Albrecht, S.S.; Kuklina, E.V.; Bansil, P.; Jamieson, D.J.; Whiteman, M.K.; Kourtis, A.P.; Posner, S.F.; Callaghan, W.M. Diabetes trends among delivery hospitalizations in the U.S., 1994–2004. Diabetes Care 2010, 33, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Kleinwechter, H.; Schäfer-Graf, U.; Bührer, C.; Hoesli, I.; Kainer, F.; Kautzky-Willer, A.; Pawlowski, B.; Schunck, F.; Somville, T.; Sorger, M. Gestationsdiabetes Mellitus (GDM)—Evidenzbasierte Leitlinie zu Diagnostik, Therapie u. Nachsorge; Deutschen Diabetes-Gesellschaft (DDG) & Deutschen Gesellschaft für Gynäkologie und Geburtshilfe (DGGG): Berlin, Germany, 2011. [Google Scholar]
- Fadl, H.E.; Ostlund, I.K.; Magnuson, A.F.; Hanson, U.S. Maternal and neonatal outcomes and time trends of gestational diabetes mellitus in Sweden from 1991 to 2003. Diabet. Med. 2010, 27, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Hapo Study Cooperative Research Group; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Tam, W.H.; Ma, R.C.; Yang, X.; Ko, G.T.; Tong, P.C.; Cockram, C.S.; Sahota, D.S.; Rogers, M.S.; Chan, J.C. Glucose intolerance and cardiometabolic risk in children exposed to maternal gestational diabetes mellitus in utero. Pediatrics 2008, 122, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.D.; Mathiesen, E.R.; Hansen, T.; Pedersen, O.; Jensen, D.M.; Lauenborg, J.; Damm, P. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: The role of intrauterine hyperglycemia. Diabetes Care 2008, 31, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Vaarasmaki, M.; Pouta, A.; Elliot, P.; Tapanainen, P.; Sovio, U.; Ruokonen, A.; Hartikainen, A.L.; McCarthy, M.; Jarvelin, M.R. Adolescent manifestations of metabolic syndrome among children born to women with gestational diabetes in a general-population birth cohort. Am. J. Epidemiol. 2009, 169, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Kautzky-Willer, A.; Prager, R.; Waldhausl, W.; Pacini, G.; Thomaseth, K.; Wagner, O.F.; Ulm, M.; Streli, C.; Ludvik, B. Pronounced insulin resistance and inadequate beta-cell secretion characterize lean gestational diabetes during and after pregnancy. Diabetes Care 1997, 20, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Abell, S.K.; De Courten, B.; Boyle, J.A.; Teede, H.J. Inflammatory and Other Biomarkers: Role in Pathophysiology and Prediction of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2015, 16, 13442–13473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, P.A.M.; Coustan, D.R. Gestational Diabetes, 1st ed.; Springer: Vienna, Australia; New York, NY, USA, 1988. [Google Scholar]
- Godfrey, K.M. The role of the placenta in fetal programming—A review. Placenta 2002, 23, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D. Epigenomic and transcriptional control of insulin resistance. J. Intern. Med. 2016, 280, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadhwa, P.D.; Buss, C.; Entringer, S.; Swanson, J.M. Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med. 2009, 27, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Dolinoy, D.C.; Das, R.; Weidman, J.R.; Jirtle, R.L. Metastable epialleles, imprinting, and the fetal origins of adult diseases. Pediatr. Res. 2007, 61, 30R–37R. [Google Scholar] [CrossRef] [PubMed]
- Lillycrop, K.A.; Slater-Jefferies, J.L.; Hanson, M.A.; Godfrey, K.M.; Jackson, A.A.; Burdge, G.C. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br. J. Nutr. 2007, 97, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Buklijas, T.; Low, F.M.; Beedle, A.S. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat. Rev. Endocrinol. 2009, 5, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Michels, K.B. Epigenetic epidemiology of the developmental origins hypothesis. Annu. Rev. Nutr. 2007, 27, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Allis, C.D.; Caparros, M.-L.; Jenuwein, T.; Lachner, M.; Reinberg, D. Overview and Concepts. In Epigenetics, 2nd ed.; Allis, C.D., Caparros, M.-L., Jenuwein, T., Reinberg, D., Lachner, M., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2015. [Google Scholar]
- Moen, G.H.; Sommer, C.; Prasad, R.B.; Sletner, L.; Groop, L.; Qvigstad, E.; Birkeland, K.I. Mechanisms in Endocrinology: Epigenetic modifications and gestational diabetes: A systematic review of published literature. Eur. J. Endocrinol. 2017, 176, R247–R267. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karmodiya, K.; Krebs, A.R.; Oulad-Abdelghani, M.; Kimura, H.; Tora, L. H3K9 and H3K14 acetylation co-occur at many gene regulatory elements, while H3K14ac marks a subset of inactive inducible promoters in mouse embryonic stem cells. BMC Genom. 2012, 13, 424. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, B.E.; Kamal, M.; Lindblad-Toh, K.; Bekiranov, S.; Bailey, D.K.; Huebert, D.J.; McMahon, S.; Karlsson, E.K.; Kulbokas, E.J., 3rd; Gingeras, T.R.; et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell 2005, 120, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Lauberth, S.M.; Nakayama, T.; Wu, X.; Ferris, A.L.; Tang, Z.; Hughes, S.H.; Roeder, R.G. H3K4me3 interactions with TAF3 regulate preinitiation complex assembly and selective gene activation. Cell 2013, 152, 1021–1036. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, B.E.; Mikkelsen, T.S.; Xie, X.; Kamal, M.; Huebert, D.J.; Cuff, J.; Fry, B.; Meissner, A.; Wernig, M.; Plath, K.; et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 2006, 125, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, K.H.; Knochel, W.; Martinez, D.E. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000, 14, 142–146. [Google Scholar] [PubMed]
- Accili, D.; Arden, K.C. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 2004, 117, 421–426. [Google Scholar] [CrossRef]
- Nakae, J.; Oki, M.; Cao, Y. The FoxO transcription factors and metabolic regulation. FEBS Lett. 2008, 582, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Li, P.; Huntley, J.; Chang, J.P.; Arden, K.C.; Olefsky, J.M. FoxO1 haploinsufficiency protects against high-fat diet-induced insulin resistance with enhanced peroxisome proliferator-activated receptor gamma activation in adipose tissue. Diabetes 2009, 58, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jin, B.; Sun, L.; Yang, H.; Cao, X.; Zhang, G. The expression of FoxO1 in placenta and omental adipose tissue of gestational diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2014, 122, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Villalta, S.A.; Agrawal, D.K. FOXO1 Mediates Vitamin D Deficiency-Induced Insulin Resistance in Skeletal Muscle. J. Bone Miner. Res. 2016, 31, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Clifton-Bligh, R.J.; McElduff, P.; McElduff, A. Maternal vitamin D deficiency, ethnicity and gestational diabetes. Diabet. Med. 2008, 25, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Maghbooli, Z.; Hossein-Nezhad, A.; Karimi, F.; Shafaei, A.R.; Larijani, B. Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes Metab. Res. Rev. 2008, 24, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Soheilykhah, S.; Mojibian, M.; Rashidi, M.; Rahimi-Saghand, S.; Jafari, F. Maternal vitamin D status in gestational diabetes mellitus. Nutr. Clin. Pract. 2010, 25, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.M.; Haeri, S.; Camargo, C.A., Jr.; Stuebe, A.M.; Boggess, K.A. First-trimester maternal vitamin D status and risk for gestational diabetes (GDM) a nested case-control study. Diabetes Metab. Res. Rev. 2012, 28, 164–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrant, H.J.; Krishnaveni, G.V.; Hill, J.C.; Boucher, B.J.; Fisher, D.J.; Noonan, K.; Osmond, C.; Veena, S.R.; Fall, C.H. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. Eur. J. Clin. Nutr. 2009, 63, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Makgoba, M.; Nelson, S.M.; Savvidou, M.; Messow, C.M.; Nicolaides, K.; Sattar, N. First-trimester circulating 25-hydroxyvitamin D levels and development of gestational diabetes mellitus. Diabetes Care 2011, 34, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Aghajafari, F.; Nagulesapillai, T.; Ronksley, P.E.; Tough, S.C.; O’Beirne, M.; Rabi, D.M. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: Systematic review and meta-analysis of observational studies. BMJ 2013, 346, f1169. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Q.; Qi, H.P.; Luo, Z.C.; Fraser, W.D. Maternal vitamin D status and adverse pregnancy outcomes: A systematic review and meta-analysis. J. Matern. Fetal Neonatal. Med. 2013, 26, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Knabl, J.; Huttenbrenner, R.; Hutter, S.; Gunthner-Biller, M.; Riedel, C.; Hiden, U.; Kainer, F.; Desoye, G.; Jeschke, U. Gestational diabetes mellitus upregulates vitamin D receptor in extravillous trophoblasts and fetoplacental endothelial cells. Reprod. Sci. 2015, 22, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.B.; Benkusky, N.A.; Pike, J.W. 1,25-Dihydroxyvitamin D3 induced histone profiles guide discovery of VDR action sites. J. Steroid Biochem. Mol. Biol. 2014, 144, 19–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Stoffers, D.A.; Nicholls, R.D.; Simmons, R.A. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J. Clin. Investig. 2008, 118, 2316–2324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawada, Y.; Asahara, S.I.; Sugiura, Y.; Sato, A.; Furubayashi, A.; Kawamura, M.; Bartolome, A.; Terashi-Suzuki, E.; Takai, T.; Kanno, A.; et al. Histone deacetylase regulates insulin signaling via two pathways in pancreatic beta cells. PLoS ONE 2017, 12, e0184435. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.Z.; Zhang, L.N.; Zhang, R.; Zhang, C.J.; He, H.P.; Zhou, H.; Wang, N.; Zhang, T.C. Histone acetyltransferase p300 promotes MRTF-A-mediates transactivation of VE-cadherin gene in human umbilical vein endothelial cells. Gene 2015, 563, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Kang, P.; Zhong, Y.; Borengasser, S.J.; Wingfield, C.; Saben, J.; Gomez-Acevedo, H.; Thakali, K.M. Transcriptomic and epigenomic landscapes during cell fusion in BeWo trophoblast cells. Placenta 2015, 36, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hai, T.; Wang, Y.; Guo, R.; Li, W.; Wang, L.; Zhou, Q. Epigenetic reprogramming, gene expression and in vitro development of porcine SCNT embryos are significantly improved by a histone deacetylase inhibitor—M-carboxycinnamic acid bishydroxamide (CBHA). Protein Cell 2014, 5, 382–393. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, K.; Alexander, J.; Azuma, R.; Xiao, R.; Snyder, N.W.; Mesaros, C.A.; Blair, I.A.; Pinney, S.E. Gestational Diabetes Alters the Metabolomic Profile in 2nd Trimester Amniotic Fluid in a Sex-Specific Manner. Int. J. Mol. Sci. 2018, 19, 2696. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Fadl, H. Perinatal outcome in relation to fetal sex in offspring to mothers with pre-gestational and gestational diabetes—A population-based study. Diabet. Med. 2014, 31, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Teague, A.M.; Chen, J.; Aston, C.E.; Leung, Y.K.; Chernausek, S.; Simmons, R.A.; Pinney, S.E. Offspring sex impacts DNA methylation and gene expression in placentae from women with diabetes during pregnancy. PLoS ONE 2018, 13, e0190698. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, S.; Li, Y.; Deng, C.; Steiner, L.A.; Xiao, H.; Wu, C.; Bungert, J.; Gallagher, P.G.; Felsenfeld, G.; et al. Chromatin boundaries require functional collaboration between the hSET1 and NURF complexes. Blood 2011, 118, 1386–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeulen, M.; Mulder, K.W.; Denissov, S.; Pijnappel, W.W.; van Schaik, F.M.; Varier, R.A.; Baltissen, M.P.; Stunnenberg, H.G.; Mann, M.; Timmers, H.T. Selective anchoring of TFIID to nucleosomes by trimethylation of histone H3 lysine 4. Cell 2007, 131, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Nian, C.; McIntosh, C.H. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 modulate beta-cell chromatin structure. J. Biol. Chem. 2009, 284, 12896–12904. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Tsai, L.T.; Zhou, Y.; Evertts, A.; Xu, S.; Griffin, M.J.; Issner, R.; Whitton, H.J.; Garcia, B.A.; Epstein, C.B.; et al. Identification of nuclear hormone receptor pathways causing insulin resistance by transcriptional and epigenomic analysis. Nat. Cell Biol. 2015, 17, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Brasacchio, D.; Okabe, J.; Tikellis, C.; Balcerczyk, A.; George, P.; Baker, E.K.; Calkin, A.C.; Brownlee, M.; Cooper, M.E.; El-Osta, A. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes 2009, 58, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- El-Osta, A.; Brasacchio, D.; Yao, D.; Pocai, A.; Jones, P.L.; Roeder, R.G.; Cooper, M.E.; Brownlee, M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J. Exp. Med. 2008, 205, 2409–2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Feng, B.; George, B.; Chakrabarti, R.; Chen, M.; Chakrabarti, S. Transcriptional coactivator p300 regulates glucose-induced gene expression in endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E127–E137. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.B.; Denko, N.; Barton, M.C. Hypoxia induces a novel signature of chromatin modifications and global repression of transcription. Mutat. Res. 2008, 640, 174–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, M.S.; Matouk, C.C.; Marsden, P.A. Epigenetics of the vascular endothelium. J. Appl. Physiol. 2010, 109, 916–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutter, S.; Knabl, J.; Andergassen, U.; Mayr, D.; Hofmann, S.; Kuhn, C.; Mahner, S.; Arck, P.; Jeschke, U. Fetal gender specific expression of tandem-repeat galectins in placental tissue from normally progressed human pregnancies and intrauterine growth restriction (IUGR). Placenta 2015, 36, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar] [PubMed]





| GDM | Control | p-Value | |||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| Birthweight (g) | 3662.1 ± 562 | 3635.9 ± 661 | 3339.8 ± 568 | 3294 ± 440 | p = 0.019 * |
| Duration of gestation at delivery (weeks) | 39.67 ± 1.30 | 39.83 ± 1.40 | 39.80 ± 1.54 | 39.75 ± 1.16 | p = 0.943 |
| Maternal BMI (pre-pregnancy) | 29.38 ± 8.03 | 26.96 ± 4.73 | 21.92 ± 3.97 | 25.04 ± 7.90 | p < 0.001 * |
| pH in umbilical artery | 7.30 ± 0.07 | 7.30 ± 0.10 | 7.28 ± 0.10 | 7.30 ± 0.08 | p = 0.826 |
| Maternal Age (years) | 31.46 ± 4.12 | 33.21 ± 5.33 | 30.30 ± 6.11 | 32.00 ± 6.13 | p = 0.177 |
| Vaginal delivery (%) | 50 | 75 | 80 | 80 | p = 0.207 |
| Antibody | Dilution | Incubation | Manufacturer |
|---|---|---|---|
| H3K4me3—polyclonal Rabbit IgG | 1:500 | 16 h at 4 °C | Abcam—ab8580 (Cambridge, UK) |
| H3K9ac—Clone Y28 Rabbit IgG | 1:200 | 16 h at 4 °C | Abcam—ab32129 (Cambridge, UK) |
| CK7—Clone OVTL Mouse IgG | 1:30 | 16 h at 4 °C | Novocastra—NCL-L-CK7-OVTL (Newcastle, UK) |
| CD31—Clone JC/70A Mouse IgG | 1:50 | 16 h at 4 °C | Abcam—ab9498 (Cambridge, UK) |
| Cy-2-labelled goat-anti-rabbit | 1:100 | 30 min at RT | Dianova—115-226-062 (Hamburg, Germany) |
| Cy-3-labelled goat-anti-mouse | 1:500 | 30 min at RT | Dianova—111-165-144 (Hamburg, Germany) |
| BeWo Stimulation for Western Blot (WB) | |||
| 1.0 µM Ethanol | 0.01 µM Vit. D | 0.1 µM Vit. D | 1.0 µM Vit. D |
| HVT Stimulation for WB | |||
| 1.0 µM Ethanol | 1.0 µM Vit. D | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hepp, P.; Hutter, S.; Knabl, J.; Hofmann, S.; Kuhn, C.; Mahner, S.; Jeschke, U. Histone H3 Lysine 9 Acetylation is Downregulated in GDM Placentas and Calcitriol Supplementation Enhanced This Effect. Int. J. Mol. Sci. 2018, 19, 4061. https://doi.org/10.3390/ijms19124061
Hepp P, Hutter S, Knabl J, Hofmann S, Kuhn C, Mahner S, Jeschke U. Histone H3 Lysine 9 Acetylation is Downregulated in GDM Placentas and Calcitriol Supplementation Enhanced This Effect. International Journal of Molecular Sciences. 2018; 19(12):4061. https://doi.org/10.3390/ijms19124061
Chicago/Turabian StyleHepp, Paula, Stefan Hutter, Julia Knabl, Simone Hofmann, Christina Kuhn, Sven Mahner, and Udo Jeschke. 2018. "Histone H3 Lysine 9 Acetylation is Downregulated in GDM Placentas and Calcitriol Supplementation Enhanced This Effect" International Journal of Molecular Sciences 19, no. 12: 4061. https://doi.org/10.3390/ijms19124061
APA StyleHepp, P., Hutter, S., Knabl, J., Hofmann, S., Kuhn, C., Mahner, S., & Jeschke, U. (2018). Histone H3 Lysine 9 Acetylation is Downregulated in GDM Placentas and Calcitriol Supplementation Enhanced This Effect. International Journal of Molecular Sciences, 19(12), 4061. https://doi.org/10.3390/ijms19124061






