A Simple Method to Reduce both Lactic Acid and Ammonium Production in Industrial Animal Cell Culture
Abstract
:1. Introduction
1.1. Summary of Methods to Reduce Lactic and Ammonium Production
- (1)
- Be universally effective—across all industrially-relevant cell lines and processes—at reducing both lactic acid and ammonium production to sufficiently low levels so as to have no negative impact on cell growth and product quality,
- (2)
- Lead to no increase and possibly even a decrease in process complexity, as discussed further below, and
- (3)
- Be commonly implemented in large-scale industrial operations run according to current Good Manufacturing Practices (cGMPs), with a solid track record of success over many years.
1.2. Process Complexity and Lack of Industrial Implementation
- (1)
- Frequent sample withdrawal, often using an automatic sampling system,
- (2)
- Sample testing for glucose and/or glutamine levels using an external sensor system,
- (3)
- Transmission of the test result into a computer control system for processing, and
- (4)
- Frequent culture additions from a glucose and/or glutamine reservoir, using a pump and transfer line.
1.3. Metabolic Engineering
1.4. Common Simple Methods
1.5. Lactate Supplementation and Adaptation (LSA) Technology
2. Results
2.1. Adaptation and Reduced Lactic Acid Production in Shaker Flask Cultures
2.2. Growth Performance of Adapted Cells in Fed-Batch Bioreactor Cultures
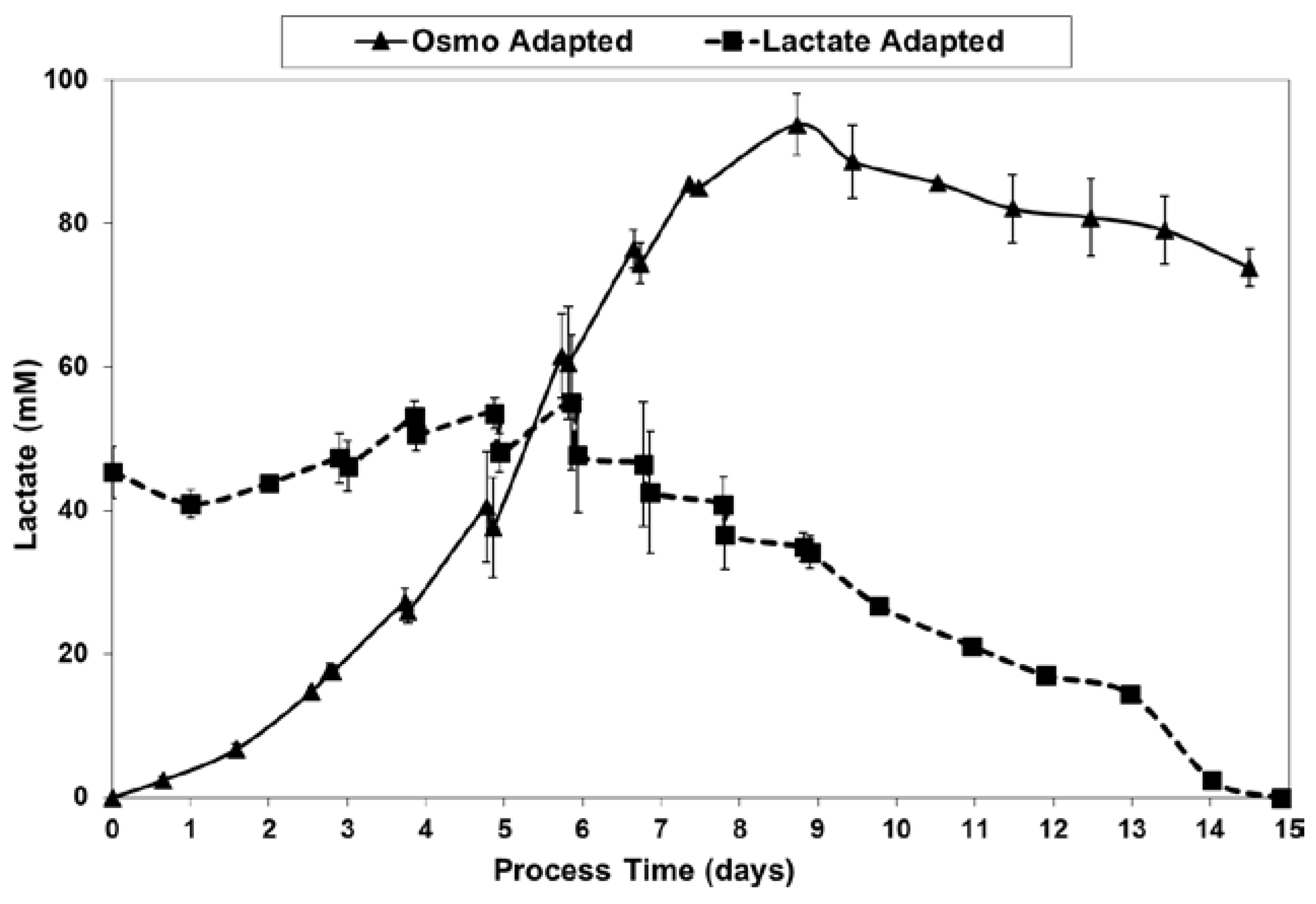
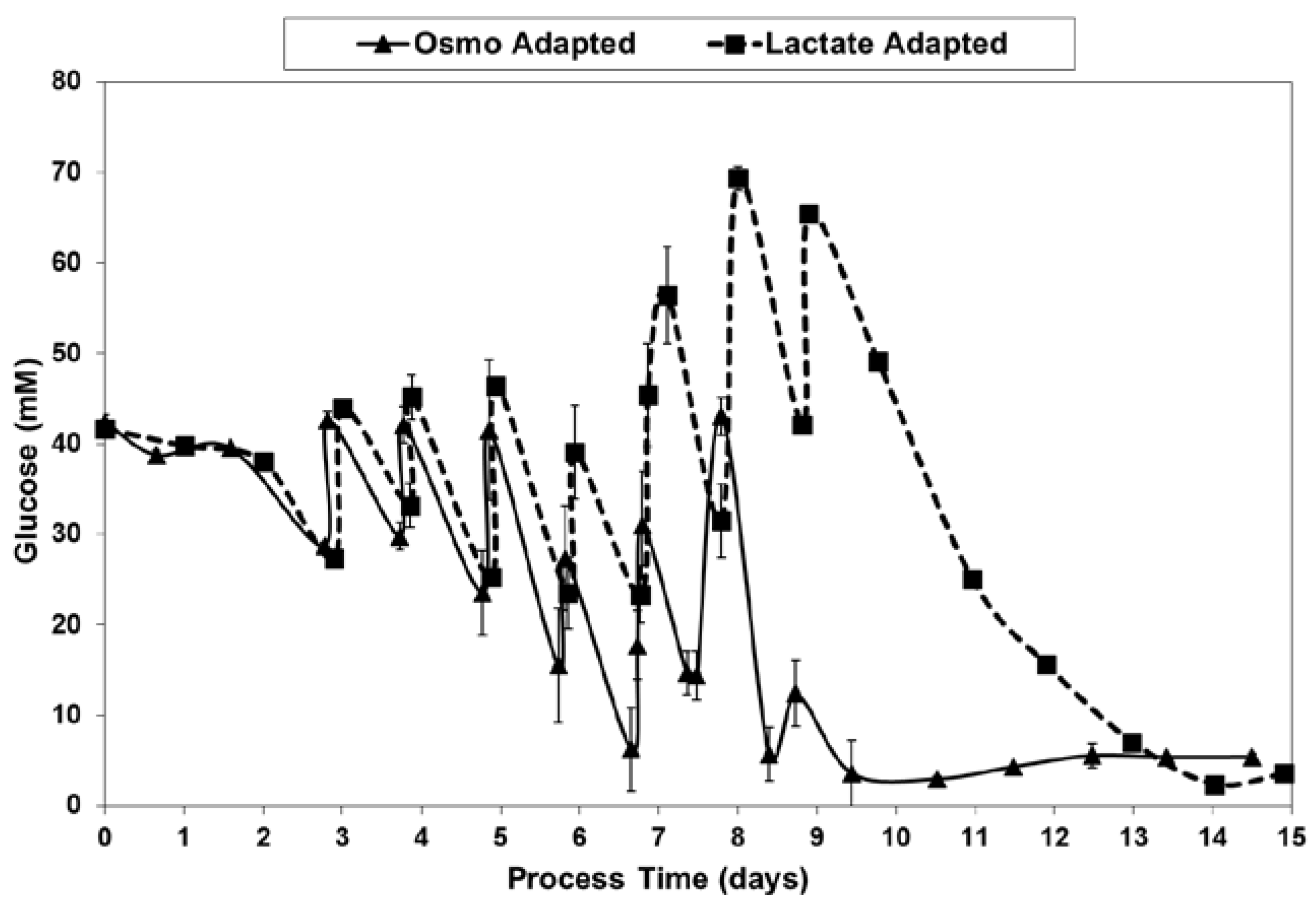
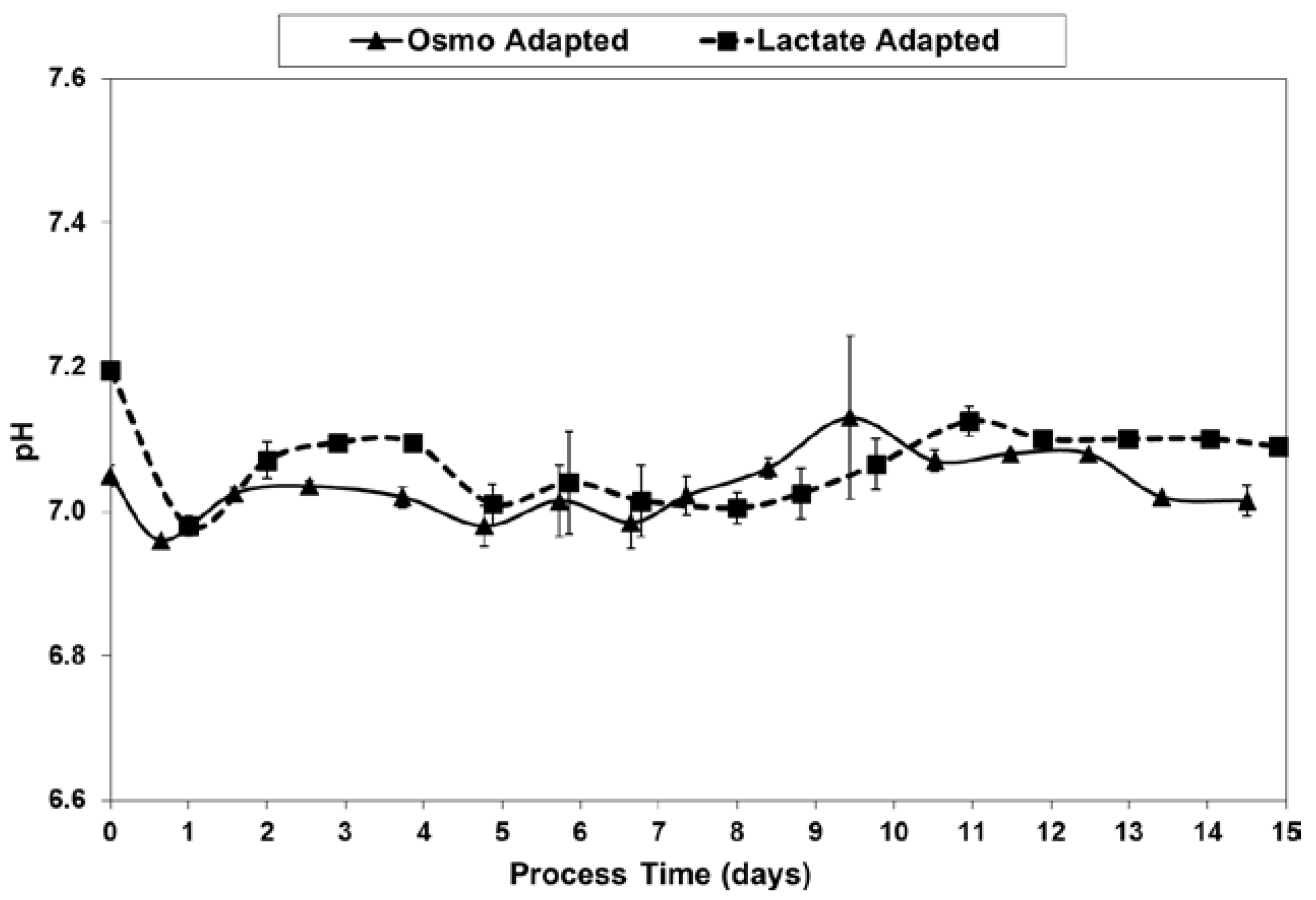
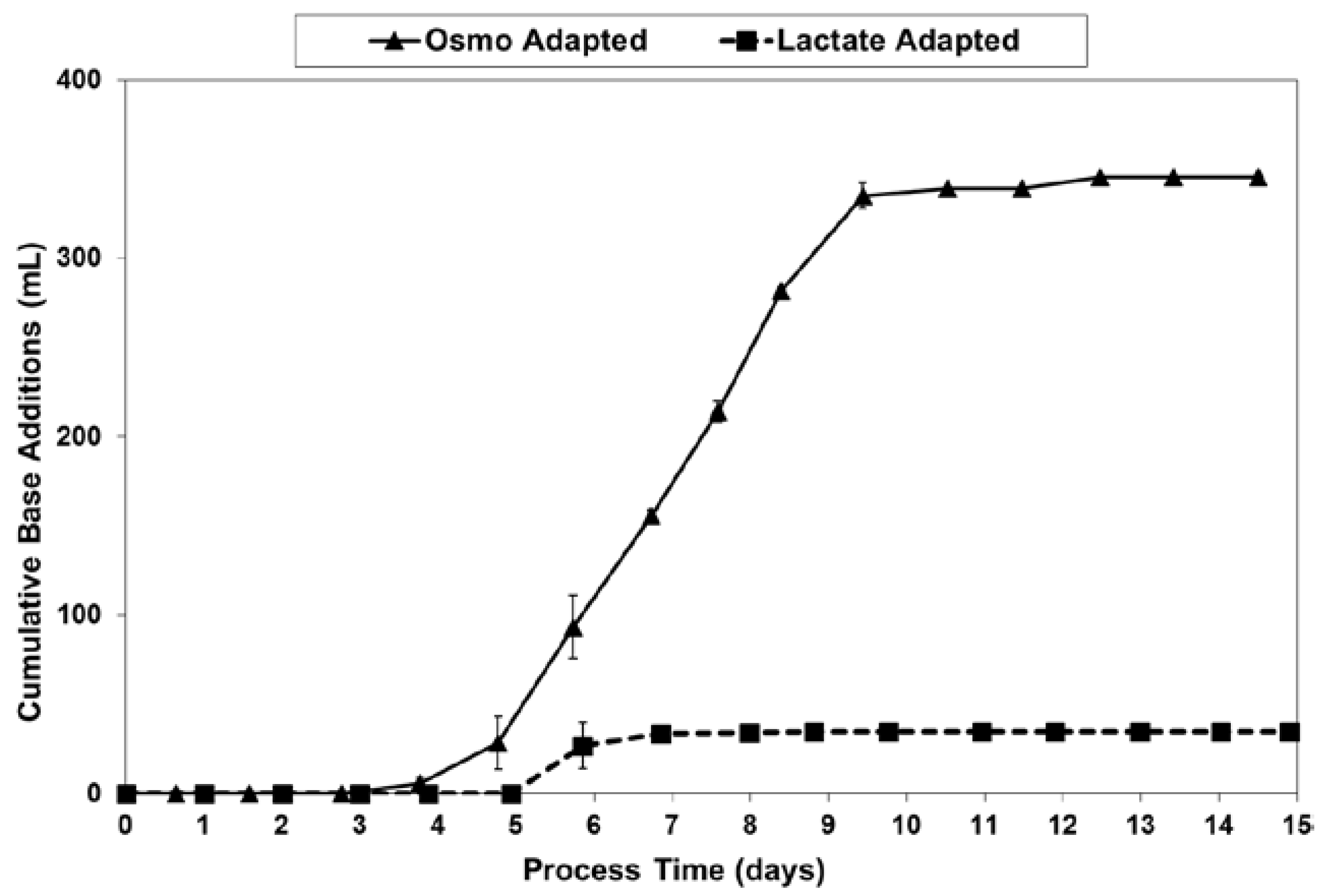
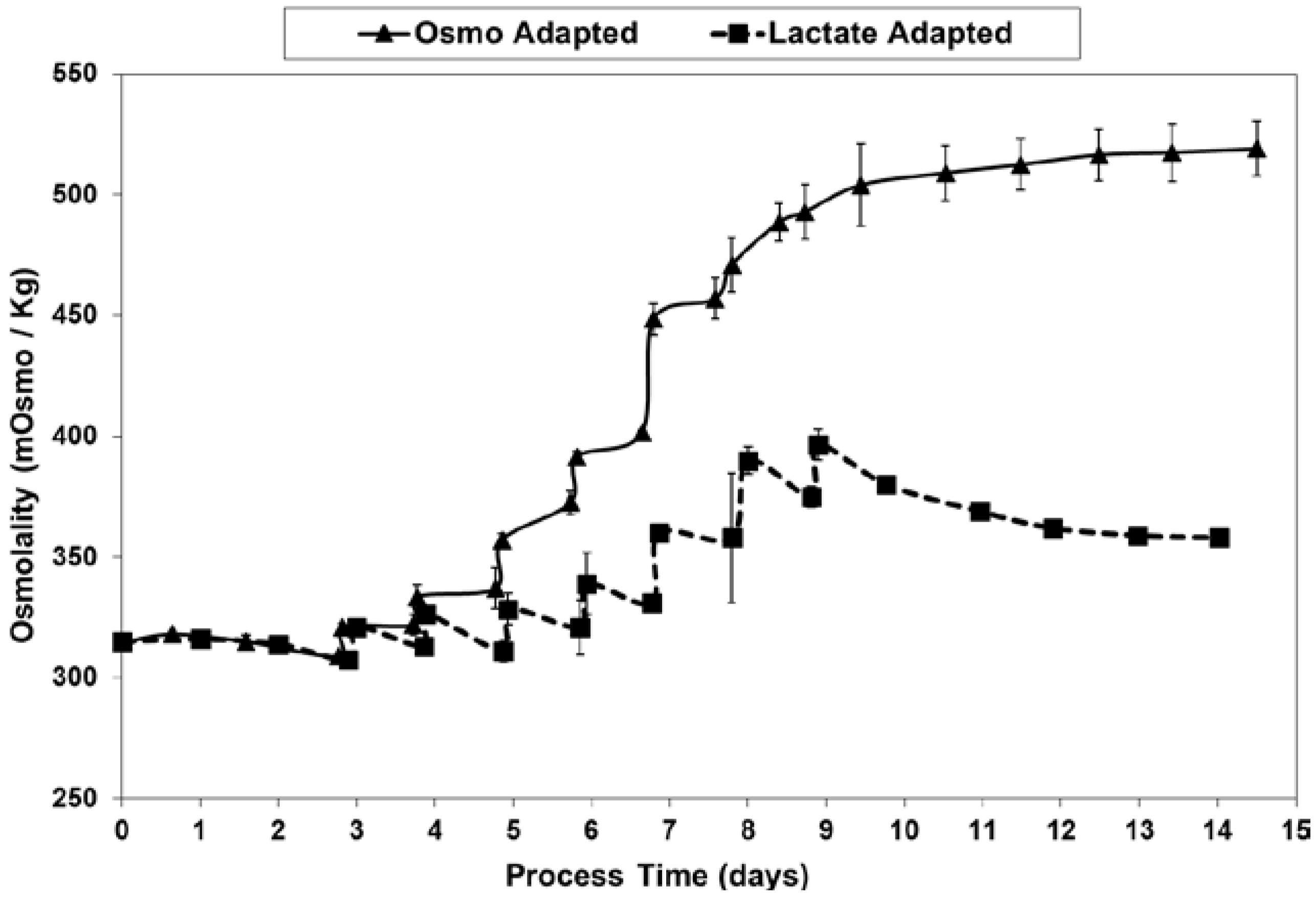
2.3. Lactic Acid Production, Base Addition, and Osmolality Profiles in Fed-Batch Bioreactor Cultures
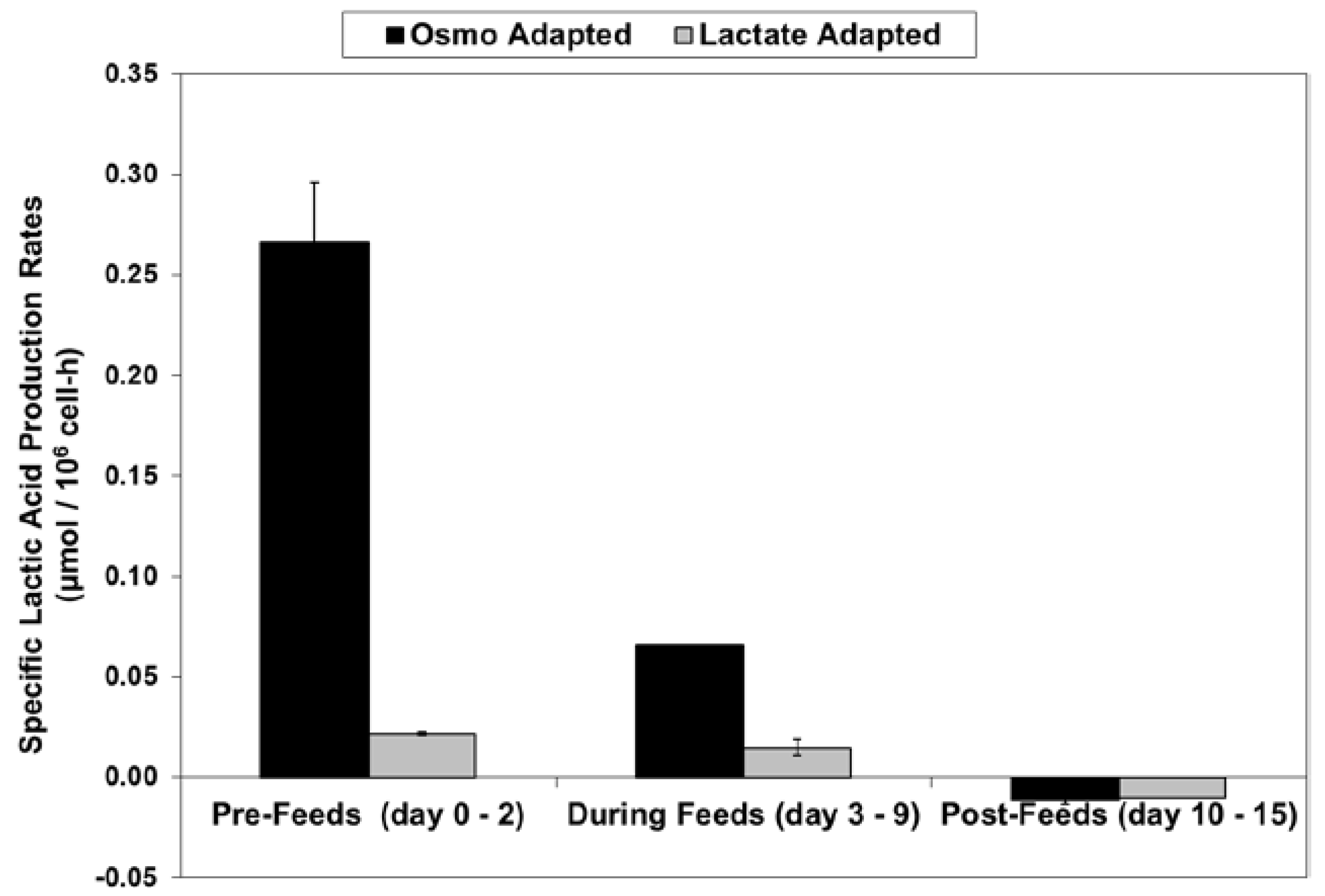
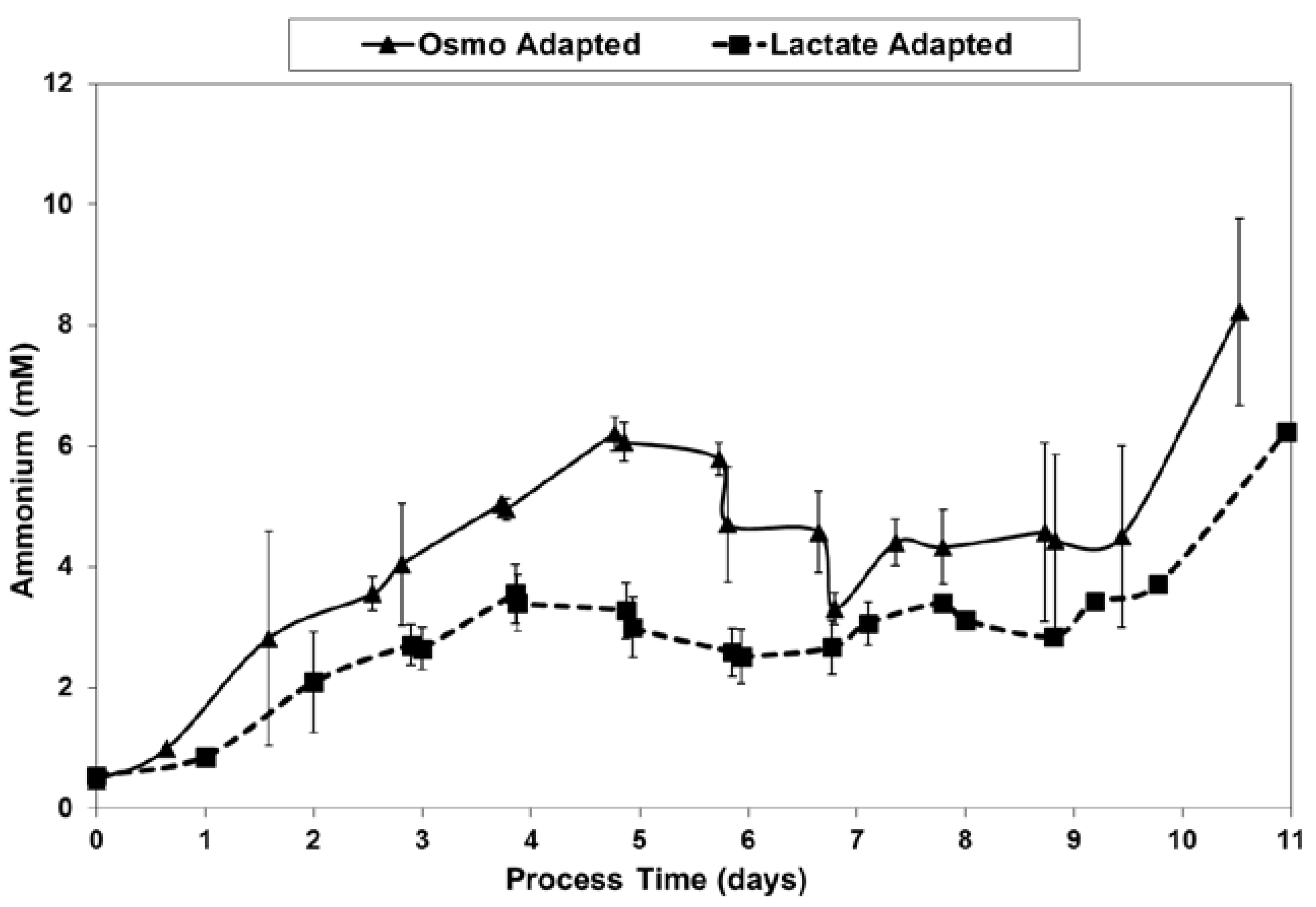
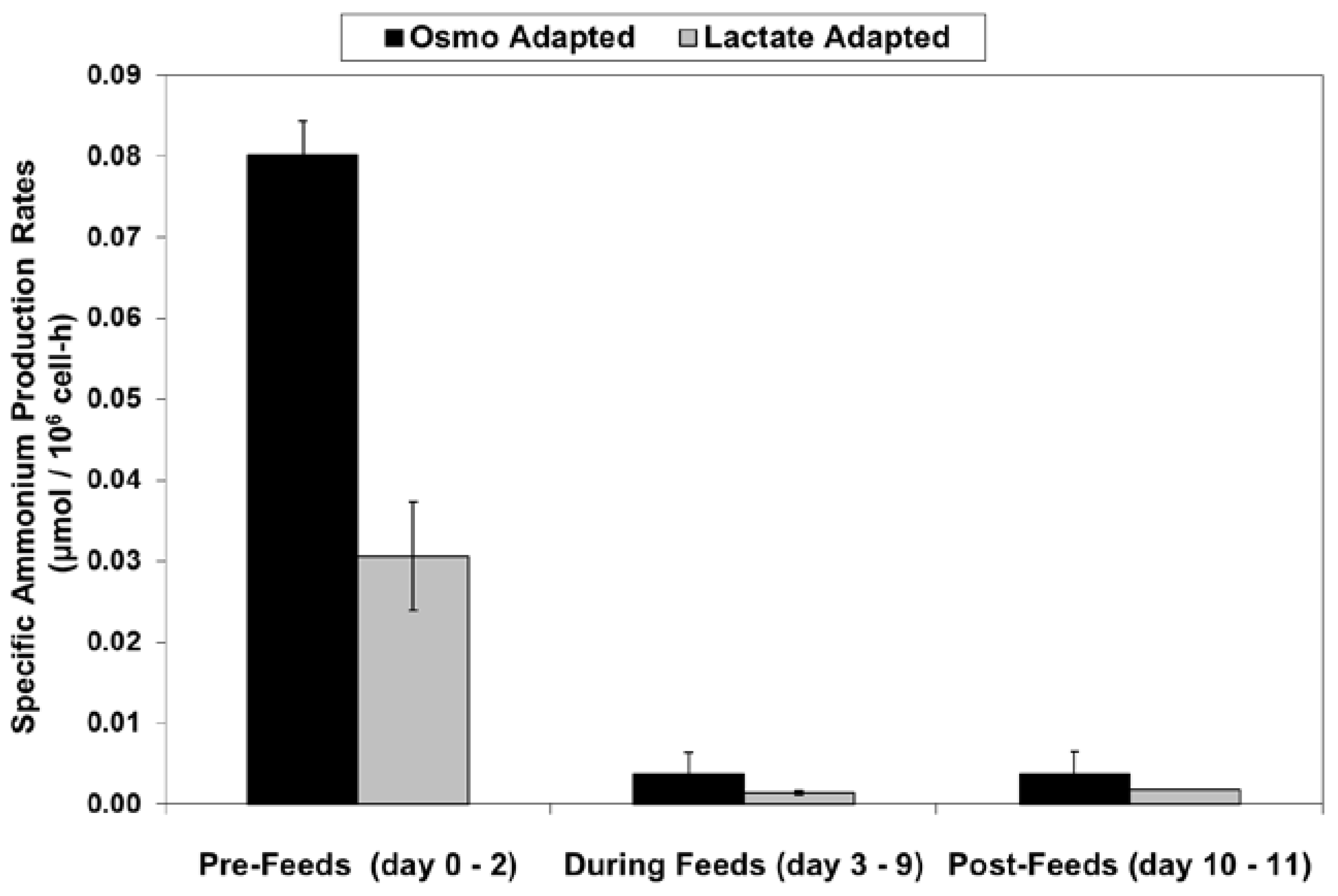
2.4. Ammonium Production and Glutamine Consumption in Fed-Batch Bioreactor Cultures
3. Discussion
4. Materials and Methods
4.1. Native Control Cells, Flask Cultures, and General Culture Methods
4.2. Adaptation of Cells
4.3. Bioreactor Cultures
4.4. Fed-Batch Experiments
4.5. Calculation of Specific Rates
5. Patents
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| cGMP | current Good Manufacturing Practice(s) |
| CHO | Chinese Hamster Ovary |
| gln | glutamine |
| gluc | glucose |
| GS | Glutamine Synthetase |
| GS-CHO | Glutamine Synthetase transfected Chinese Hamster Ovary |
| HIPDOG | HI-end pH-controlled Delivery of Glucose |
| IVCD | integrated viable cell days |
| LA | lactate adapted cells |
| Lac | lactate |
| Lac A | lactic acid |
| Laci | initial lactate |
| LC | lactate consumption |
| LSA | Lactate Supplementation and Adaptation |
| NH4+ | ammonium |
| OA | osmolality adapted cells |
| NR | not reported |
| P | nutrient or metabolite |
| qGln | specific glutamine uptake rate in μmol/106 cells-h |
| qGluc | specific glucose uptake rate in μmol/106 cells-h |
| qLac | specific lactic acid production rate in μmol/106 cells-h |
| qNH4+ | specific ammonium production rate in μmol/106 cells-h |
| TCA | tricarboxylic acid |
| TCD | Total Cell Density |
| VCD | Viable Cell Density |
| Yl/g | lactate produced per glucose consumed (mole/mole) |
| Kd | specific death rate in h−1 |
| μN | specific net growth rate in h−1 |
| μT | specific total growth rate in h−1 |
References
- Chang, R.S.; Geyer, R.P. Propagation of the conjunctival and HeLa cells in various carbohydrate media. Proc. Soc. Exp. Biol. Med. 1957, 96, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Leibovitz, A. The growth and maintenance of tissue-cell cultures in free gas exchange with the atmosphere. Am. J. Hyg. 1963, 78, 173–180. [Google Scholar] [PubMed]
- Fleischaker, R.J. An Experimental Study in the use of Instrumentation to Analyze Metabolism and Product Formation in Cell Culture. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, June 1982. [Google Scholar]
- Glacken, M.W.; Fleischaker, R.J.; Sinskey, A.J. Reduction of waste product excretion via nutrient control: Possible strategies for maximizing product and cell yields on serum in cultures of mammalian cells. Biotechnol. Bioeng. 1986, 28, 1376–1389. [Google Scholar] [CrossRef] [PubMed]
- Glacken, M.W.; Adema, E.; Sinskey, A.J. Mathematical descriptions of hybridoma culture kinetics: I. Initial metabolic rates. Biotechnol. Bioeng. 1988, 32, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Glacken, M.W.; Huang, C.; Sinskey, A.J. Mathematical descriptions of hybridoma culture kinetics. III. Simulation of fed-batch bioreactors. J. Biotechnol. 1989, 10, 39–66. [Google Scholar] [CrossRef]
- Lao, M.S.; Toth, D. Effects of ammonium and lactate on growth and metabolism of a recombinant Chinese hamster ovary cell culture. Biotechnol. Prog. 1997, 13, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Omasa, T.; Higashiyama, K.; Shioya, S.; Suga, K. Effects of lactate concentration on hybridoma culture in lactate-controlled fed-batch operation. Biotechnol. Bioeng. 1992, 39, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, S.S.; Riley, M.R.; Palsson, B.O. Effects of ammonia and lactate on hybridoma growth, metabolism, and antibody production. Biotechnol. Bioeng. 1992, 39, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Vijayasankaran, N.; Shen, A.; Kiss, R.; Amanullah, A. Cell culture process for monoclonal antibody production. mAbs 2010, 2, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Le, H.; Kabbur, S.; Pollastrini, L.; Sun, Z.; Mills, K.; Johnson, K.; Karypis, G.; Hu, W.-S. Multivariate analysis of cell culture bioprocess data–lactate consumption as process indicator. J. Biotechnol. 2012, 162, 20–223. [Google Scholar] [CrossRef] [PubMed]
- Yuk, I.H.; Russell, S.; Tang, Y.; Hsu, W.-T.; Mauger, J.B.; Aulakh, R.P.S.; Luo, J.; Gawlitzek, M.; Joly, J.C. Effect of cooper on CHO cells: Cellular requirements and product quality considerations. Biotechnol. Prog. 2015, 31, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Eagle, H.; Barban, S.; Levy, M.; Schultze, H.O. The utilization of carbohydrates by human cell cultures. J. Biol. Chem. 1958, 233, 551–558. [Google Scholar] [PubMed]
- Reitzer, L.J.; Wice, B.M.; Kennell, D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J. Biol. Chem. 1979, 254, 2669–2676. [Google Scholar] [PubMed]
- Duval, D.; Demangel, C.; Miossec, S.; Geahel, I. Role of metabolic waste products in the control of cell proliferation and antibody production by mouse hybridoma cells. Hybridoma 1992, 11, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Moellering, B.J.; Tedesco, J.L.; Townsend, R.R.; Hardy, M.R.; Scott, R.W.; Prior, C.P. Electrophoretic differences in a MAb expressed in three media. BioPharm 1990, 3, 30–38. [Google Scholar]
- Zielke, H.R.; Zielke, C.L.; Ozand, P.T. Glutamine: A major energy source for cultured mammalian cells. Fed. Proc. 1984, 43, 121–125. [Google Scholar] [PubMed]
- Christie, A.; Butler, M. The adaptation of BHK cells to a non-ammoniagenic glutamate-based culture medium. Biotechnol. Bioeng. 1999, 64, 298–309. [Google Scholar] [CrossRef]
- Altamirano, C.; Cairo, J.J.; Godia, F. Decoupling cell growth and product formation in Chinese hamster ovary cells through metabolic control. Biotechnol. Bioeng. 2001, 76, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, C.; Paredes, C.; Cairo, J.J.; Godia, F. Improvement of CHO cell culture medium formulation: Simultaneous substitution of glucose and glutamine. Biotechnol. Prog. 2000, 16, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Altramirano, C.; Illanes, A.; Becerra, S.; Cairo, J.J.; Godia, F. Considerations on the lactate consumption by CHO cells in the presence of galactose. J. Biotechnol. 2006, 125, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, H.; Park, Y.S.; Iijima, S.; Kobayashi, T. Growth characteristics in fed-batch culture of hybridoma cells with control of glucose and glutamine concentrations. Biotechnol. Bioeng. 1994, 44, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ljunggren, J.; Haggstrom, L. Catabolic control of hybridoma cells by glucose and glutamine limited fed batch culture. Biotechnol. Bioeng. 1994, 44, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Maranga, L.; Goochee, C.F. Metabolism of PER.C6 cells cultivated under fed-batch conditions at low glucose and glutamine levels. Biotechnol. Bioeng. 2006, 94, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Europa, A.F.; Gambhir, A.; Fu, P.C.; Hu, W.-S. Multiple steady states with distinct cellular metabolism in continuous culture of mammalian cells. Biotechnol. Bioeng. 2000, 67, 25–34. [Google Scholar] [CrossRef]
- Cruz, H.J.; Moreira, J.L.; Carrondo, M.J. Metabolic shifts by nutrient manipulation in continuous cultures of BHK cells. Biotechnol. Bioeng. 1999, 66, 104–113. [Google Scholar] [CrossRef]
- Gagnon, M.; Hiller, G.; Luan, Y.T.; Kittredge, A.; DeFelice, J.; Drapeau, D. High-end pH-controlled delivery of glucose effectively suppresses lactate accumulation in CHO fed-batch cultures. Biotechnol. Bioeng. 2011, 108, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Rehm, J.; Hu, W.-S. High viable cell concentration fed-batch cultures of hybridoma cells through on-line nutrient feeding. Biotechnol. Bioeng. 1995, 46, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Khattak, S.F.; Xing, Z.; He, A.; Kayne, P.S.; Qian, N.-X.; Pan, S.-H.; Li, Z.J. Cell culture and gene transcription effects of copper sulfate on Chinese Hamster Ovary cells. Biotechnol. Prog. 2011, 27, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Vijayasankaran, N.; Autsen, J.; Santuray, R.; Hudson, T.; Amanullah, A.; Li, F. Comparative metabolic analysis to understand lactate metabolism shift in Chinese Hamster Ovary cell culture process. Biotechnol. Bioeng. 2012, 109, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, J.; Ren, D.; Tsai, W.L.; Li, F.; Amanullah, A.; Hudson, T. Probing of c-terminal lysine variation in a recombinant monoclonal antibody production using Chinese Hamster Ovary Cells with chemically-defined media. Biotech. Bioeng. 2012, 109, 2306–2315. [Google Scholar] [CrossRef] [PubMed]
- Yuk, I.H.; Zhang, J.D.; Ebeling, M.; Berrera, M.; Gomez, N.; Werz, S.; Meiringer, C.; Shao, Z.; Swanberg, J.C.; Lee, K.H.; et al. Effects of copper on CHO cells: Insights from gene expression analyses. Biotechnol. Prog. 2014, 30, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Bollati-Fogolin, M.; Forno, G.; Nimtz, M.; Conradt, H.S.; Etcheverrigaray, M.; Kratje, R. Temperature reduction in cultures of hGM-CSF-expressing CHO cells: Effect on productivity and product quality. Biotechnol. Prog. 2005, 21, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.K.; Choi, S.L.; Song, J.Y.; Lee, G.M. Effect of culture pH on erythropoietin production by Chinese Hamster Ovary cells grown in suspension at 32.5 and 37.0 degrees Celsius. Biotechnol. Bioeng. 2005, 89, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Schmid, G.; Blanch, H.W.; Wilke, C.R. Hybridoma growth, metabolism, and product formation in HEPES-buffered medium: II. Effect of pH. Biotechnol. Lett. 1990, 12, 633–638. [Google Scholar] [CrossRef]
- Luan, Y.T.; Mulharasan, R.; Magee, W.E. Factors governing lactic acid formation in long term cultivation of hybridoma cells. Biotechnol. Lett. 1987, 9, 751–756. [Google Scholar] [CrossRef]
- Miller, W.M.; Blanch, H.W.; Wilke, C.R. A kinetic analysis of hybridoma growth and metabolism in batch and continuous suspension culture: Effect of nutrient concentration, dilution rate, and pH. Biotechnol. Bioeng. 1988, 32, 947–965. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wong, C.L.; Vijayasankaran, N.; Hudson, T.; Amanullah, A. Feeding lactate for CHO cell culture processes: Impact on culture metabolism and performance. Biotechnol. Bioeng. 2012, 109, 1173–1186. [Google Scholar] [CrossRef] [PubMed]
- Goochee, C.F.; Monica, T. Environmental effects on protein glycosylation. Biotechnology 1990, 8, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Andersen, D.C.; Goochee, C.F. The effect of cell-culture conditions on the oligosaccharide structures of secreted glycoproteins. Curr. Opin. Biotechnol. 1994, 5, 546–549. [Google Scholar] [CrossRef]
- Andersen, D.C.; Goochee, C.F. The effect of ammonia on the O-linked glycosylation of granulocyte colony-stimulating factor produced by Chinese hamster ovary cells. Biotechnol. Bioeng. 1995, 47, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Bibila, T.A.; Robinson, D.K. In pursuit of the optimal fed-batch process for monoclonal antibody production. Biotechnol. Prog. 1995, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cruz, H.J.; Freitas, C.M.; Alves, P.M.; Moreira, J.L.; Carrondo, M.J. Effects of ammonia and lactate on growth, metabolism, and productivity of BHK cells. Enzyme Microb. Technol. 2000, 27, 43–52. [Google Scholar] [CrossRef]
- Cruz, H.J.; Peixoto, C.M.; Nimtz, M.; Alves, P.M.; Dias, E.M.; Moreira, J.L.; Carrondo, M.J.T. Metabolic shifts do not influence the glycosylation patterns of recombinant fusion protein expressed in BHK cells. Biotechnol. Bioeng. 2000, 69, 129–139. [Google Scholar] [CrossRef]
- Cruz, H.J.; Moreira, J.L.; Carrondo, M.J.T. Metabolically optimized BHK cell fed-batch cultures. J. Biotechnol. 2000, 80, 109–118. [Google Scholar] [CrossRef]
- Genzel, Y.; Ritter, J.B.; Konig, S.; Alt, R.; Reichl, U. Substitution of glutamine by pyruvate to reduce ammonia formation and growth inhibition of mammalian cells. Biotechol. Prog. 2005, 21, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Ryll, T.; Valley, U.; Wagner, R. Biochemistry of growth inhibition by ammonium ions in mammalian cells. Biotechnol. Bioeng. 1994, 44, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Butler, M. Effects of Ammonia on CHO cell growth, Erythropoietin production, and Glycosylation. Biotechnol. Bioeng. 2000, 68, 370–380. [Google Scholar] [CrossRef]
- Dutton, R.L.; Scharer, J.M.; Moo-Young, M. Hybridoma growth and productivity: Effects of conditioned medium and of inoculum size. Cytotechnology 1999, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, C.; Buckland, B.; Aunins, J. Fed-batch culture of recombinant NS0 myeloma cells with high monoclonal antibody production. Biotechnol. Bioeng. 1997, 55, 783–792. [Google Scholar] [CrossRef]
- Veeravalli, K.; Laird, M.W.; Fedesco, M.; Zhang, Y.; Yu, C.X. Strain engineering to prevent norleucine incorporation during recombinant protein production in Escherichia coli. Biotechnol. Prog. 2015, 31, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Veeravalli, K.; Laird, M.W. Towards an era of utilizing methionine overproducing hosts for recombinant protein production in Escherichia coli. Bioengineered 2015, 6, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.H.; Lo, J.C.; Nissom, P.M.; Wong, V.V. Glutamine or glucose starvation in hybridoma cultures induces death receptor and mitochondrial apoptotic pathways. Biotechnol. Lett. 2006, 28, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, G.B.; Balcarcel, R.R.; Follstad, B.D.; Stephanopoulos, G.; Wang, D.I.C. Metabolic effects on recombinant interferon-γ glycosylation in continuous culture of Chinese hamster ovary cells. Biotechnol. Bioeng. 2000, 62, 336–347. [Google Scholar] [CrossRef]
- Ma, N.; Ellet, J.; Okediadi, C.; Hermes, P.; McCormick, E.; Casnocha, S. A single nutrient feed supports both chemically defined NS0 and CHO fed-batch processes: Improved productivity and lactate metabolism. Biotechnol. Prog. 2009, 25, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Bebbington, C.R.; Renner, G.; Thomson, S.; King, D.; Abrams, D.; Yarranton, G.T. High-level expression of a recombinant antibody from myeloma cells using a glutamine synthetase gene as an amplifiable selectable marker. Bio/Technology 1992, 10, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Paredes, C.; Prats, E.; Cairo, J.J.; Azorin, F.; Cornudella, L.; Godia, F. Modification of glucose and glutamine metabolism in hybridoma cells through metabolic engineering. Cytotechnology 1999, 30, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Young, J.D. Metabolic flux rewiring in mammalian cell cultures. Curr. Opin. Biotechnol. 2013, 24, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, Y.G.; Lee, G.M. CHO cells in biotechnology for production of recombinant proteins: Current state and further potential. Appl. Microbiol. Biotechnol. 2012, 93, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Dietmair, S.; Nielsen, L.K.; Timmins, N.E. Engineering a mammalian super producer. J. Chem. Technol. Biotechnol. 2011, 86, 905–914. [Google Scholar] [CrossRef]
- Quek, L.E.; Dietmair, S.; Kromer, J.O.; Nielsen, L.K. Metabolic flux analysis in mammalian cell culture. Metabol. Eng. 2010, 12, 61–171. [Google Scholar] [CrossRef] [PubMed]
- Wayte, J.; Boraston, R.; Bland, H.; Varley, J.; Brown, M. pH: Effects on growth and productivity of cell lines producing monoclonal antibodies: Control in large-scale fermenters. Genet. Eng. Biotechnol. 1997, 17, 125–132. [Google Scholar]
- Birch, J.R.; Edwards, D.J. The effect of pH on the growth and carbohydrate metabolism of a lymphoblastoid cell line. Dev. Biol. Stand. 1980, 46, 59–63. [Google Scholar] [PubMed]
- Low, K.; Harbour, C. Growth Kinetics of Hybridoma cells: 2. The effects of varying energy source-concentrations. Dev. Biol. Stand. 1985, 60, 73–79. [Google Scholar] [PubMed]
- Chaderjian, W.R.; Chin, E.T.; Harris, R.J.; Etcheverry, T.M. Effect of copper sulfate on performance of a serum-free CHO cell culture process and the level of free thiol in the recombinant antibody expressed. Biotechnol. Prog. 2005, 21, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Kaschak, T.; Boyd, D.; Lu, F.; Derfus, G.; Kluck, B.; Nogal, B.; Emery, C.; Summers, C.; Zheng, K.; Bayer, R.; et al. Characterization of the basic charge variants of a human IgG1. mAbs 2011, 3, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Croughan, M.S.; (Keck Graduate Institute, Claremont, CA, USA). Personal communication based on 30 years in industry, including 20 years of consulting experience with over 80 clients, 2017.
- Gong, X.; Li, D.; Li, X.; Fang, Q.; Han, X.; Wu, Y.; Yang, S.; Shen, B.Q. Fed-batch culture optimization of a growth-associated hybridoma cell line in chemically defined protein-free media. Cytotechnology 2006, 52, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Wang, D. Applications of improved stoichiometric model in medium design and fed-batch cultivation of animal cells in bioreactor. Cytotechnology 1994, 15, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Wlaschin, K.; Hu, W.-S. Fedbatch culture and dynamic nutrient feeding. In Advances in Biochemical Engineering/Biotechnology; Scheper, T., Hu, W.-S., Eds.; Springer: Berlin, Germany, 2006; Volume 101, pp. 43–74. ISBN 0724-6145. [Google Scholar]
- Zhang, L.; Shen, H.; Zhang, Y. Fed-batch culture of hybridoma cells in serum-free medium using an optimized feeding strategy. J. Chem. Technol. Biotechnol. 2004, 79, 171–181. [Google Scholar] [CrossRef]
- Mather, J.P.; Tsao, M.C. Method for Culturing Chinese Hamster Ovary Cells to Improve Production of Recombinant Proteins. U.S. Patent 5122469, 16 June 1992. [Google Scholar]
- Yuk, I.H.; Zhang, B.; Yang, Y.; Dutina, G.; Leach, K.D.; Vijayasankaran, N.; Shen, A.; Andersen, D.C.; Snedecor, B.R.; Joly, J.C. Controlling glycation of recombinant antibody in fed-batch cell cultures. Biotechnol. Bioeng. 2011, 108, 2600–2610. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Toh, P.C.; Burnett, I.; Li, F.; Hudson, T.; Amanullah, A.; Li, J. Automatic dynamic fed-batch process and media optimization for high productivity cell culture process development. Biotechnol. Bioeng. 2013, 110, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Bibila, T.A.; Ranucci, C.S.; Glazomitsky, K.; Buckland, B.C.; Aunins, J.G. Monoclonal antibody process development using medium concentrates. Biotechnol. Prog. 1994, 10, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Burky, J.E.; Wesson, M.C.; Young, A.; Farnsworth, S.; Dionne, B.; Zhu, Y.; Hartman, T.E.; Qu, L.; Zhou, W.; Sauer, P.W. Protein-free fed-batch culture of non-GS NS0 cell lines for production of recombinant antibodies. Biotechnol. Bioeng. 2007, 96, 281–293. [Google Scholar] [CrossRef] [PubMed]
- DeZengotita, V.M.; Miller, W.M.; Aunins, J.G.; Zhou, W. Phosphate feeding improves high-cell-concentration NS0 myeloma culture performance for monoclonal antibody production. Biotechnol. Bioeng. 2000, 69, 566–576. [Google Scholar] [CrossRef]
- Pascoe, D.E.; Arnott, D.; Papoutsakis, E.T.; Miller, W.M.; Andersen, D.C. Proteome analysis of antibody-producing CHO cell lines with different metabolic profiles. Biotechnol. Bioeng. 2007, 98, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Rawn, J.D. Biochemistry; Harbor & Row Publishers, Inc.: New York, NY, USA, 1983; pp. 576, 589, 823–824. ISBN 0-06-045335-4. [Google Scholar]
- Nelson, D.L.; Cox, M.M. Principles of Bioenergetics and Glycolysis and the Catabolism of Hexoses. In Lehninger Principles of Biochemistry, 3rd ed.; Worth Publishers: New York, NY, USA, 2000; pp. 494, 497, 542. ISBN 1-57259-153-6. [Google Scholar]
- Segel, I.H. Biochemical Calculations, 2nd ed.; John Wiley and Sons, Inc.: New York, NY, USA, 1968; p. 155. ISBN 0-471-77421-9. [Google Scholar]
- Mulukutla, B.C.; Gramer, M.; Hu, W.-S. On metabolic shift to lactate consumption in fed-batch culture of mammalian cells. Metab. Eng. 2012, 14, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Lee, D.Y.; Kim, I.Y.; Kim, H.J.; Park, H.W.; Choe, T.B.; Kim, I.-H. Enhancement of erythropoietin production in recombinant Chinese Hamster Ovary cells by sodium lactate addition. Biotechnol. Bioproc. Eng. 2007, 12, 60–72. [Google Scholar] [CrossRef]
- Schumpp, B.; Schlaeger, E.J. Growth study of lactate and ammonia double-resistant clones of HL-60 cells. Cytotechnology 1992, 8, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Smelko, J.P.; Wiltberger, K.R.; Hickman, E.F.; Morris, B.J.; Blackburn, T.J.; Ryll, T. Performance of high-intensity fed-batch mammalian cell cultures in disposable bioreactor systems. Biotechnol. Prog. 2011, 27, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Sauer, P.W.; Burky, J.E.; Wesson, M.C.; Sternard, H.D.; Qu, L. A high-yielding, generic fed-batch cell culture process for production of recombinant antibodies. Biotechnol. Bioeng. 2000, 67, 585–597. [Google Scholar] [CrossRef]
- Kelley, B. Industrialization of mAb production technology—The bioprocessing industry at a crossroads. mAbs 2009, 1, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-S. Cell Culture Bioprocess Engineering; University of Minnesota: Minneapolis, MO, USA, 2012; pp. 1–17. ISBN 978-0-9856626-0-8. [Google Scholar]
- Wurm, F.M. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat. Biotechnol. 2004, 22, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Jayapal, K.P.; Wlaschin, K.F.; Hu, W.-S. Recombinant protein therapeutics from CHO cells—20 years and counting. Chem. Eng. Prog. 2007, 103, 40–47. [Google Scholar]
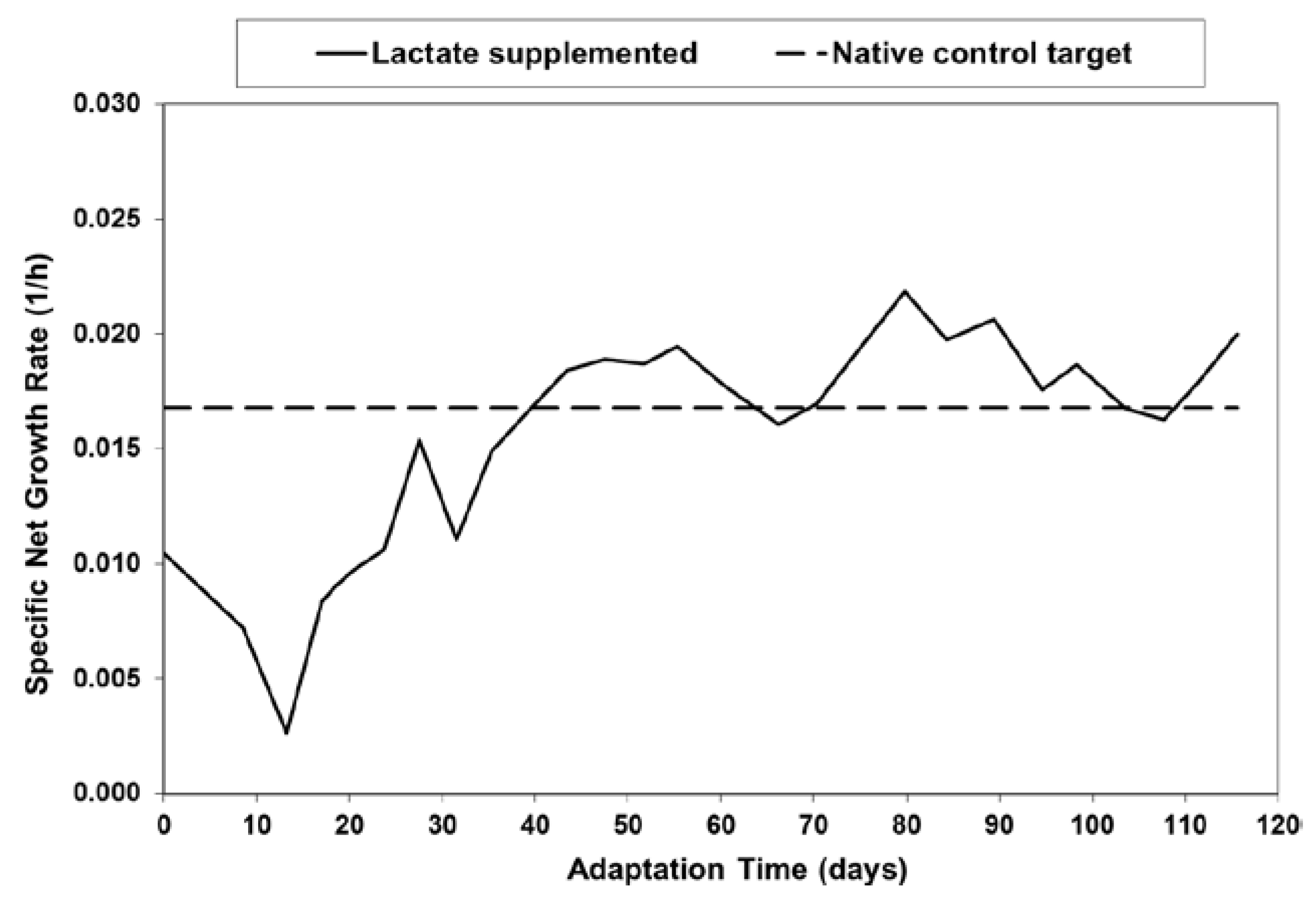
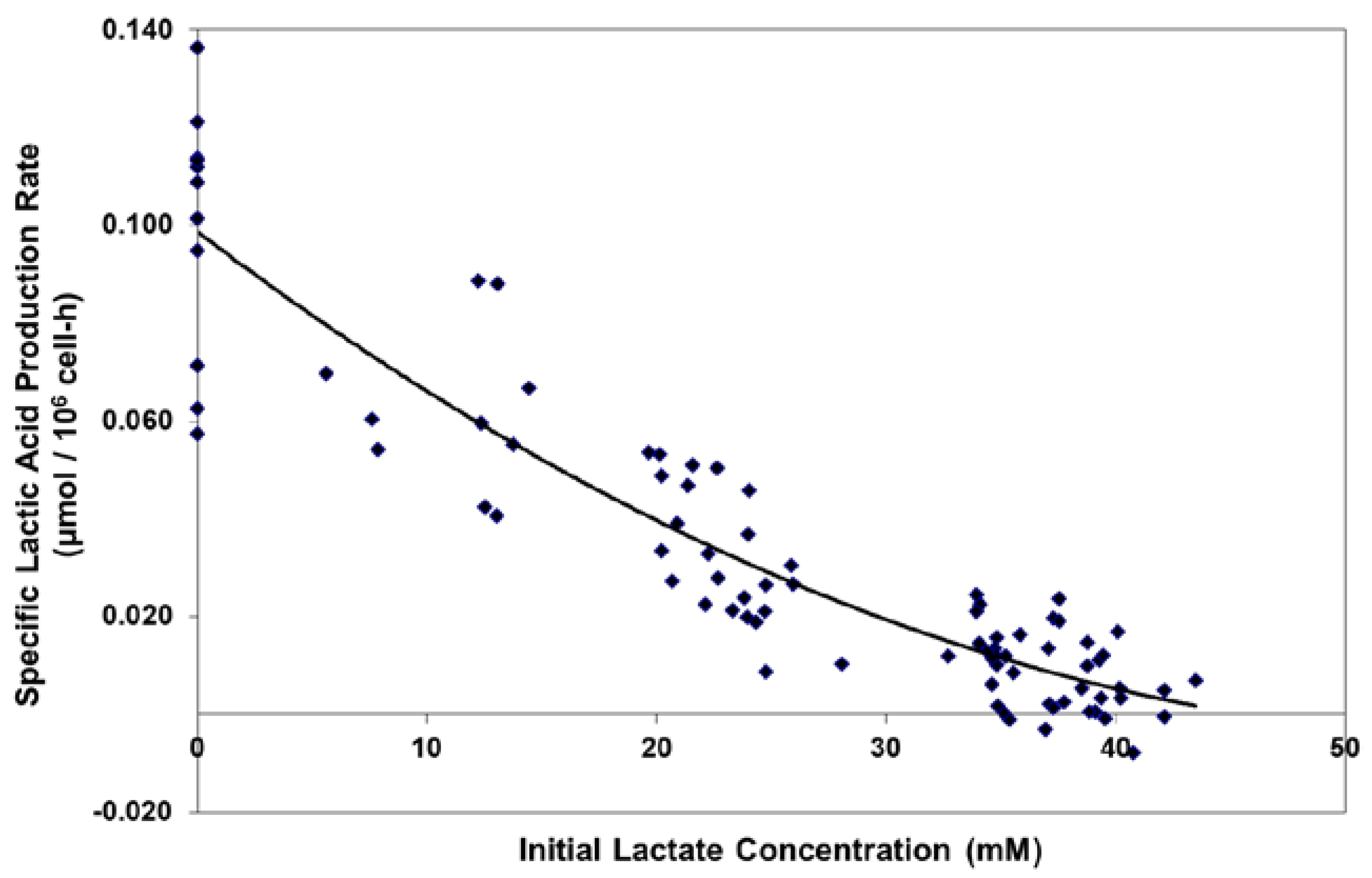
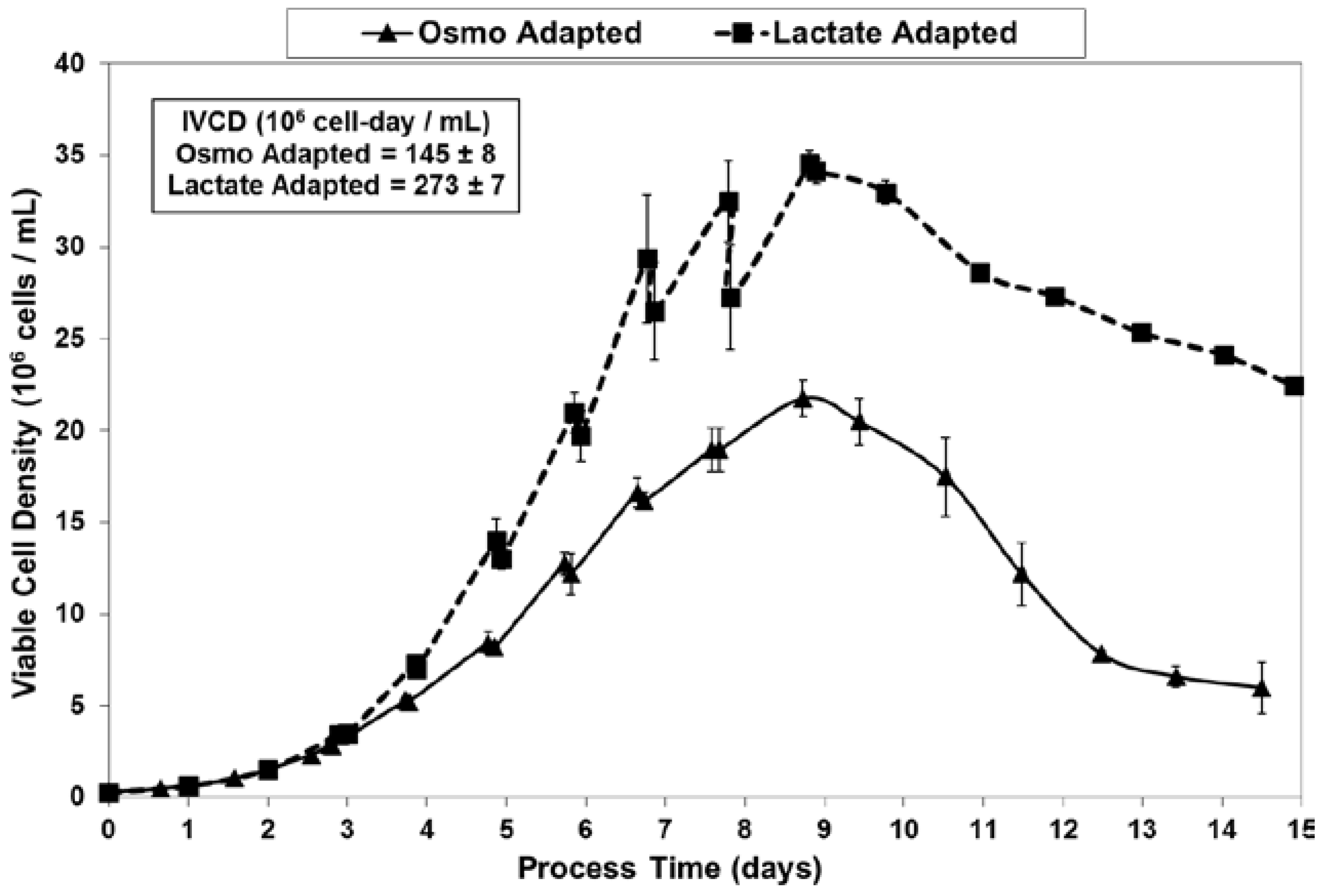
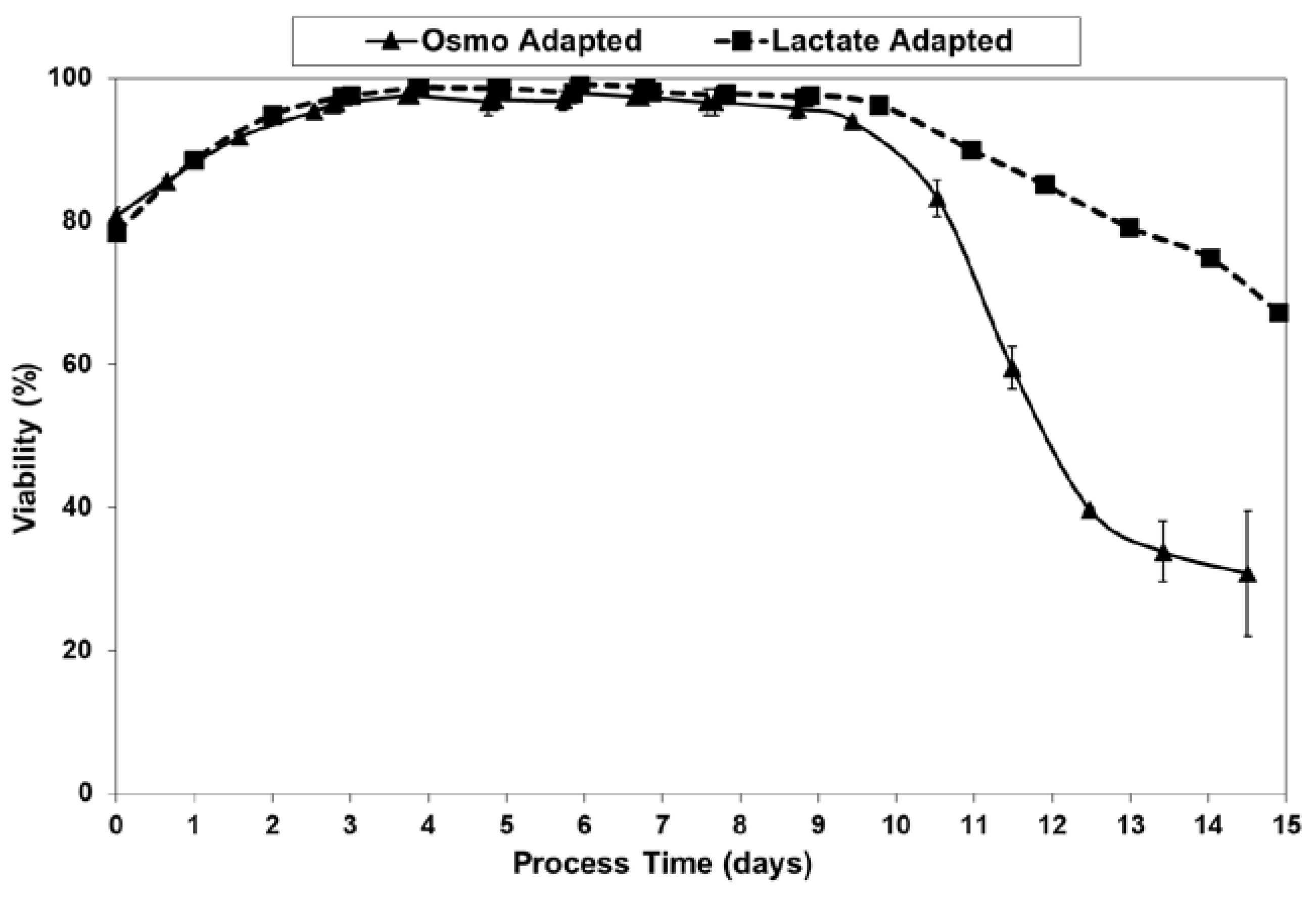

| Method | References | Effect on Lactic Acid (Lac A) Production | Effect on Ammonium (NH4+) Production | Likely Universally Reduces Lac A and NH4+ Production? | No Change or Decrease in Process Complexity? | Commonly ImplemenTed in cGMP Operations? |
|---|---|---|---|---|---|---|
| Replacement of glucose (gluc), glutamine (gln), or both with alternative sugars and/or amino acids | [1,2,13,14,15,16] (gluc only) | Reduced | NR | No | Yes | No |
| [17,18] (gln only) | NR | Reduced | No | Yes | No | |
| [3,19,20] (gluc only) | Reduced | Increased | No | Yes | No | |
| [21] (gluc only) | Reduced | Unchanged | No | Yes | No | |
| [20] (both) | Reduced | Reduced | Possibly | Yes | No | |
| On-line feedback control of glucose and/or glutamine at very low levels using glucose sensor, glutamine sensor, and/or other sensors and concentrated feeds | [22,23,24] (gluc only) | Reduced | Increased | No | No | No |
| [23] (gln only) | Reduced | Reduced | No | No | No | |
| [4,22,23,24,25,26] (both) | Reduced | Reduced | Yes | No | No | |
| [3,27] (gluc only with other sensors) | Reduced | Increased | No | No | 27 at Pfizer | |
| [28] (both with other sensors) | Reduced | Unchanged | Yes | No | Not in completely full form | |
| Copper supplementation | [12,29,30,31,32] | Reduced | NR | No | Yes | Yes |
| Reduction in temperature | [33] | Reduced | Unchanged | No | Yes | Yes |
| [34] | Reduced | Reduced | No | Yes | Yes | |
| Reduction in pH | [34,35,36] | Reduced | Unchanged | No | Yes | Yes |
| [37] | Reduced | Increased | No | Yes | Yes | |
| Selection of clones with lactate consumption phenotype | [10,11,12,27,30,38] | Reduced | Mixed | No | Yes | Yes |
| Cell—Line | μn (p ≈ 0.01) (1/h) | qLac (p < 0.001) (μmol/106 Cell-h) | qGluc (p < 0.001) (μmol/106 Cell-h) | Yl/g (p < 0.001) |
|---|---|---|---|---|
| Lactate Adapted | 0.025 ± 0.001 | 0.022 ± 0.018 | 0.054 ± 0.006 | 0.388 ± 0.344 |
| Osmo Adapted | 0.027 ± 0.002 | 0.112 ± 0.032 | 0.078 ± 0.012 | 1.411 ± 0.284 |
| Feed Solution | Component Levels in Given Feed Solution | Measured (*) or Theoretical (+) Osmolality | |||
|---|---|---|---|---|---|
| Efficient Feed B | CD CHO AGT | Glutamine | Glucose | ||
| HCD-1 | 1× | 12 g/L | 8 mM | 21 g/L | 540 mOsmo * |
| HCD-2 | 1× | 24 g/L | 8 mM | 24 g/L | 700 mOsmo * |
| HCD-3 | - | - | 200 mM | - | 200 mOsmo + |
| HCD-4 | - | - | 300 mM | 500 g/L | 3080 mOsmo + |
| Feed Solution | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 |
|---|---|---|---|---|---|---|---|---|
| HCD-1 | 80 mL | 80 mL | 80 mL | 160 mL | ||||
| HCD-2 | 320 mL | 185 mL | 80 mL | |||||
| HCD-3 | 16 mL | 16 mL | 32 mL | |||||
| HCD-4 | 20 mL | 20 mL |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freund, N.W.; Croughan, M.S. A Simple Method to Reduce both Lactic Acid and Ammonium Production in Industrial Animal Cell Culture. Int. J. Mol. Sci. 2018, 19, 385. https://doi.org/10.3390/ijms19020385
Freund NW, Croughan MS. A Simple Method to Reduce both Lactic Acid and Ammonium Production in Industrial Animal Cell Culture. International Journal of Molecular Sciences. 2018; 19(2):385. https://doi.org/10.3390/ijms19020385
Chicago/Turabian StyleFreund, Nathaniel W., and Matthew S. Croughan. 2018. "A Simple Method to Reduce both Lactic Acid and Ammonium Production in Industrial Animal Cell Culture" International Journal of Molecular Sciences 19, no. 2: 385. https://doi.org/10.3390/ijms19020385
APA StyleFreund, N. W., & Croughan, M. S. (2018). A Simple Method to Reduce both Lactic Acid and Ammonium Production in Industrial Animal Cell Culture. International Journal of Molecular Sciences, 19(2), 385. https://doi.org/10.3390/ijms19020385




