Synthesis and Evaluation of the 4-Substituted 2-Hydroxy-5-Iodochalcones and Their 7-Substituted 6-Iodoflavonol Derivatives for Inhibitory Effect on Cholinesterases and β-Secretase
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biology
2.2.1. Inhibitory Effect of Chalcones 2a–p against AChE and BChE Activities
2.2.2. Inhibitory Effect of Flavonols 3a–p against AChE and BChE Activities
2.2.3. Inhibitory Activities of Compounds 2h, 2j, 2n, 2p, 3b, 3c, 3l and 3p against BACE-1
2.2.4. Enzyme Kinetic Analysis of the Most Active Compounds for ChEs and BACE-1
2.3. Molecular Docking Studies into AChE and BChE Active Sites
2.3.1. Molecular Docking of Compounds 2f and 3b into AChE Active Site
2.3.2. Molecular Docking of Compounds 2h and 3p into the Active Site of BChE
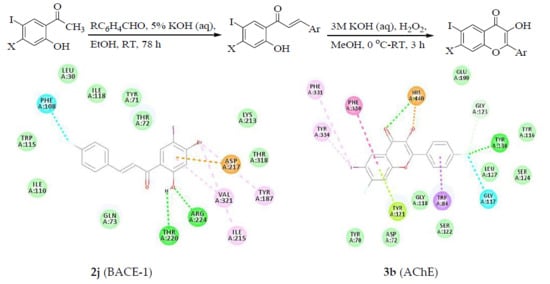
2.3.3. Docking of Chalcones (2h, 2j, 2n and 2p) and Flavonols (3b, 3c, 3l and 3p) into BACE-1
3. Materials and Methods
3.1. General
3.2. Typical Procedure for the Synthesis of the 4-Substituted 2-Hydroxy-5-Iodoacetophenones 1a–d
1-(4-F.luoro-2-hydroxy-5-iodophenyl)ethanone (1a)
1-(4-C.hloro-2-hydroxy-5-iodophenyl)ethanone (1b)
1-(4-B.romo-2-hydroxy-5-iodophenyl)ethanone (1c)
1-(2-H.ydroxy-5-iodo-4-methoxyphenyl)ethanone (1d)
3.3. Typical Procedure for the Synthesis of the 2-hydroxy-5-iodo-4-methoxychalcones 2a–p
(E)-1-(4-Fluoro-2-hydroxy-5-iodophenyl)-3-phenylpropenone (2a)
(E)-1-(4-Fluoro-2-hydroxy-5-iodophenyl)-3-(4-fluorophenyl)propenone (2b)
(E)-3-(4-chlorophenyl)-1-(4-fluoro-2-hydroxy-5-iodophenyl)prop-2-en-1-one (2c)
(E)-1-(4-Fluoro-2-hydroxy-5-iodophenyl)-3-(4-methoxyphenyl)propenone (2d)
(E)-1-(4-Chloro-2-hydroxy-5-iodophenyl)-3-phenylpropenone (2e)
(E)-1-(4-Chloro-2-hydroxy-5-iodophenyl)-3-(4-fluorophenyl)propenone (2f)
(E)-1-(4-Chloro-2-hydroxy-5-iodophenyl)-3-(4-chlorophenyl)propenone (2g)
(E)-1-(4-Chloro-2-hydroxy-5-iodophenyl)-3-(4-methoxyphenyl)propenone (2h)
(E)-1-(4-Bromo-2-hydroxy-5-iodophenyl)-3-phenylpropenone (2i)
(E)-1-(4-Bromo-2-hydroxy-5-iodophenyl)-3-(4-fluorophenyl)propenone (2j)
(E)-1-(4-Bromo-2-hydroxy-5-iodophenyl)-3-(4-chlorophenyl)propenone (2k)
(E)-1-(4-Bromo-2-hydroxy-5-iodophenyl)-3-(4-methoxyphenyl)propenone (2l)
(E)-1-(2-Hydroxy-5-iodo-4-methoxyphenyl)-3-phenylprop-2-en-1-one (2m)
(E)-3-(4-Fluorophenyl)-1-(2-hydroxy-5-iodo-4-methoxyphenyl)prop-2-en-1-one (2n)
(E)-3-(4-Chlorophenyl)-1-(2-hydroxy-5-iodo-4-methoxyphenyl)prop-2-en-1-one (2o)
(E)-1-(2-Hydroxy-5-iodo-4-methoxyphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one (2p)
3.4. Typical Procedure for the Synthesis of the 7-substituted 2-aryl-3-hydroxy-6-iodochromen-4-ones 3a–p
7-Flu.oro-3-hydroxy-6-iodo-2-phenyl-4H-chromen-4-one (3a)
7-Flu.oro-2-(4-fluorophenyl)-3-hydroxy-6-iodo-4H-chromen-4-one (3b)
2-(4-C.hlorophenyl)-7-fluoro-3-hydroxy-6-iodo-4H-chromen-4-one (3c)
7-Flu.oro-3-hydroxy-6-iodo-2-(4-methoxyphenyl)-4H-chromen-4-one (3d)
7-Chl.oro-3-hydroxy-6-iodo-2-phenyl-4H-chromen-4-one (3e)
7-Chl.oro-2-(4-fluorophenyl)-3-hydroxy-6-iodo-4H-chromen-4-one (3f)
7-Chl.oro-2-(4-chlorophenyl)-3-hydroxy-6-iodo-4H-chromen-4-one (3g)
7-Chl.oro-3-hydroxy-6-iodo-2-(4-methoxyphenyl)-4H-chromen-4-one (3h)
7-Bro.mo-3-hydroxy-6-iodo-2-phenyl-4H-chromen-4-one (3i)
7-Bro.mo-2-(4-fluorophenyl)-3-hydroxy-6-iodo-4H-chromen-4-one (3j)
7-Bro.mo-2-(4-chlorophenyl)-3-hydroxy-6-iodo-4H-chromen-4-one (3k)
7-Bro.mo-3-hydroxy-6-iodo-2-(4-methoxyphenyl)-4H-chromen-4-one (3l)
3-Hyd.roxy-6-iodo-7-methoxy-2-phenyl-4H-chromen-4-one (3m)
2-(4-F.luorophenyl)-3-hydroxy-6-iodo-7-methoxy-4H-chromen-4-one (3n)
2-(4-C.hlorophenyl)-3-hydroxy-6-iodo-7-methoxy-4H-chromen-4-one (3o)
3-Hyd.roxy-6-iodo-7-methoxy-2-(4-methoxyphenyl)-4H-chromen-4-one (3p)
3.5. In Vitro Cholinesterase (AChE and BChE) Inhibition Assays
3.6. In Vitro BACE-1 Inhibitory Assays
3.7. Kinetic Studies against ChEs and BACE-1
3.7.1. Kinetic Evaluation of 2h, 3b and 3p against ChEs
3.7.2. Kinetic Evaluation of 2j and 3l against BACE-1
3.8. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hasan, A.; Khan, M.; Sher, M.; Maharvi, G.M.; Nawaz, S.A.; Choudhary, M.I.; Atta-Ur-Rahman; Supuran, C.T. Synthesis and inhibitory potential towards acetylcholinesterase, butyrylcholinesterase and lipoxygenase of some variably substituted chalcones. J. Enz. Inh. Med. Chem. 2005, 20, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Jalili-Balej, L.; Babaei, E.; Abdpour, S.; Bukhari, S.N.A.; Oroumadi, A.; Ramazani, A.; Sharifzadeh, M.; Abdollahi, M.; Khoobi, M. A review on flavonoid-based scaffolds as multi-target-directed ligands (MTDLs) for Alzheimer’s disease. Eur. J. Med. Chem. 2018, 152, 570–589. [Google Scholar] [CrossRef] [PubMed]
- Emmerzaal, T.L.; Kiliaan, A.J.; Gustafson, D.R. 2003–2013: A decade of body mass index, Alzheimer’s disease, and dementia. J. Alzheimer’s Dis. 2015, 43, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Gammon, K. Neurodegenerative disease: Brain windfall. Nature 2014, 515, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Durães, F.; Pinto, M.; Sousa, E. Old drugs as new treatments for neurodegenerative diseases. Pharmaceuticals 2018, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Fosso, M.Y.; LeVine Rd, H.; Green, K.D.; Tsodikov, O.V.; Garneau-Tsodikova, S. Effects of structural modifications on the metal binding, anti-amyloid activity, and cholinesterase inhibitory activity of chalcones. Org. Biomol. Chem. 2015, 13, 9418–9426. [Google Scholar] [CrossRef] [PubMed]
- Chuiko, G.; Podgornaya, V.; Zhelnin, Y. Acetylcholinesterase and butyrylcholinesterase activities in brain and plasma of freshwater teleosts: Cross-species and cross-family differences. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 135, 55–61. [Google Scholar] [CrossRef]
- Sugimoto, H.; Yamanishi, Y.; Limura, H.; Kawakami, Y. Donepezil hydrochloride (E2020) and other acetylcholinesterase inhibitors. Curr. Med. Chem. 2000, 7, 303–317. [Google Scholar] [CrossRef] [PubMed]
- McGleenon, B.M.; Dynan, K.B.; Passmore, A.P. Acetylcholinesterase inhibitors in Alzheimer’s disease. Br. J. Clin. Pharmacol. 1999, 48, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Raveh, L.; Grauver, E.; Grunwald, J.; Cohen, E.; Ashani, Y. The stoichiometry of protection against soman and VX toxicity in monkeys pretreated with human butyrylcholinesterase. Toxicol. Appl. Pharm. 1997, 145, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Q.; Holloway, H.W.; Utsuki, T.; Brossi, A.; Greig, N.H. Synthesis of novel phenserine-based-selective inhibitors of butyrylcholinesterase for Alzheimer’s disease. J. Med. Chem. 1999, 42, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ayllon, M.S.; Small, D.H.; Avila, J.; Saez-Valero, J. Revisiting the role of acetylcholinesterase in Alzheimer’s disease: Cross-talk with P.-tau and β-amyloid. Front. Mol. Neurosci. 2011, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guillozet, A.L.; Smiley, J.F.; Mash, D.C.; Mesulam, M.-M.C. Butyrycholinesterase in the life cycle of amyloid plaques. Ann. Neurol. 1997, 42, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Youn, K.; Park, J.-H.; Lee, J.; Jeong, W.-S.; Ho, C.-T.; Jun, M. The identification of biochanin A as a potent and selective β-site app-cleaving enzyme 1 (Bace1) inhibitor. Nutrients 2016, 8, 637. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.E.; Cho, J.K.; Curtis-Long, M.J.; Ryu, H.W.; Kim, J.H.; Kim, H.J.; Yuk, H.K.; Kim, D.W.; Park, K.H. Inhibitory evaluation of sulfonamide chalcones on β-secretase and acylcholinesterase. Molecules 2013, 18, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.; Matos, M.J.; Garrido, J.; Uriarte, E.; Borges, F. Chromone: A valid scaffold in medicinal chemistry. Chem. Rev. 2014, 114, 4960–4992. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Ali, M.T.; Shawan, M.M.A.K.; Sarwar, M.G.; Khan, M.A.K.; Halim, M.A. Halogen-directed drug design for Alzheimer’s disease: A combined density functional and molecular docking study. SpringerPlus 2016, 5, 1346–1359. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shi, T.; Wang, Y.; Yang, H.; Yan, X.; Luo, X.; Jiang, H.; Zhu, W. Halogen bonding—A novel interaction for rational drug design? J. Med. Chem. 2009, 52, 2854–2862. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, Y.; Xu, Z.; Li, H.; Liu, H.; Zhu, W. Halogen bonding for rational drug design and new drug discovery. Expert Opin. Drug Discov. 2012, 7, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, R.; Zimmermann, M.O.; Lange, A.; Joerger, A.C.; Boeckler, F.M. Principles and applications of halogen bonding in medicinal chemistry and chemical biology. J. Med. Chem. 2012, 56, 1363–1388. [Google Scholar] [CrossRef] [PubMed]
- Kolář, M.; Hobza, P.; Bronowska, A. Plugging the explicit σ-holes in molecular docking. Chem. Comm. 2013, 49, 981–983. [Google Scholar] [CrossRef] [PubMed]
- CCDC 1880847. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 28 November 2018).
- CCDC 1880859. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 28 November 2018).
- Andersson, C.D.; Forsgren, N.; Akfur, C.; Allgardsson, A.; Berg, L.; Engdahl, C.; Qian, W.; Ekström, F.; Linusson, A. Divergent structure-activity relationships of structurally similar acetylcholinesterase inhibitors. J. Med. Chem. 2013, 56, 7615–7624. [Google Scholar] [CrossRef] [PubMed]
- Rampa, A.; Bartolini, M.; Proccoli, L.; Naldi, M.; Iriepa, I.; Moraleda, I.; Belluti, F.; Gobbi, S.; Tarozzi, A.; Bisi, A. Exploiting the chalcone scaffold to develop multifunctional agents for Alzheimer’s disease. Molecules 2018, 23, 1902. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.G. Advances in the treatment of Alzheimer’s disease: Benefits of dual cholinesterase inhibition. Eur. Neurol. 2002, 47, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Flavonols and flavones as BACE-1 inhibitors: Structure–activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Biophys. Acta. 2008, 1780, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Mai, Y.C.; Li, Y.; Hou, J.Q.; Huang, S.L.; Ou, T.M.; Tan, J.H.; An, L.K.; Li, D.; Gu, L.Q.; et al. Synthesis and evaluation of novel rutaecarpine derivatives and related alkaloids derivatives as selective acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2010, 45, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.H. Molecular interactions of cholinesterases inhibitors using in silico methods: Currents status and future prospects. New Biotech. 2009, 25, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.M.; Li, X.M.; Li, F.; Wu, J.-J.; Kong, L.-Y.; Wang, X.-B. Synthesis and evaluation of multi-target-directed ligands for the treatment of Alzheimer’s disease based on the fusion of donepezil and melatonin. Bioorg. Med. Chem. 2016, 24, 4324–4338. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.V.; Sethna, S. Chromones and fluvones. Part, I. Iodination of 5- and 7-hydroxy -2-methylchromone. J. Chem. Soc. 1959, 2676–2678. [Google Scholar] [CrossRef]
- Ali, S.M.; Mohd, I. Selective nuclear iodination of 2′-hydroxychalcones with iodine monochloride under basic conditions. Chem. Ind. 1986, 12, 426–427. [Google Scholar] [CrossRef]
- Arduini, F.; Errico, I.; Amine, A.; Micheli, L.; Palleschi, G.; Moscone, D. Enzymatic spectrophotometric method for aflatoxin B detection based on acetylcholinesterase inhibition. Anal. Chem. 2007, 79, 3409–3415. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, G.R.; Rao, A.A.; Srinivas, K.; Nirmala, G.; Lakshmi, G.; Suryanarayna, D.; Rao, P.V.N.; Kaldhar, D.G.S.V.G.L.; Kumar, S.V.; Devi, T.U.; et al. Butyrylcholinesterase in metabolic syndrome. Med. Hypotheses 2010, 75, 648–651. [Google Scholar] [CrossRef] [PubMed]











| Ar | Designation of X for 2a–p | |||
| C6H5- | F (2a) | Cl (2e) | Br (2i) | OCH3 (2m) |
| 4-FC6H4- | F (2b) | Cl (2f) | Br (2j) | OCH3 (2n) |
| 4-ClC6H4- | F (2c) | Cl (2g) | Br (2k) | OCH3 (2o) |
| 4-MeOC6H4- | F (2d) | Cl (2h) | Br (2l) | OCH3 (2p) |
| Ar | Designation of X for 3a–p | |||
| C6H5- | F (3a) | Cl (3e) | Br (3i) | OCH3 (3m) |
| 4-FC6H4- | F (3b) | Cl (3f) | Br (3j) | OCH3 (3n) |
| 4-ClC6H4- | F (3c) | Cl (3g) | Br (3k) | OCH3 (3o) |
| 4-MeOC6H4- | F (3d) | Cl (3h) | Br (3l) | OCH3 (3p) |
| Compound | IC50 (µM) | SI | ||
|---|---|---|---|---|
| AChE | BChE | BChE/AChE | AChE/BChE | |
| 2a | 38.78 ± 0.01 | 28.06 ± 0.10 | 0.72 | 1.38 |
| 2b | 16.60 ± 0.03 | 19.73 ± 0.05 | 1.19 | 0.84 |
| 2c | 10.50 ± 0.08 | 14.58 ± 0.14 | 1.39 | 0.72 |
| 2d | 10.95 ± 0.30 | 8.49 ± 0.05 | 0.78 | 1.29 |
| 2e | 10.64 ± 0.07 | 25.19 ± 0.30 | 2.37 | 0.46 |
| 2f | 9.57 ± 0.20 | 17.86 ± 0.08 | 1.87 | 0.54 |
| 2g | 11.00 ± 0.06 | >100 | - | - |
| 2h | 11.72 ± 0.07 | 4.17 ± 0.15 | 0.36 | 2.81 |
| 2i | 58.14 ± 0.21 | 14.11 ± 0.11 | 0.24 | 4.12 |
| 2j | 10.98 ± 0.03 | 5.73 ± 0.05 | 0.52 | 1.92 |
| 2k | 11.56 ± 0.05 | 10.44 ± 0.09 | 0.90 | 1.11 |
| 2l | 10.51 ± 0.03 | 15.25 ± 0.01 | 1.45 | 0.69 |
| 2m | 10.01 ± 0.09 | 9.52 ± 0.03 | 0.95 | 1.05 |
| 2n | 9.38 ± 0.07 | 5.91 ± 0.05 | 0.63 | 1.59 |
| 2o | 11.07 ± 0.12 | 11.63 ± 0.17 | 1.05 | 0.95 |
| 2p | 10.96 ± 0.03 | 4.89 ± 0.02 | 0.45 | 2.24 |
| Donepezil | 4.77 ± 0.06 | 6.04 ± 0.03 | 1.27 | 0.79 |
| Compound | IC50 (µM) | SI | ||
|---|---|---|---|---|
| AChE | BChE | BChE/AChE | AChE/BChE | |
| 3a | 29.71 ± 0.01 | 27.01 ± 0.06 | 0.91 | 1.10 |
| 3b | 3.23 ± 0.02 | 29.78 ± 0.04 | 9.22 | 0.11 |
| 3c | 3.45 ± 0.07 | 37.51 ± 0.08 | 10.8 | 0.09 |
| 3d | 58.18 ± 0.03 | 29.41 ± 0.04 | 0.50 | 1.98 |
| 3e | 47.85 ± 0.04 | 6.64 ± 0.03 | 0.14 | 7.21 |
| 3f | 38.39 ± 0.02 | 18.17 ± 0.10 | 0.47 | 2.11 |
| 3g | 59.10 ± 0.05 | 7.69 ± 0.01 | 0.13 | 7.69 |
| 3h | 14.33 ± 0.03 | 5.72 ± 0.01 | 0.40 | 2.51 |
| 3i | 9.96 ± 0.01 | 5.76 ± 0.04 | 0.57 | 1.73 |
| 3j | 11.16 ± 0.01 | 27.01 ± 0.07 | 2.42 | 0.41 |
| 3k | 29.24 ± 0.03 | 5.25 ± 0.01 | 0.18 | 5.57 |
| 3l | 28.99 ± 0.02 | 4.88 ± 0.01 | 0.17 | 5.94 |
| 3m | 9.25 ± 0.09 | 10.50 ± 0.02 | 1.14 | 0.88 |
| 3n | 6.15 ± 0.01 | 7.93 ± 0.06 | 1.29 | 0.76 |
| 3o | 55.27 ± 0.04 | 13.71 ± 0.14 | 0.24 | 4.03 |
| 3p | 7.19 ± 0.02 | 3.29 ± 0.03 | 0.45 | 2.18 |
| Donepezil | 4.79 ± 0.05 | 6.04 ± 0.03 | 1.26 | 0.79 |
| Compound | BACE-1 |
|---|---|
| 2h | 13.82 ± 0.03 |
| 2j | 4.703 ± 0.06 |
| 2n | 25.07 ± 0.1 |
| 2p | 70.79 ± 0.2 |
| 3b | 32.18 ± 0.15 |
| 3c | 19.69 ± 0.05 |
| 3l | 15.74 ± 0.12 |
| 3p | 22.44 ± 0.07 |
| Quercetin | 12.66 ± 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mphahlele, M.J.; Agbo, E.N.; Gildenhuys, S. Synthesis and Evaluation of the 4-Substituted 2-Hydroxy-5-Iodochalcones and Their 7-Substituted 6-Iodoflavonol Derivatives for Inhibitory Effect on Cholinesterases and β-Secretase. Int. J. Mol. Sci. 2018, 19, 4112. https://doi.org/10.3390/ijms19124112
Mphahlele MJ, Agbo EN, Gildenhuys S. Synthesis and Evaluation of the 4-Substituted 2-Hydroxy-5-Iodochalcones and Their 7-Substituted 6-Iodoflavonol Derivatives for Inhibitory Effect on Cholinesterases and β-Secretase. International Journal of Molecular Sciences. 2018; 19(12):4112. https://doi.org/10.3390/ijms19124112
Chicago/Turabian StyleMphahlele, Malose J., Emmanuel N. Agbo, and Samantha Gildenhuys. 2018. "Synthesis and Evaluation of the 4-Substituted 2-Hydroxy-5-Iodochalcones and Their 7-Substituted 6-Iodoflavonol Derivatives for Inhibitory Effect on Cholinesterases and β-Secretase" International Journal of Molecular Sciences 19, no. 12: 4112. https://doi.org/10.3390/ijms19124112
APA StyleMphahlele, M. J., Agbo, E. N., & Gildenhuys, S. (2018). Synthesis and Evaluation of the 4-Substituted 2-Hydroxy-5-Iodochalcones and Their 7-Substituted 6-Iodoflavonol Derivatives for Inhibitory Effect on Cholinesterases and β-Secretase. International Journal of Molecular Sciences, 19(12), 4112. https://doi.org/10.3390/ijms19124112