Adsorption Behavior of Surfactant on Lignite Surface: A Comparative Experimental and Molecular Dynamics Simulation Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Adsorption Isotherms
2.2. XPS Analysis
2.3. Molecular Dynamics Simulation of NPEO10 Adsorbed on the Coal Surface
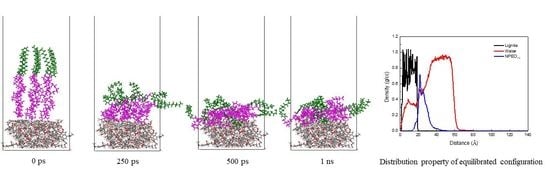
2.3.1. Structure of NPEO10 Adsorbed on the Coal Surface
2.3.2. Interaction Energies between Surfactant and Coal
2.3.3. Mobility of Water Molecules
3. Experiment and Methods
3.1. Materials
3.2. Surfactant Adsorption
3.3. XPS Measurements
3.4. Molecular Dynamics Simulation Methodology
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ohm, T.-I.; Chae, J.-S.; Lim, J.-H.; Moon, S.-H. Evaluation of a hot oil immersion drying method for the upgrading of crushed low-rank coal. J. Mech. Sci. Technol. 2012, 26, 1299–1303. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, J.; Shen, W.; Wu, J.; Wang, R.; Zhou, J.; Cen, K. Improving the slurrying ability of XiMeng brown coal by medium- to low-temperature thermal treatment. Fuel Process. Technol. 2014, 119, 218–227. [Google Scholar] [CrossRef]
- Dey, S. Enhancement in hydrophobicity of low rank coal by surfactants—A critical overview. Fuel Process. Technol. 2012, 94, 151–158. [Google Scholar] [CrossRef]
- Jia, R.; Harris, G.H.; Fuerstenau, D.W. An improved class of universal collectors for the flotation of oxidized and/or low-rank coal. Int. J. Miner. Process. 2000, 58, 99–118. [Google Scholar] [CrossRef]
- You, X.; Li, L.; Liu, J.; Wu, L.; He, M.; Lyu, X. Investigation of particle collection and flotation kinetics within the Jameson cell downcomer. Powder Technol. 2017, 310, 221–227. [Google Scholar] [CrossRef]
- Xia, W.; Yang, J.; Liang, C. A short review of improvement in flotation of low rank/oxidized coals by pretreatments. Powder Technol. 2013, 237, 1–8. [Google Scholar] [CrossRef]
- Ye, Y.; Jin, R.; Miller, J. Thermal treatment of low-rank coal and its relationship to flotation response. Coal Prep. 1988, 6, 1–16. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Ling, X.; Hao, J.; Xie, W. Adsorption Performance of Nonionic Surfactant on the Lignite Particles with Different Density. Energy Fuels 2017, 31, 6580–6586. [Google Scholar] [CrossRef]
- Ceylan, K.; Kucuk, M.Z. Effectiveness of the dense medium and the froth flotation methods in cleaning some Turkish lignites. Energy Convers. Manag. 2004, 45, 1407–1418. [Google Scholar] [CrossRef]
- Vamvuka, D.; Agridiotis, V. The effect of chemical reagents on lignite flotation. Int. J. Miner. Process. 2001, 61, 209–224. [Google Scholar] [CrossRef]
- Cebeci, Y. The investigation of the floatability improvement of Yozgat Ayridam lignite using various collectors. Fuel 2002, 81, 281–289. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, X. Flotation of lignite pretreated by sorbitan monooleate. Physicochem. Probl. Miner. Process. 2014, 50, 759–766. [Google Scholar]
- Xia, W.; Ni, C.; Xie, G. Effective Flotation of Lignite Using a Mixture of Dodecane and 4-Dodecylphenol (DDP) as a Collector. Int. J. Coal Prep. Util. 2015, 36, 262–271. [Google Scholar] [CrossRef]
- Ni, C.; Bu, X.; Xia, W.; Liu, B.; Peng, Y.; Xie, G. Improving lignite flotation performance by enhancing the froth properties using polyoxyethylene sorbitan monostearate. Int. J. Miner. Process. 2016, 155, 99–105. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Liu, J.; Sun, Y.; Sun, W. Flotation and adsorption of muscovite using mixed cationic–nonionic surfactants as collector. Powder Technol. 2015, 276, 26–33. [Google Scholar] [CrossRef]
- Chen, B.; Diao, Z.; Lu, H. Using the ReaxFF reactive force field for molecular dynamics simulations of the spontaneous combustion of lignite with the Hatcher lignite model. Fuel 2014, 116, 7–13. [Google Scholar] [CrossRef]
- Zhang, Z. Structure and dynamics in brown coal matrix during moisture removal process by molecular dynamics simulation. Mol. Phys. 2011, 109, 447–455. [Google Scholar] [CrossRef]
- Zhang, L.; Li, B.; Xia, Y.; Liu, S. Wettability modification of Wender lignite by adsorption of dodecyl poly ethoxylated surfactants with different degree of ethoxylation: A molecular dynamics simulation study. J. Mol. Graph. Model. 2017, 76, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.; Sathish, P.; Tanwar, J.; Moon, K.; Fuerstenau, D. A molecular dynamics study of the interaction of oleate and dodecylammonium chloride surfactants with complex aluminosilicate minerals. J. Colloid Interface Sci. 2011, 362, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Y.-L.; He, D.-D.; Liu, G.-S. Adsorption of cationic collectors and water on muscovite (001) surface: A molecular dynamics simulation study. Miner. Eng. 2013, 53, 101–107. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Sun, W.; Sun, Y. Molecular dynamics simulation study of the interaction of mixed cationic/anionic surfactants with muscovite. Appl. Surf. Sci. 2015, 327, 364–370. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, H.; Chen, J.; Zhao, K.; Wang, L.; Hao, Y. Elemental mercury removal from syngas at high-temperature using activated char pyrolyzed from biomass and lignite. Korean J. Chem. Eng. 2016, 33, 3134–3140. [Google Scholar] [CrossRef]
- Liu, J.; Wu, J.; Zhu, J.; Wang, Z.; Zhou, J.; Cen, K. Removal of oxygen functional groups in lignite by hydrothermal dewatering: An experimental and DFT study. Fuel 2016, 178, 85–92. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Yuan, S.; Wang, Z.; Zhou, J.; Cen, K. Theoretical investigation of noncovalent interactions between low-rank coal and water. Energy Fuels 2016, 30, 7118–7124. [Google Scholar] [CrossRef]
- Li, J.; Cui, H.; Song, X.; Zhang, G.; Wang, X.; Song, Q.; Wei, N.; Tian, J. Adsorption and intercalation of organic pollutants and heavy metal ions into MgAl-LDHs nanosheets with high capacity. RSC Adv. 2016, 6, 92402–92410. [Google Scholar] [CrossRef]
- Cao, X.; Yan, B.; Wang, Q.; Wang, Y.; Qiu, J.; Huang, Y.; Li, L.; Zhang, Y.; Hu, S.; Lü, X.; et al. Adsorption of Cr(VI) from Aqueous Solutions on Organic Modified Laponite. Chem. J. Chin. Univ. Chin. Ed. 2017, 38, 173–181. [Google Scholar]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-Y.; Wang, P.; Ma, J.-P.; Liu, Q.-K.; Dong, Y.-B. A nanoporous Ag (I)-MOF showing unique selective adsorption of benzene among its organic analogues. Chem. Commun. 2014, 50, 13672–13675. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, S.; Zhao, X.; Yuan, G.; Mimura, H. Adsorption mechanism of chlorides on carbon nanotubes based on first-principles calculations. Chem. Phys. Lett. 2013, 580, 94–98. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, C.; Cheng, W.; Zhang, Q.; Nie, W. Effects of oxygen element and oxygen-containing functional groups on surface wettability of coal dust with various metamorphic degrees based on XPS experiment. J. Anal. Methods Chem. 2015, 2015, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-P.; Zhang, Y.-Y.; Li, H.; Gao, J.-X.; Cheng, X.-L. Molecular dynamics investigation of thermite reaction behavior of nanostructured Al/SiO2 system. Acta Phys. Sin. 2014, 63, 086401. [Google Scholar]
- Tao, C.-G.; Feng, H.-J.; Zhou, J.; Lv, L.-H.; Lu, X.-H. Molecular simulation of oxygen adsorption and diffusion in polypropylene. Acta Phys. Chim. Sin. 2009, 25, 1373–1378. [Google Scholar]
- Sun, H. COMPASS: An ab Initio Force-Field Optimized for Condensed-Phase Applications Overview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Wender, I. Catalytic Synthesis of Chemicals from Coal. Catal. Rev. Sci. Eng. 2006, 14, 97–129. [Google Scholar] [CrossRef]
- Kumagai, H.; Hayashi, J.; Chiba, T.; Nakamura, K. Change in physical and chemical characteristics of brown coal along with a progress of moisture release. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 1999; p. U611. [Google Scholar]
- Tang, H.-Y.; Wang, X.-H.; Feng, L.; Cao, Z.-X.; Liu, X.-C. Theoretical study on the interactions between the lignite monomer and water molecules. Russ. J. Phys. Chem. A 2015, 89, 1605–1613. [Google Scholar] [CrossRef]
- Berendsen, H.; Vangunsteren, W.; Devlieg, J. Dynamic simulation of complex molecular-systems. ACS Symp. Ser. 1987, 353, 106–122. [Google Scholar]









| T, k | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm, mg g–1 | b, L mg–1 | b, L mol–1 | r12 | n | r22 | |
| 308 | 41.67 | 60.00 | 3.96 × 107 | 0.9990 | 0.12 | 0.5144 |
| 318 | 60.24 | 41.50 | 2.74 × 107 | 0.9996 | 0.10 | 0.7136 |
| 328 | 81.30 | 30.75 | 2.03 × 107 | 0.9992 | 0.09 | 0.6077 |
| Types | Before | After | ||
|---|---|---|---|---|
| Peak BE/eV | Contents/% | Peak BE/eV | Contents/% | |
| C 1s | 284.78 | 76.58 | 284.82 | 80.57 |
| O 1s | 532.47 | 19.00 | 532.79 | 17.33 |
| N 1s | 399.65 | 1.12 | 400.00 | 0.48 |
| S 2p | 163.30 | 0.11 | 168.12 | 0.25 |
| Si 2p | 103.36 | 1.82 | 103.14 | 0.76 |
| Al 2p | 74.86 | 1.37 | 74.73 | 0.61 |
| rOH | θ(HOH) | q(O) | q(H) |
|---|---|---|---|
| 0.1 nm | 109°28′ | −0.82 e | 0.41 e |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Zhang, W.; Cao, X.; You, X.; Li, L. Adsorption Behavior of Surfactant on Lignite Surface: A Comparative Experimental and Molecular Dynamics Simulation Study. Int. J. Mol. Sci. 2018, 19, 437. https://doi.org/10.3390/ijms19020437
He M, Zhang W, Cao X, You X, Li L. Adsorption Behavior of Surfactant on Lignite Surface: A Comparative Experimental and Molecular Dynamics Simulation Study. International Journal of Molecular Sciences. 2018; 19(2):437. https://doi.org/10.3390/ijms19020437
Chicago/Turabian StyleHe, Meng, Wei Zhang, Xiaoqiang Cao, Xiaofang You, and Lin Li. 2018. "Adsorption Behavior of Surfactant on Lignite Surface: A Comparative Experimental and Molecular Dynamics Simulation Study" International Journal of Molecular Sciences 19, no. 2: 437. https://doi.org/10.3390/ijms19020437
APA StyleHe, M., Zhang, W., Cao, X., You, X., & Li, L. (2018). Adsorption Behavior of Surfactant on Lignite Surface: A Comparative Experimental and Molecular Dynamics Simulation Study. International Journal of Molecular Sciences, 19(2), 437. https://doi.org/10.3390/ijms19020437