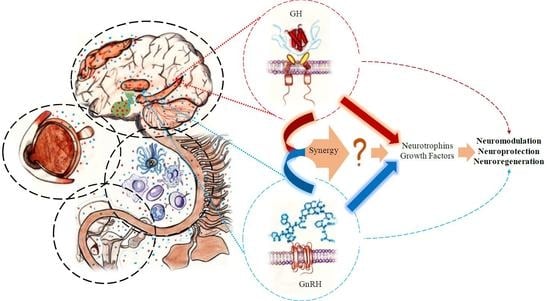
Growth Hormone (GH) and Gonadotropin-Releasing Hormone (GnRH) in the Central Nervous System: A Potential Neurological Combinatory Therapy?
Abstract
:1. Introduction
2. Expression and Neurotrophic Effects of Growth Hormone (GH) in the Central Nervous System (CNS)
2.1. Cerebral Cortex
2.2. Subcortical Organs
2.2.1. Hypothalamus
2.2.2. Cerebellum
2.2.3. Hippocampus
2.3. Spinal Cord
3. Expression and Neurotrophic Effects of GnRH in the CNS
3.1. Cerebral Cortex
3.2. Subcortical Organs
3.2.1. Hypothalamus
3.2.2. Cerebellum
3.2.3. Hippocampus
3.3. Spinal Cord
4. GH and GnRH Effects on Other Neural Tissues
4.1. Neuroretina
4.2. Brain Tumors
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheung, L.W.; Wong, A.S. Gonadotropin-releasing hormone: GnRH receptor signaling in extrapituitary tissues. FEBS J. 2008, 275, 5479–5495. [Google Scholar] [CrossRef] [PubMed]
- Quintanar, J.L.; Salinas, E. Neurotrophic effects of GnRH on neurite outgrowth and neurofilament protein expression in cultured cerebral cortical neurons of rat embryos. Neurochem. Res. 2008, 33, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Skinner, D.C.; Albertson, A.J.; Navratil, A.; Smith, A.; Mignot, M.; Talbott, H.; Scanlan-Blake, N. Effects of gonadotrophin-releasing hormone outside the hypothalamic-pituitary-reproductive axis. J. Neuroendocrinol. 2009, 21, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Rojas, A.; Huerta-Reyes, M. Human gonadotropin-releasing hormone receptor-activated cellular functions and signaling pathways in extra-pituitary tissues and cancer cells (review). Oncol. Rep. 2009, 22, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S. Extrapituitary growth hormone. Endocrine 2010, 38, 335–359. [Google Scholar] [CrossRef] [PubMed]
- Arámburo, C.; Alba-Betancourt, C.; Luna, M.; Harvey, S. Expression and function of growth hormone in the nervous system: A brief review. Gen. Comp. Endocrinol. 2014, 203, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.; Baudet, M.L. Extrapituitary growth hormone and growth? Gen. Comp. Endocrinol. 2014, 205, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.J.; Dai, W.; O’Mara, M.L.; Abankwa, D.; Chhabra, Y.; Pelekanos, R.A.; Gardon, O.; Tunny, K.A.; Blucher, K.M.; Morton, C.J.; et al. Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science 2014, 344, 1249783. [Google Scholar] [CrossRef] [PubMed]
- Stojilkovic, S.S.; Bjelobaba, I.; Zemkova, H. Ion channels of pituitary gonadotrophs and their roles in signaling and secretion. Front. Endocrinol. (Lausanne) 2017, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E.; Locatelli, V.; Rizzi, L. Neurotrophic and neuroregenerative effects of GH/IGF1. Int. J. Mol. Sci. 2017, 18, 2441. [Google Scholar] [CrossRef] [PubMed]
- Falleti, M.G.; Maruff, P.; Burman, P.; Harris, A. The effects of growth hormone (GH) deficiency and GH replacement on cognitive performance in adults: A meta-analysis of the current literature. Psychoneuroendocrinology 2006, 31, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Lem, A.J.; van der Kaay, D.C.; Hokken-Koelega, A.C. Bone mineral density and body composition in short children born SGA during growth hormone and gonadotropin releasing hormone analog treatment. J. Clin. Endocrinol. Metab. 2013, 98, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Du, X.F.; Yang, X.H.; Li, J.; Hao, M.; Guo, Y.H. Growth hormone co-treatment within a gnrh agonist long protocol improves implantation and pregnancy rates in patients undergoing IVF-ET. Arch. Gynecol. Obstet. 2016, 294, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Q.; Cheng, X.; Luo, Y.; Wen, Y. Effects and safety of combination therapy with gonadotropin-releasing hormone analogue and growth hormone in girls with idiopathic central precocious puberty: A meta-analysis. J. Endocrinol. Investig. 2016, 39, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Lobie, P.E.; Zhu, T.; Graichen, R.; Goh, E.L. Growth hormone, insulin-like growth factor I and the CNS: Localization, function and mechanism of action. Growth Horm. IGF Res. 2000, 10 (Suppl. B), S51–S56. [Google Scholar] [CrossRef]
- Webb, E.A.; O’Reilly, M.A.; Clayden, J.D.; Seunarine, K.K.; Chong, W.K.; Dale, N.; Salt, A.; Clark, C.A.; Dattani, M.T. Effect of growth hormone deficiency on brain structure, motor function and cognition. Brain 2012, 135, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Kinney-Forshee, B.A.; Kinney, N.E.; Steger, R.W.; Bartke, A. Could a deficiency in growth hormone signaling be beneficial to the aging brain? Physiol. Behav. 2004, 80, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Shevah, O.; Kornreich, L.; Galatzer, A.; Laron, Z. The intellectual capacity of patients with laron syndrome (LS) differs with various molecular defects of the growth hormone receptor gene. Correlation with CNS abnormalities. Horm. Metab. Res. 2005, 37, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Laron, Z. Childhood-onset growth hormone deficiency, cognitive function and brain N-acetylaspartate. Psychoneuroendocrinology 2006, 31, 680. [Google Scholar] [CrossRef] [PubMed]
- Nashiro, K.; Guevara-Aguirre, J.; Braskie, M.N.; Hafzalla, G.W.; Velasco, R.; Balasubramanian, P.; Wei, M.; Thompson, P.M.; Mather, M.; Nelson, M.D.; et al. Brain structure and function associated with younger adults in growth hormone receptor-deficient humans. J. Neurosci. 2017, 37, 1696–1707. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwpoort, I.C.; Drent, M.L. Cognition in the adult with childhood-onset GH deficiency. Eur. J. Endocrinol. 2008, 159 (Suppl. S1), S53–S57. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Martinez, E.; Sonntag, W.E.; Wilson, A.; Donahue, A.; Molina, D.P.; Brunso-Bechtold, J.; Nicolle, M.M. Early-onset GH deficiency results in spatial memory impairment in mid-life and is prevented by GH supplementation. J. Endocrinol. 2010, 204, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Alonso, B.; Casteleiro, N.; Couto, P.; Castanon, B.; Zas, E.; Reimunde, P. Effects of recombinant growth hormone (GH) replacement and psychomotor and cognitive stimulation in the neurodevelopment of GH-deficient (GHD) children with cerebral palsy: A pilot study. Ther. Clin. Risk Manag. 2011, 7, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Reimunde, P.; Quintana, A.; Castanon, B.; Casteleiro, N.; Vilarnovo, Z.; Otero, A.; Devesa, A.; Otero-Cepeda, X.L.; Devesa, J. Effects of growth hormone (GH) replacement and cognitive rehabilitation in patients with cognitive disorders after traumatic brain injury. Brain Inj. 2011, 25, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Quik, E.H.; Valk, G.D.; Drent, M.L.; Stalpers, L.J.; Kenemans, J.L.; Koppeschaar, H.P.; van Dam, P.S. Reduced growth hormone secretion after cranial irradiation contributes to neurocognitive dysfunction. Growth Horm. IGF Res. 2012, 22, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Moreau, O.K.; Cortet-Rudelli, C.; Yollin, E.; Merlen, E.; Daveluy, W.; Rousseaux, M. Growth hormone replacement therapy in patients with traumatic brain injury. J. Neurotrauma. 2013, 30, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, J.E.; Kristrom, B.; Jonsson, B.; Tuvemo, T.; Albertsson-Wikland, K. Growth hormone treatment improves cognitive function in short children with growth hormone deficiency. Horm. Res. Paediatr. 2015, 83, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, F. Growth hormone in the brain: Characteristics of specific brain targets for the hormone and their functional significance. Front. Neuroendocrinol. 2000, 21, 330–348. [Google Scholar] [CrossRef] [PubMed]
- Ajo, R.; Cacicedo, L.; Navarro, C.; Sanchez-Franco, F. Growth hormone action on proliferation and differentiation of cerebral cortical cells from fetal rat. Endocrinology 2003, 144, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Declercq, J.; Brouwers, B.; Pruniau, V.P.; Stijnen, P.; de Faudeur, G.; Tuand, K.; Meulemans, S.; Serneels, L.; Schraenen, A.; Schuit, F.; et al. Metabolic and behavioural phenotypes in nestin-cre mice are caused by hypothalamic expression of human growth hormone. PLoS ONE 2015, 10, e0135502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cady, G.; Landeryou, T.; Garratt, M.; Kopchick, J.J.; Qi, N.; Garcia-Galiano, D.; Elias, C.F.; Myers, M.G., Jr.; Miller, R.A.; Sandoval, D.A.; et al. Hypothalamic growth hormone receptor (GHR) controls hepatic glucose production in nutrient-sensing leptin receptor (LEPRb) expressing neurons. Mol. Metab. 2017, 6, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T. Effects of growth hormone on cerebral development: Morphological studies. Horm. Res. 1996, 45, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Stephen, J.; Vilboux, T.; Mian, L.; Kuptanon, C.; Sinclair, C.M.; Yildirimli, D.; Maynard, D.M.; Bryant, J.; Fischer, R.; Vemulapalli, M.; et al. Mutations in kiaa0753 cause Joubert syndrome associated with growth hormone deficiency. Hum. Genet. 2017, 136, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Devesa, P.; Agasse, F.; Xapelli, S.; Almenglo, C.; Devesa, J.; Malva, J.O.; Arce, V.M. Growth hormone pathways signaling for cell proliferation and survival in hippocampal neural precursors from postnatal mice. BMC Neurosci. 2014, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Sanders, E.J.; Parker, E.; Arámburo, C.; Harvey, S. Retinal growth hormone is an anti-apoptotic factor in embryonic retinal ganglion cell differentiation. Exp. Eye Res. 2005, 81, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Sanders, E.J.; Lin, W.Y.; Parker, E.; Harvey, S. Growth hormone promotes the survival of retinal cells in vivo. Gen. Comp. Endocrinol. 2011, 172, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Lea, R.W.; Dawson, T.; Martinez-Moreno, C.G.; El-Abry, N.; Harvey, S. Growth hormone and cancer: GH production and action in glioma? Gen. Comp. Endocrinol. 2015, 220, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Aberg, D. Role of the growth hormone/insulin-like growth factor 1 axis in neurogenesis. Endocr. Dev. 2010, 17, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, F.; De la Herran-Arita, A.K.; Juarez-Aguilar, E.; Regalado-Santiago, C.; Millan-Aldaco, D.; Blanco-Centurion, C.; Drucker-Colin, R. Growth hormone improves hippocampal adult cell survival and counteracts the inhibitory effect of prolonged sleep deprivation on cell proliferation. Brain Res. Bull. 2011, 84, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Almenglo, C.; Devesa, P.; Devesa, J.; Arce, V.M. GPE promotes the proliferation and migration of mouse embryonic neural stem cells and their progeny in vitro. Int. J. Mol. Sci. 2017, 18, 1280. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, D.G.; Vukovic, J.; Waters, M.J.; Bartlett, P.F. GH mediates exercise-dependent activation of SVZ neural precursor cells in aged mice. PLoS ONE 2012, 7, e49912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porlan, E.; Perez-Villalba, A.; Delgado, A.C.; Ferron, S.R. Paracrine regulation of neural stem cells in the subependymal zone. Arch. Biochem. Biophys. 2013, 534, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, D.G.; Golmohammadi, M.G.; Large, B.; Waters, M.J.; Rietze, R.L. Exercise increases neural stem cell number in a growth hormone-dependent manner, augmenting the regenerative response in aged mice. Stem Cell. 2009, 27, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Christophidis, L.J.; Gorba, T.; Gustavsson, M.; Williams, C.E.; Werther, G.A.; Russo, V.C.; Scheepens, A. Growth hormone receptor immunoreactivity is increased in the subventricular zone of juvenile rat brain after focal ischemia: A potential role for growth hormone in injury-induced neurogenesis. Growth Horm. IGF Res. 2009, 19, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.J.; Blackmore, D.G. Growth hormone (GH), brain development and neural stem cells. Pediatr. Endocrinol. Rev. 2011, 9, 549–553. [Google Scholar] [PubMed]
- Blackmore, D.G.; Reynolds, B.A.; Golmohammadi, M.G.; Large, B.; Aguilar, R.M.; Haro, L.; Waters, M.J.; Rietze, R.L. Growth hormone responsive neural precursor cells reside within the adult mammalian brain. Sci. Rep. 2012, 2, 250. [Google Scholar] [CrossRef] [PubMed]
- Walser, M.; Hansen, A.; Svensson, P.A.; Jernas, M.; Oscarsson, J.; Isgaard, J.; Aberg, N.D. Peripheral administration of bovine GH regulates the expression of cerebrocortical beta-globin, GABAb receptor 1, and the lissencephaly-1 protein (lis-1) in adult hypophysectomized rats. Growth Horm. IGF Res. 2011, 21, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Gronbladh, A.; Johansson, J.; Nostl, A.; Nyberg, F.; Hallberg, M. GH improves spatial memory and reverses certain anabolic androgenic steroid-induced effects in intact rats. J. Endocrinol. 2013, 216, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, G.S.; Grover, L.M. Growth hormone enhances excitatory synaptic transmission in area ca1 of rat hippocampus. J. Neurophysiol. 2006, 95, 2962–2974. [Google Scholar] [CrossRef] [PubMed]
- Molina, D.P.; Ariwodola, O.J.; Linville, C.; Sonntag, W.E.; Weiner, J.L.; Brunso-Bechtold, J.K.; Adams, M.M. Growth hormone modulates hippocampal excitatory synaptic transmission and plasticity in old rats. Neurobiol. Aging 2012, 33, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Le Greves, M.; Steensland, P.; Le Greves, P.; Nyberg, F. Growth hormone induces age-dependent alteration in the expression of hippocampal growth hormone receptor and n-methyl-d-aspartate receptor subunits gene transcripts in male rats. Proc. Natl. Acad. Sci. USA 2002, 99, 7119–7123. [Google Scholar] [CrossRef] [PubMed]
- Le Greves, M.; Zhou, Q.; Berg, M.; Le Greves, P.; Fholenhag, K.; Meyerson, B.; Nyberg, F. Growth hormone replacement in hypophysectomized rats affects spatial performance and hippocampal levels of NMDA receptor subunit and psd-95 gene transcript levels. Exp. Brain Res. 2006, 173, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lund, P.K.; Burgess, S.B.; Rudisch, B.E.; McIlwain, D.L. Growth hormone, insulin-like growth factor I, and motoneuron size. J. Neurobiol. 1997, 32, 202–212. [Google Scholar] [CrossRef]
- Baudet, M.L.; Rattray, D.; Harvey, S. Growth hormone and its receptor in projection neurons of the chick visual system: Retinofugal and tectobulbar tracts. Neuroscience 2007, 148, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.; Martinez-Moreno, C.G.; Mora, J.; Aizouki, M.; Luna, M.; Aramburo, C.; Harvey, S. Internalization and synaptogenic effect of GH in retinal ganglion cells (RGCs). Gen. Comp. Endocrinol. 2016, 234, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Grimbly, C.; Martin, B.; Karpinski, E.; Harvey, S. Growth hormone production and action in N1E-115 neuroblastoma cells. J. Mol. Neurosci. 2009, 39, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Arce, V.M.; Devesa, P.; Devesa, J. Role of growth hormone (GH) in the treatment on neural diseases: From neuroprotection to neural repair. Neurosci. Res. 2013, 76, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Reimunde, P.; Devesa, P.; Barbera, M.; Arce, V. Growth hormone (GH) and brain trauma. Horm. Behav. 2013, 63, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Diaz-Getino, G.; Rey, P.; Garcia-Cancela, J.; Loures, I.; Nogueiras, S.; Hurtado de Mendoza, A.; Salgado, L.; Gonzalez, M.; Pablos, T.; et al. Brain recovery after a plane crash: Treatment with growth hormone (GH) and Neurorehabilitation: A Case Report. Int. J. Mol. Sci. 2015, 16, 30470–30482. [Google Scholar] [CrossRef] [PubMed]
- Alba-Betancourt, C.; Arámburo, C.; Avila-Mendoza, J.; Ahumada-Solórzano, S.M.; Carranza, M.; Rodriguez-Mendez, A.J.; Harvey, S.; Luna, M. Expression, cellular distribution, and heterogeneity of growth hormone in the chicken cerebellum during development. Gen. Comp. Endocrinol. 2011, 170, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Alba-Betancourt, C.; Luna-Acosta, J.L.; Ramirez-Martinez, C.E.; Avila-Gonzalez, D.; Granados-Avalos, E.; Carranza, M.; Martinez-Coria, H.; Arámburo, C.; Luna, M. Neuro-protective effects of growth hormone (GH) after hypoxia-ischemia injury in embryonic chicken cerebellum. Gen. Comp. Endocrinol. 2013, 183, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Svensson, A.L.; Bucht, N.; Hallberg, M.; Nyberg, F. Reversal of opiate-induced apoptosis by human recombinant growth hormone in murine fetus primary hippocampal neuronal cell cultures. Proc. Natl. Acad. Sci. USA 2008, 105, 7304–7308. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, F. The role of the somatotrophic axis in neuroprotection and neuroregeneration of the addictive brain. Int. Rev. Neurobiol. 2009, 88, 399–427. [Google Scholar] [CrossRef] [PubMed]
- Nylander, E.; Gronbladh, A.; Zelleroth, S.; Diwakarla, S.; Nyberg, F.; Hallberg, M. Growth hormone is protective against acute methadone-induced toxicity by modulating the NMDA receptor complex. Neuroscience 2016, 339, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.; Sharma, H.S.; Stalberg, E.; Badgaiyan, R.D.; Westman, J.; Nyberg, F. Growth hormone attenuates alterations in spinal cord evoked potentials and cell injury following trauma to the rat spinal cord. An experimental study using topical application of rat growth hormone. Amino Acids 2000, 19, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, F.; Sharma, H.S. Repeated topical application of growth hormone attenuates blood-spinal cord barrier permeability and edema formation following spinal cord injury: An experimental study in the rat using Evans blue, ([125])I-sodium and lanthanum tracers. Amino Acids 2002, 23, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S. A select combination of neurotrophins enhances neuroprotection and functional recovery following spinal cord injury. Ann. N. Y. Acad. Sci. 2007, 1122, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S. Selected combination of neurotrophins potentiate neuroprotection and functional recovery following spinal cord injury in the rat. Acta Neurochir. Suppl. 2010, 106, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Muresanu, D.F.; Sharma, A.; Lafuente, J.V.; Patnaik, R.; Tian, Z.R.; Nyberg, F.; Sharma, H.S. Nanowired Delivery of growth hormone attenuates pathophysiology of spinal cord injury and enhances insulin-like growth Factor-1 concentration in the plasma and the spinal cord. Mol. Neurobiol. 2015, 52, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Avila-Mendoza, J.; Mora, J.; Carranza, M.; Luna, M.; Arámburo, C. Growth hormone reverses excitotoxic damage induced by kainic acid in the green iguana neuroretina. Gen. Comp. Endocrinol. 2016, 234, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moreno, C.G.; Avila-Mendoza, J.; Wu, Y.; Arellanes-Licea Edel, C.; Louie, M.; Luna, M.; Arámburo, C.; Harvey, S. Neuroprotection by GH against excitotoxic-induced cell death in retinal ganglion cells. Gen. Comp. Endocrinol. 2016, 234, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moreno, C.G.; Fleming, T.; Carranza, M.; Avila-Mendoza, J.; Luna, M.; Harvey, S.; Arámburo, C. Growth hormone protects against kainate excitotoxicity and induces BDNF and NT3 expression in chicken neuroretinal cells. Exp. Eye Res. 2017, 166, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, M.; Zhang, X.; Sun, X.; Ling, F. The effect and mechanism of growth hormone replacement on cognitive function in rats with traumatic brain injury. PLoS ONE 2014, 9, e108518. [Google Scholar] [CrossRef] [PubMed]
- Bohlooly, Y.M.; Olsson, B.; Bruder, C.E.; Linden, D.; Sjogren, K.; Bjursell, M.; Egecioglu, E.; Svensson, L.; Brodin, P.; Waterton, J.C.; et al. Growth hormone overexpression in the central nervous system results in hyperphagia-induced obesity associated with insulin resistance and dyslipidemia. Diabetes 2005, 54, 51–62. [Google Scholar] [CrossRef]
- Hojvat, S.; Baker, G.; Kirsteins, L.; Lawrence, A.M. Growth hormone (GH) immunoreactivity in the rodent and primate CNS: Distribution, characterization and presence posthypophysectomy. Brain Res. 1982, 239, 543–557. [Google Scholar] [CrossRef]
- Harvey, S.; Lavelin, I.; Pines, M. Growth hormone (GH) action in the brain: Neural expression of a GH-response gene. J. Mol. Neurosci. 2002, 18, 89–95. [Google Scholar] [CrossRef]
- Scheepens, A.; Sirimanne, E.S.; Breier, B.H.; Clark, R.G.; Gluckman, P.D.; Williams, C.E. Growth hormone as a neuronal rescue factor during recovery from CNS injury. Neuroscience 2001, 104, 677–687. [Google Scholar] [CrossRef]
- Coculescu, M. Blood-brain barrier for human growth hormone and insulin-like growth factor-I. J. Pediatr. Endocrinol. Metab. 1999, 12, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Yu, Y.; Cain, C.M.; Nyberg, F.; Couraud, P.O.; Kastin, A.J. Permeation of growth hormone across the blood-brain barrier. Endocrinology 2005, 146, 4898–4904. [Google Scholar] [CrossRef] [PubMed]
- Lobie, P.E.; Garcia-Aragon, J.; Lincoln, D.T.; Barnard, R.; Wilcox, J.N.; Waters, M.J. Localization and ontogeny of growth hormone receptor gene expression in the central nervous system. Dev. Brain Res. 1993, 74, 225–233. [Google Scholar] [CrossRef]
- Ransome, M.I.; Goldshmit, Y.; Bartlett, P.F.; Waters, M.J.; Turnley, A.M. Comparative analysis of cns populations in knockout mice with altered growth hormone responsiveness. Eur. J. Neurosci. 2004, 19, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Furigo, I.C.; Metzger, M.; Teixeira, P.D.; Soares, C.R.; Donato, J., Jr. Distribution of growth hormone-responsive cells in the mouse brain. Brain Struct. Funct. 2017, 222, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Sharma, H.S.; Olsson, Y.; Gordh, T.; Thoren, P.; Sjoquist, P.O.; Roos, P.; Adem, A.; Nyberg, F. Vascular permeability to growth hormone in the rat central nervous system after focal spinal cord injury. Influence of a new anti-oxidant h 290/51 and age. Neurosci. Res. 1995, 23, 185–194. [Google Scholar] [CrossRef]
- Ye, P.; Umayahara, Y.; Ritter, D.; Bunting, T.; Auman, H.; Rotwein, P.; D’Ercole, A.J. Regulation of insulin-like growth factor I (IGF-I) gene expression in brain of transgenic mice expressing an IGF-I-luciferase fusion gene. Endocrinology 1997, 138, 5466–5475. [Google Scholar] [CrossRef] [PubMed]
- Walser, M.; Schioler, L.; Oscarsson, J.; Aberg, M.A.; Svensson, J.; Aberg, N.D.; Isgaard, J. Different modes of GH administration influence gene expression in the male rat brain. J. Endocrinol. 2014, 222, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Walser, M.; Schioler, L.; Oscarsson, J.; Aberg, M.A.; Wickelgren, R.; Svensson, J.; Isgaard, J.; Aberg, N.D. Mode of GH administration and gene expression in the female rat brain. J. Endocrinol. 2017, 233, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Persson, A.I.; Aberg, N.D.; Oscarsson, J.; Isaksson, O.G.; Ronnback, L.; Frick, F.; Sonesson, C.; Eriksson, P.S. Expression of delta opioid receptor mRNA and protein in the rat cerebral cortex and cerebellum is decreased by growth hormone. J. Neurosci. Res. 2003, 71, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Heredia, M.; Fuente, A.; Criado, J.; Yajeya, J.; Devesa, J.; Riolobos, A.S. Early growth hormone (GH) treatment promotes relevant motor functional improvement after severe frontal cortex lesion in adult rats. Behav. Brain Res. 2013, 247, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Heredia, M.; Palomero, J.; de la Fuente, A.; Criado, J.M.; Yajeya, J.; Devesa, J.; Devesa, P. Motor improvement of skilled forelimb use induced by treatment with growth hormone and rehabilitation is dependent on the onset of the treatment after cortical ablation. Neural Plast. 2018, in press. [Google Scholar]
- Aberg, N.D.; Carlsson, B.; Rosengren, L.; Oscarsson, J.; Isaksson, O.G.; Ronnback, L.; Eriksson, P.S. Growth hormone increases connexin-43 expression in the cerebral cortex and hypothalamus. Endocrinology 2000, 141, 3879–3886. [Google Scholar] [CrossRef] [PubMed]
- Addison, M.L.; Rissman, E.F. Sexual dimorphism of growth hormone in the hypothalamus: Regulation by estradiol. Endocrinology 2012, 153, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- Yoshizato, H.; Fujikawa, T.; Soya, H.; Tanaka, M.; Nakashima, K. The growth hormone (GH) gene is expressed in the lateral hypothalamus: Enhancement by GH-releasing hormone and repression by restraint stress. Endocrinology 1998, 139, 2545–2551. [Google Scholar] [CrossRef] [PubMed]
- Quinnies, K.M.; Bonthuis, P.J.; Harris, E.P.; Shetty, S.R.; Rissman, E.F. Neural growth hormone: Regional regulation by estradiol and/or sex chromosome complement in male and female mice. Biol. Sex Differ. 2015, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Kamegai, J.; Minami, S.; Sugihara, H.; Hasegawa, O.; Higuchi, H.; Wakabayashi, I. Growth hormone receptor gene is expressed in neuropeptide y neurons in hypothalamic arcuate nucleus of rats. Endocrinology 1996, 137, 2109–2112. [Google Scholar] [CrossRef] [PubMed]
- Kastrup, Y.; Le Greves, M.; Nyberg, F.; Blomqvist, A. Distribution of growth hormone receptor mRNA in the brain stem and spinal cord of the rat. Neuroscience 2005, 130, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Kamegai, J.; Minami, S.; Sugihara, H.; Higuchi, H.; Wakabayashi, I. Growth hormone induces expression of the c-fos gene on hypothalamic neuropeptide-y and somatostatin neurons in hypophysectomized rats. Endocrinology 1994, 135, 2765–2771. [Google Scholar] [CrossRef] [PubMed]
- Wells, T.; Flavell, D.M.; Wells, S.E.; Carmignac, D.F.; Robinson, I.C. Effects of growth hormone secretagogues in the transgenic growth-retarded (TGR) rat. Endocrinology 1997, 138, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.E.; Flavell, D.M.; Bisset, G.W.; Houston, P.A.; Christian, H.; Fairhall, K.M.; Robinson, I.C. Transgenesis and neuroendocrine physiology: A transgenic rat model expressing growth hormone in vasopressin neurones. J. Physiol. 2003, 551, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, D.T.; el-Hifnawi, E.; Sinowatz, F.; Waters, M.J. Immunohistochemical localization of growth hormone receptor binding protein in the mammalian cerebellum. Ann. Anat. 1994, 176, 419–427. [Google Scholar] [CrossRef]
- Persson, A.I.; Thorlin, T.; Eriksson, P.S. Comparison of immunoblotted delta opioid receptor proteins expressed in the adult rat brain and their regulation by growth hormone. Neurosci. Res. 2005, 52, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Anneren, G.; Tuvemo, T.; Gustafsson, J. Growth hormone therapy in young children with down syndrome and a clinical comparison of down and Prader-Willi syndromes. Growth Horm. IGF Res. 2000, 10 (Suppl. B), S87–S91. [Google Scholar] [CrossRef]
- Myrelid, A.; Bergman, S.; Elfvik Stromberg, M.; Jonsson, B.; Nyberg, F.; Gustafsson, J.; Anneren, G. Late effects of early growth hormone treatment in down syndrome. Acta Paediatr. 2010, 99, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Myrelid, A.; Frisk, P.; Stridsberg, M.; Anneren, G.; Gustafsson, J. Normal growth hormone secretion in overweight young adults with down syndrome. Growth Horm. IGF Res. 2010, 20, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Aberg, N.D.; Brywe, K.G.; Isgaard, J. Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. Sci. World J. 2006, 6, 53–80. [Google Scholar] [CrossRef] [PubMed]
- Aberg, N.D.; Johansson, I.; Aberg, M.A.; Lind, J.; Johansson, U.E.; Cooper-Kuhn, C.M.; Kuhn, H.G.; Isgaard, J. Peripheral administration of GH induces cell proliferation in the brain of adult hypophysectomized rats. J. Endocrinol. 2009, 201, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Devesa, P.; Reimunde, P.; Gallego, R.; Devesa, J.; Arce, V.M. Growth hormone (GH) treatment may cooperate with locally-produced GH in increasing the proliferative response of hippocampal progenitors to kainate-induced injury. Brain Inj. 2011, 25, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Arellanes-Licea, E.C.; Avila-Mendoza, J.; Ramirez-Martinez, E.C.; Ramos, E.; Uribe-Gonzalez, N.; Arámburo, C.; Morales, T.; Luna, M. Upregulation of GH but not IGF1 in the hippocampus of the lactating dam after kainic acid injury. Endocr. Connect. 2018. [Google Scholar] [CrossRef] [PubMed]
- Donahue, A.N.; Aschner, M.; Lash, L.H.; Syversen, T.; Sonntag, W.E. Growth hormone administration to aged animals reduces disulfide glutathione levels in hippocampus. Mech. Ageing Dev. 2006, 127, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Li, R.C.; Guo, S.Z.; Raccurt, M.; Moudilou, E.; Morel, G.; Brittian, K.R.; Gozal, D. Exogenous growth hormone attenuates cognitive deficits induced by intermittent hypoxia in rats. Neuroscience 2011, 196, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Gronbladh, A.; Nylander, E.; Hallberg, M. The neurobiology and addiction potential of anabolic androgenic steroids and the effects of growth hormone. Brain Res. Bull. 2016, 126, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Molina, D.P.; Ariwodola, O.J.; Weiner, J.L.; Brunso-Bechtold, J.K.; Adams, M.M. Growth hormone and insulin-like growth factor-I alter hippocampal excitatory synaptic transmission in young and old rats. Age 2013, 35, 1575–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malek, M.; Sarkaki, A.; Zahedi-Asl, S.; Rajaei, Z.; Farbood, Y. Effect of intra-hippocampal injection of human recombinant growth hormone on synaptic plasticity in the nucleus basalis magnocellularis-lesioned aged rats. Arq. Neuropsiquiatr. 2017, 75, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Elbornsson, M.; Horvath, A.; Gotherstrom, G.; Bengtsson, B.A.; Johannsson, G.; Svensson, J. Seven years of growth hormone (GH) replacement improves quality of life in hypopituitary patients with adult-onset GH deficiency. Eur. J. Endocrinol. 2017, 176, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, F.; Hallberg, M. Growth hormone and cognitive function. Nat. Rev. Endocrinol. 2013, 9, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Wass, J.A.; Reddy, R. Growth hormone and memory. J. Endocrinol. 2010, 207, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; McFarlane, H.G.; Kopchick, J.J. Spatial learning and memory in male mice with altered growth hormone action. Horm. Behav. 2017, 93, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Lema, H.; Zas, E.; Munin, B.; Taboada, P.; Devesa, P. Learning and memory recoveries in a young girl treated with growth hormone and neurorehabilitation. J. Clin. Med. 2016, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Rhodin, A.; von Ehren, M.; Skottheim, B.; Gronbladh, A.; Ortiz-Nieto, F.; Raininko, R.; Gordh, T.; Nyberg, F. Recombinant human growth hormone improves cognitive capacity in a pain patient exposed to chronic opioids. Acta Anaesthesiol. Scand. 2014, 58, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Ramis, M.; Sarubbo, F.; Sola, J.; Aparicio, S.; Garau, C.; Miralles, A.; Esteban, S. Cognitive improvement by acute growth hormone is mediated by NMDA and AMPA receptors and MEK pathway. Prog. Neuropsychopharmacol. Biol. Psychiatr. 2013, 45, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhang, Y.; Yu, X.; Song, J.; Xu, C.; Wan, Y. Changes in growth hormone (GH), GH receptor, and GH signal transduction in hippocampus of congenital hypothyroid rats. J. Neurosci. Res. 2011, 89, 248–255. [Google Scholar] [CrossRef] [PubMed]
- DiCarlo, L.M.; Vied, C.; Nowakowski, R.S. The stability of the transcriptome during the estrous cycle in four regions of the mouse brain. J. Comp. Neurol. 2017, 525, 3360–3387. [Google Scholar] [CrossRef] [PubMed]
- Gisabella, B.; Farah, S.; Peng, X.; Burgos-Robles, A.; Lim, S.H.; Goosens, K.A. Growth hormone biases amygdala network activation after fear learning. Transl. Psychiatr. 2016, 6, e960. [Google Scholar] [CrossRef] [PubMed]
- Lechan, R.M.; Molitch, M.E.; Jackson, I.M. Distribution of immunoreactive human growth hormone-like material and thyrotropin-releasing hormone in the rat central nervous system: Evidence for their coexistence in the same neurons. Endocrinology 1983, 112, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.; Hull, K. Neural growth hormone: An update. J. Mol. Neurosci. 2003, 20, 1–14. [Google Scholar] [CrossRef]
- Zhai, Q.; Lai, Z.; Roos, P.; Nyberg, F. Characterization of growth hormone binding sites in rat brain. Acta Paediatr. Suppl. 1994, 406, 92–95. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.L.; Hoke, V.B.; Kopchick, J.J.; Fuller, C.R.; Lund, P.K. Differential inhibition of postnatal brain, spinal cord and body growth by a growth hormone antagonist. BMC Neurosci. 2004, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koohestani, F.; Brown, C.M.; Meisami, E. Postnatal growth hormone deficiency in growing rats causes marked decline in the activity of spinal cord acetylcholinesterase but not butyrylcholinesterase. Int. J. Dev. Neurosci. 2012, 30, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Tsitouras, P.D.; Zhong, Y.G.; Spungen, A.M.; Bauman, W.A. Serum testosterone and growth hormone/insulin-like growth factor-I in adults with spinal cord injury. Horm. Metab. Res. 1995, 27, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.S.; Wang, Y.H.; Lien, I.N. Suppression of the hypothalamus-pituitary somatotrope axis in men with spinal cord injuries. Metabolism 1995, 44, 1116–1120. [Google Scholar] [CrossRef]
- Bauman, W.A.; Zhang, R.L.; Spungen, A.M. Provocative stimulation of growth hormone: A monozygotic twin study discordant for spinal cord injury. J. Spinal Cord Med. 2007, 30, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Rouleau, P.; Ung, R.V.; Lapointe, N.P.; Guertin, P.A. Hormonal and immunological changes in mice after spinal cord injury. J. Neurotrauma. 2007, 24, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Bogdanovic, N.; Nyberg, F.; Suliman, I.; Islam, A.; Roos, P.; Winblad, B.; Adem, A. Effects of long-term ovariectomy and ovarian steroids on somatogenic binding sites in rat brain and liver. Neurosci. Lett. 1995, 194, 193–196. [Google Scholar] [CrossRef]
- Hanci, M.; Kuday, C.; Oguzoglu, S.A. The effects of synthetic growth hormone on spinal cord injury. J. Neurosurg. Sci. 1994, 38, 43–49. [Google Scholar] [PubMed]
- Isla, A.; Budke, M.; Garcia-Grande, A.; Gomez de la Riva, A.; Morales, C.; Rey, J. [Protective effects of the growth hormone (GH) on the irradiated spinal cord in rats]. Neurocirugia 2007, 18, 89–94. [Google Scholar] [CrossRef]
- Liu, X.; Green, K.J.; Ford, Z.K.; Queme, L.F.; Lu, P.; Ross, J.L.; Lee, F.B.; Shank, A.T.; Hudgins, R.C.; Jankowski, M.P. Growth hormone regulates the sensitization of developing peripheral nociceptors during cutaneous inflammation. Pain 2017, 158, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Alonso, A.; Lopez, N.; Garcia, J.; Puell, C.I.; Pablos, T.; Devesa, P. Growth hormone (GH) and rehabilitation promoted distal innervation in a child affected by caudal regression syndrome. Int. J. Mol. Sci. 2017, 18, 230. [Google Scholar] [CrossRef] [PubMed]
- Quintanar, J.L.; Salinas, E.; Gonzalez, R. Expression of gonadotropin-releasing hormone receptor in cerebral cortical neurons of embryos and adult rats. Neurosci. Lett. 2007, 11, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chadwick, W.; Park, S.S.; Zhou, Y.; Silver, N.; Martin, B.; Maudsley, S. Gonadotropin-releasing hormone receptor system: Modulatory role in aging and neurodegeneration. CNS Neurol. Disord. Drug Targets 2010, 9, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Kanaho, Y.; Enomoto, M.; Endo, D.; Maehiro, S.; Park, M.K.; Murakami, S. Neurotrophic effect of gonadotropin-releasing hormone on neurite extension and neuronal migration of embryonic gonadotropin-releasing hormone neurons in chick olfactory nerve bundle culture. J. Neurosci. Res. 2009, 87, 2237–2244. [Google Scholar] [CrossRef] [PubMed]
- Quintanar, J.L.; Salinas, E.; Gonzalez, R. Gonadotropin-releasing hormone receptor in spinal cord neurons of embryos and adult rats. Neurosci. Lett. 2009, 461, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Schang, A.L.; Bleux, C.; Chenut, M.C.; Ngo-Muller, V.; Querat, B.; Jeanny, J.C.; Counis, R.; Cohen-Tannoudji, J.; Laverriere, J.N. Identification and analysis of two novel sites of rat GnRH receptor gene promoter activity: The pineal gland and retina. Neuroendocrinol. 2013, 97, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Corchuelo, S.; Martinez, E.R.M.; Butzge, A.J.; Doretto, L.B.; Ricci, J.M.B.; Valentin, F.N.; Nakaghi, L.S.O.; Somoza, G.M.; Nobrega, R.H. Characterization of GnRH/GnIH elements in the olfacto-retinal system and ovary during zebrafish ovarian maturation. Mol. Cell. Endocrinol. 2017, 450, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Van Groeninghen, J.C.; Kiesel, L.; Winkler, D.; Zwirner, M. Effects of luteinising-hormone-releasing hormone on nervous-system tumours. Lancet 1998, 352, 372–373. [Google Scholar] [CrossRef]
- Marelli, M.M.; Moretti, R.M.; Mai, S.; Muller, O.; Van Groeninghen, J.C.; Limonta, P. Novel insights into gnrh receptor activity: Role in the control of human glioblastoma cell proliferation. Oncol. Rep. 2009, 21, 1277–1282. [Google Scholar] [CrossRef]
- Skinner, D.C.; Malpaux, B.; Delaleu, B.; Caraty, A. Luteinizing hormone (LH)-releasing hormone in third ventricular cerebrospinal fluid of the ewe: Correlation with LH pulses and the LH surge. Endocrinology 1995, 136, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.T.; Kow, L.M.; Pfaff, D.W. Modulatory actions of luteinizing hormone-releasing hormone on electrical activity of preoptic neurons in brain slices. Neuroscience 1988, 27, 623–628. [Google Scholar] [CrossRef]
- Xu, C.; Xu, X.Z.; Nunemaker, C.S.; Moenter, S.M. Dose-dependent switch in response of gonadotropin-releasing hormone (GnRH) neurons to GnRH mediated through the type I GnRH receptor. Endocrinology 2004, 145, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, V.; Evans, N.P.; Dahl, G.E.; McFadden, K.L.; Mauger, D.T.; Karsch, F.J. Evidence for short or ultrashort loop negative feedback of gonadotropin-releasing hormone secretion. Neuroendocrinology 1995, 3, 248–258. [Google Scholar] [CrossRef]
- Voogd, J.; Glickstein, M. The anatomy of the cerebellum. Trends Neurosci. 1998, 21, 370–375. [Google Scholar] [CrossRef]
- Pasha, K.V.; Vijayan, E. Acute and short-term effects of intraventricular injection of somatostatin and LHRH on glutamate and GABA levels in rat brain. Biochem. Int. 1992, 26, 7–15. [Google Scholar] [PubMed]
- Prange-Kiel, J.; Jarry, H.; Schoen, M.; Kohlmann, P.; Lohse, C.; Zhou, L.; Rune, G.M. Gonadotropin-releasing hormone regulates spine density via its regulatory role in hippocampal estrogen synthesis. J. Cell Biol. 2008, 180, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.N.; Lu, F.; Wu, J.N.; Liu, D.D.; Hsieh, W.Y. Activation of gonadotropin-releasing hormone receptors induces a long-term enhancement of excitatory postsynaptic currents mediated by ionotropic glutamate receptors in the rat hippocampus. Neurosci. Lett. 1999, 260, 33–36. [Google Scholar] [CrossRef]
- Fester, L.; Rune, G.M. Sexual neurosteroids and synaptic plasticity in the hippocampus. Brain Res. 2015, 1621, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Quintanar, J.L.; Calderon-Vallejo, D.; Hernandez-Jasso, I. Effects of GnRH on neurite outgrowth, neurofilament and spinophilin proteins expression in cultured spinal cord neurons of rat embryos. Neurochem. Res. 2016, 41, 2693–2698. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Soto, I.; Salinas, E.; Hernandez-Jasso, I.; Quintanar, J.L. Leuprolide acetate, a GnRH agonist, improves experimental autoimmune encephalomyelitis: A possible therapy for multiple sclerosis. Neurochem. Res. 2012, 37, 2190–2197. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.; Yu, C.; Kim, D.S.; Li, J.; Drewing, A.; Li, L. Zebrafish olfacto-retinal centrifugal axon projection and distribution: Effects of gonadotropin-releasing hormone and dopaminergic signaling. Dev. Neurosci. 2016, 38, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Vallejo, D.; Quintanar-Stephano, A.; Hernandez-Jasso, I.; Jimenez-Hernandez, V.; Ruiz-Ornelas, J.; Jimenez, I.; Quintanar, J.L. Functional and structural recovery of the injured spinal cord in rats treated with gonadotropin-releasing hormone. Neurochem. Res. 2015, 40, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Diaz Galindo, C.; Gomez-Gonzalez, B.; Salinas, E.; Calderon-Vallejo, D.; Hernandez-Jasso, I.; Bautista, E.; Quintanar, J.L. Leuprolide acetate induces structural and functional recovery of injured spinal cord in rats. Neural Regen. Res. 2015, 10, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.C.; Fletcher, P.C.; Daly, E.M.; Picchioni, M.M.; Brammer, M.; Giampietro, V.; Rymer, J.; McGuire, P.K.; Maki, P.M.; Murphy, D.G. A study of visuospatial working memory pre- and post-Gonadotropin Hormone Releasing Hormone agonists (GnRHa) in young women. Horm. Behav. 2008, 54, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Grigorova, M.; Sherwin, B.B.; Tulandi, T. Effects of treatment with leuprolide acetate depot on working memory and executive functions in young premenopausal women. Psychoneuroendocrinology 2006, 31, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Maeng, L.Y.; Taha, M.B.; Cover, K.K.; Glynn, S.S.; Murillo, M.; Lebron-Milad, K.; Milad, M.R. Acute gonadotropin-releasing hormone agonist treatment enhances extinction memory in male rats. Psychoneuroendocrinology 2017, 82, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Yang, J.M.; Wu, J.N.; Chen, Y.C.; Kao, Y.H.; Tung, C.S.; Yang, S.N. Activation of gonadotropin-releasing hormone receptors produces neuronal excitation in the rat hippocampus. Chin. J. Physiol. 2013, 42, 67–71. [Google Scholar]
- Osada, T.; Kimura, F. Lhrh effects on hippocampal neurons are modulated by estrogen in rats. Endocr. J. 1995, 42, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Kubek, M.J.; Wilber, J.F.; Leesthma, J.E. The identification of gonadotropin-releasing hormone (GnRH) in hypothalamic and extrahypothalamic loci of the human nervous system. Horm. Metab. Res. 1979, 11, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Caraty, A.; Skinner, D.C. Gonadotropin-releasing hormone in third ventricular cerebrospinal fluid: Endogenous distribution and exogenous uptake. Endocrinology 2008, 149, 5227–5234. [Google Scholar] [CrossRef] [PubMed]
- Albertson, A.J.; Talbott, H.; Wang, Q.; Jensen, D.; Skinner, D.C. The gonadotropin-releasing hormone type I receptor is expressed in the mouse cerebellum. Cerebellum 2008, 7, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Ciechanowska, M.; Lapot, M.; Malewski, T.; Mateusiak, K.; Misztal, T.; Przekop, F. Expression of the GnRH and GnRH receptor (GnRH-R) genes in the hypothalamus and of the GnRH-R gene in the anterior pituitary gland of anestrous and luteal phase ewes. Anim. Reprod. Sci. 2008, 108, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Wilber, J.F.; Montoya, E.; Plotnikoff, N.P.; White, W.F.; Gendrick, R.; Renaud, L.; Martin, J.B. Gonadotropin-releasing hormone and thyrotropin-releasing hormone: Distribution and effects in the central nervous system. Recent Prog. Horm. Res. 1976, 32, 117–159. [Google Scholar] [PubMed]
- D’Aniello, B.; Pinelli, C.; King, J.A.; Rastogi, R.K. Neuroanatomical organization of GnRH neuronal systems in the lizard (Podarcis s. sicula) brain during development. Brain Res. 1994, 657, 221–226. [Google Scholar] [CrossRef]
- Chu, C.; Zhou, J.; Zhao, Y.; Liu, C.; Chang, P.; Zhou, Q.; Zhao, L.; Huang, W. Expression of FSH and its co-localization with FSH receptor and GnRH receptor in rat cerebellar cortex. J. Mol. Histol. 2013, 44, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, P.; Crumeyrolle, M.; Latouche, J.; Jordan, D.; Fillion, G.; L’Heritier, A.; Kordon, C.; Dussaillant, M.; Rostene, W.; Haour, F. Characterization and distribution of receptors for gonadotropin-releasing hormone in the rat hippocampus. Neuroendocrinology 1988, 48, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Jennes, L. Effects of gonadotropin releasing hormone and estradiol on c-fos expression in the rat hippocampus. Mol. Cell. Neurosci. 1990, 1, 139–145. [Google Scholar] [CrossRef]
- Prange-Kiel, J.; Schmutterer, T.; Fester, L.; Zhou, L.; Imholz, P.; Brandt, N.; Vierk, R.; Jarry, H.; Rune, G.M. Endocrine regulation of estrogen synthesis in the hippocampus? Prog. Histochem. Cytochem. 2013, 48, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Dolan, S.; Evans, N.P.; Richter, T.A.; Nolan, A.M. Expression of gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor in sheep spinal cord. Neurosci. Lett. 2003, 346, 120–122. [Google Scholar] [CrossRef]
- Kaiser, U.B.; Jakubowiak, A.; Steinberger, A.; Chin, W.W. Regulation of rat pituitary gonadotropin-releasing hormone receptor mRNA levels in vivo and in vitro. Endocrinology 1993, 133, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Sanders, E.J.; Parker, E.; Harvey, S. Retinal ganglion cell survival in development: Mechanisms of retinal growth hormone action. Exp. Eye Res. 2006, 83, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.; Baudet, M.L.; Sanders, E.J. Retinal growth hormone in perinatal and adult rats. J. Mol. Neurosci. 2006, 28, 257–264. [Google Scholar] [CrossRef]
- Sanders, E.J.; Parker, E.; Harvey, S. Endogenous growth hormone in human retinal ganglion cells correlates with cell survival. Mol. Vis. 2009, 15, 920–926. [Google Scholar] [PubMed]
- Harvey, S.; Baudet, M.L.; Sanders, E.J. Growth hormone and developmental ocular function: Clinical and basic studies. Pediatr. Endocrinol. Rev. 2007, 5, 510–515. [Google Scholar] [PubMed]
- Harvey, S.; Martinez-Moreno, C.G. Growth hormone and ocular dysfunction: Endocrine, paracrine or autocrine etiologies? Growth Horm. IGF Res. 2016, 29, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Servili, A.; Herrera-Perez, P.; Kah, O.; Munoz-Cueto, J.A. The retina is a target for GnRH-3 system in the european sea bass, dicentrarchus labrax. Gen. Comp. Endocrinol. 2012, 175, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Witkin, J.W. Immunocytochemical demonstration of luteinizing hormone-releasing hormone in optic nerve and nasal region of fetal rhesus macaque. Neurosci. Lett. 1987, 79, 73–77. [Google Scholar] [CrossRef]
- Santacana, M.; de la Vega, A.G.; Heredia, M.; Valverde, F. Presence of LHRH (luteinizing hormone-releasing hormone) fibers in the optic nerve, optic chiasm and optic tract of the adult rat. Dev. Brain Res. 1996, 91, 292–299. [Google Scholar] [CrossRef]
- Wirsig-Wiechmann, C.R.; Wiechmann, A.F. Vole retina is a target for gonadotropin-releasing hormone. Brain Res. 2002, 950, 210–217. [Google Scholar] [CrossRef]
- Sakoguchi, T.; Hama, S.; Tominaga, A.; Kinoshita, Y.; Sugiyama, K.; Arita, K.; Kurisu, K. Growth hormone receptor expression in brain tumors. Hiroshima J. Med. Sci. 2012, 61, 1–6. [Google Scholar] [PubMed]
- Brittain, A.L.; Basu, R.; Qian, Y.; Kopchick, J.J. Growth hormone and the epithelial-to-mesenchymal transition. J. Clin. Endocrinol. Metab. 2017, 102, 3662–3673. [Google Scholar] [CrossRef] [PubMed]
- Grundker, C.; Emons, G. The role of gonadotropin-releasing hormone in cancer cell proliferation and metastasis. Front. Endocrinol. 2017, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Sun, C.M.; Li, X.T.; Liu, C.J.; Zhou, Y.X. Growth hormone therapy and risk of recurrence/progression in intracranial tumors: A meta-analysis. Neurol. Sci. 2015, 36, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, A. Growth hormone therapy and brain tumors. Pediatr. Endocrinol. Rev. 2017, 14 (Suppl. S1), 229–234. [Google Scholar] [CrossRef] [PubMed]
- Jaszberenyi, M.; Schally, A.V.; Block, N.L.; Nadji, M.; Vidaurre, I.; Szalontay, L.; Rick, F.G. Inhibition of U-87 mg glioblastoma by an-152 (AEZS-108), a targeted cytotoxic analog of luteinizing hormone-releasing hormone. Oncotarget 2013, 4, 422–432. [Google Scholar] [CrossRef] [PubMed]


| Structure/Effect | Cortex | Hypothalamus | Cerebellum | Hippocampus | Spinal Cord | Neuro Retina | Brain Tumors |
|---|---|---|---|---|---|---|---|
| Structure Development | + [29] | + [30,31] | + [32,33] | + [34] | + [7] | + [35,36] | + [37] |
| Proliferation & Differentiation | + [29] | n/d | + [32] | + [38,39,40,41,42,43,44,45,46] | n/d | n/d | n/d |
| Axon/Dendrite growth & Synaptic actions | + [47,48] | n/d | + [32] | + [49,50,51,52] | + [53] | + [54,55] | + [56] |
| Neuroprotection & Neuroregeneration | + [57,58,59] | n/d | + [32,60,61] | + [62,63,64] | + [65,66,67,68,69] | + [70,71,72] | n/d |
| Cognitive & Behavior | + [73] | + [31,74] | + [33] | + [57] | n/d | n/d | n/d |
| Structure/Effect | Cortex | Hypothalamus | Cerebellum | Hippocampus | Spinal Cord | Neuro Retina | Brain Tumors |
|---|---|---|---|---|---|---|---|
| Structure Development | + [137] | + [138,139] | n/d | + [138] | + [140] | + [141,142] | + [143,144] |
| Proliferation & Differentiation | n/d | n/d | n/d | n/d | n/d | n/d | n/d |
| Axon/Dendrite growth & Synaptic actions | + [2,145] | + [139,146,147,148] | + [149,150] | + [151,152,153] | + [154,155] | + [142,156] | n/d |
| Neuroprotection & Neuroregeneration | + [2] | n/d | n/d | n/d | + [155,157,158] | n/d | n/d |
| Cognitive & Behavior | + [159,160,161] | n/d | n/d | + [162,163] | n/d | n/d | n/d |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Moreno, C.G.; Calderón-Vallejo, D.; Harvey, S.; Arámburo, C.; Quintanar, J.L. Growth Hormone (GH) and Gonadotropin-Releasing Hormone (GnRH) in the Central Nervous System: A Potential Neurological Combinatory Therapy? Int. J. Mol. Sci. 2018, 19, 375. https://doi.org/10.3390/ijms19020375
Martínez-Moreno CG, Calderón-Vallejo D, Harvey S, Arámburo C, Quintanar JL. Growth Hormone (GH) and Gonadotropin-Releasing Hormone (GnRH) in the Central Nervous System: A Potential Neurological Combinatory Therapy? International Journal of Molecular Sciences. 2018; 19(2):375. https://doi.org/10.3390/ijms19020375
Chicago/Turabian StyleMartínez-Moreno, Carlos G., Denisse Calderón-Vallejo, Steve Harvey, Carlos Arámburo, and José Luis Quintanar. 2018. "Growth Hormone (GH) and Gonadotropin-Releasing Hormone (GnRH) in the Central Nervous System: A Potential Neurological Combinatory Therapy?" International Journal of Molecular Sciences 19, no. 2: 375. https://doi.org/10.3390/ijms19020375
APA StyleMartínez-Moreno, C. G., Calderón-Vallejo, D., Harvey, S., Arámburo, C., & Quintanar, J. L. (2018). Growth Hormone (GH) and Gonadotropin-Releasing Hormone (GnRH) in the Central Nervous System: A Potential Neurological Combinatory Therapy? International Journal of Molecular Sciences, 19(2), 375. https://doi.org/10.3390/ijms19020375