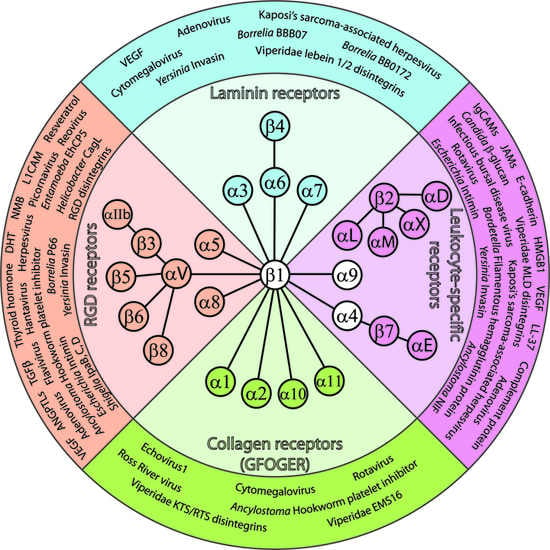
Beyond the Matrix: The Many Non-ECM Ligands for Integrins
Abstract
:1. Introduction
2. Integrin-Mediated Cell-Cell Interactions
2.1. Integrin-Counterreceptor Interactions in Leukocyte Extravasation
2.2. Non-ECM Integrin Ligands as Primers for Phagocytosis
2.3. Non-ECM Integrin Ligands as Triggers for NETosis
2.4. Non-ECM Integrin Ligands in Immune Surveillance
2.5. Integrin-Mediated Stem Cell Homing
2.6. Integrin-Counterreceptor Interactions in Tumor Cell Migration
2.7. Integrin-Counterreceptor Interactions in Erythrocyte Development
3. Non-ECM Integrin Ligands of Viruses
3.1. Non-ECM Integrin Ligands of Picornaviridae
3.2. Non-ECM Integrin Ligands of Flaviviridae
3.3. Non-ECM Integrin Ligands of Herpesviridae
3.4. Non-ECM Integrin Ligands of Togaviridae
3.5. Non-ECM Integrin Ligands of Adenoviridae
3.6. Non-ECM Integrin Ligands of Hantaviridae
3.7. Non-ECM Integrin Ligands of Birnaviridae
3.8. Non-ECM Integrin Ligands of Reoviridae
4. Non-ECM Integrin Ligands in Venoms
5. Bacterial Use of Non-ECM Integrin Ligands
5.1. Non-ECM Integrin Ligands of Borrelia burgdorferi
5.2. Non-ECM Integrin Ligands of Helicobacter pylori
5.3. Non-ECM Integrin Ligands of Yersinia
6. Protists and Multicellular Parasites That Use Non-ECM Integrin Ligands
6.1. Non-ECM Integrin Ligands of Entamoeba histolytica
6.2. Non-ECM Integrin Ligands of Hookworms
6.3. Non-ECM Integrin Ligands of Blood-Sucking Parasites
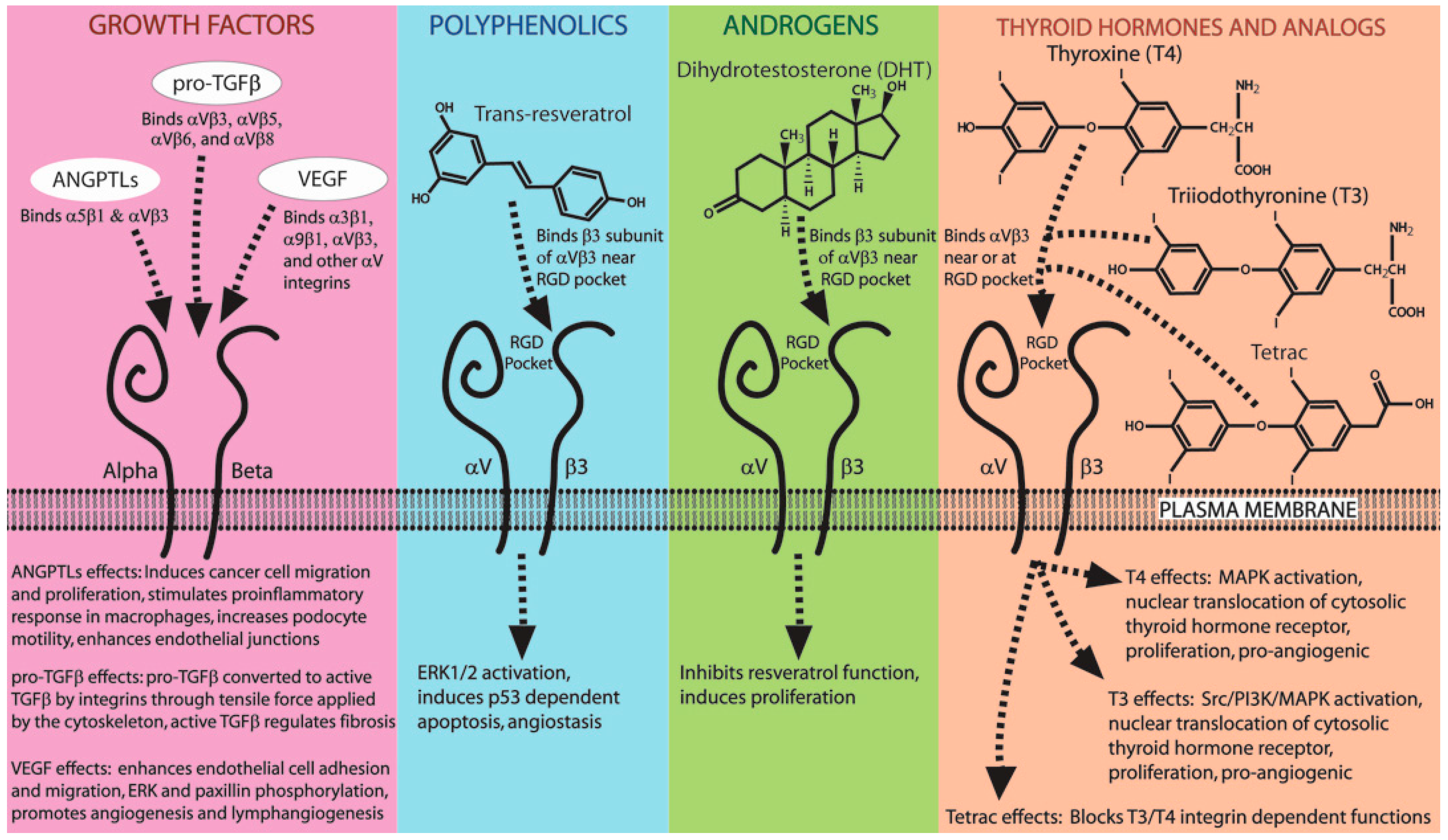
7. Hormones, Small Molecules, and Growth Factors That Mimic Integrin Ligands
7.1. Small Molecules and Hormones That Bind Integrins (Resveratrol, Thyroid Hormone, DHT)
7.2. Growth Factors That Bind Integrins (ANGPTLs, TGFβ, VEGF)
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef] [PubMed]
- Harburger, D.S.; Calderwood, D.A. Integrin signalling at a glance. J. Cell Sci. 2009, 122, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Pancer, Z.; Kruse, M.; Muller, I.; Muller, W.E. On the origin of Metazoan adhesion receptors: Cloning of integrin α subunit from the sponge Geodia cydonium. Mol. Biol. Evol. 1997, 14, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Brower, D.L.; Brower, S.M.; Hayward, D.C.; Ball, E.E. Molecular evolution of integrins: Genes encoding integrin β subunits from a coral and a sponge. Proc. Natl. Acad. Sci. USA 1997, 94, 9182–9187. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, W.; Blumbach, B.; Diehl-Seifert, B.; Koziol, C.; Batel, R.; Steffen, R.; Muller, I.M.; Muller, W.E. Increased expression of integrin and receptor tyrosine kinase genes during autograft fusion in the sponge Geodia cydonium. Cell Adhes. Commun. 1999, 7, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Sebe-Pedros, A.; Roger, A.J.; Lang, F.B.; King, N.; Ruiz-Trillo, I. Ancient origin of the integrin-mediated adhesion and signaling machinery. Proc. Natl. Acad. Sci. USA 2010, 107, 10142–10147. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The emergence of integrins: A personal and historical perspective. Matrix Biol. 2004, 23, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: A family of cell surface receptors. Cell 1987, 48, 549–554. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 1992, 69, 11–25. [Google Scholar] [CrossRef]
- Sullivan, D.P.; Muller, W.A. Neutrophil and monocyte recruitment by PECAM, CD99, and other molecules via the LBRC. Semin. Immunopathol. 2014, 36, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Imhof, B.A.; Aurrand-Lions, M. Adhesion mechanisms regulating the migration of monocytes. Nat. Rev. Immunol. 2004, 4, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Zundler, S.; Fischer, A.; Schillinger, D.; Binder, M.T.; Atreya, R.; Rath, T.; Lopez-Posadas, R.; Voskens, C.J.; Watson, A.; Atreya, I.; et al. The α4β1 Homing Pathway Is Essential for Ileal Homing of Crohn’s Disease Effector T Cells In Vivo. Inflamm. Bowel Dis. 2017, 23, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Rettig, M.P.; Ansstas, G.; DiPersio, J.F. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia 2012, 26, 34–53. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, A.; Rettig, M.P.; Cooper, M.L.; Holt, M.S.; Ritchey, J.K.; Eissenberg, L.; DiPersio, J.F. Bortezomib is a rapid mobilizer of hematopoietic stem cells in mice via modulation of the VCAM-1/VLA-4 axis. Blood 2014, 124, 2752–2754. [Google Scholar] [CrossRef] [PubMed]
- Bungartz, G.; Stiller, S.; Bauer, M.; Muller, W.; Schippers, A.; Wagner, N.; Fassler, R.; Brakebusch, C. Adult murine hematopoiesis can proceed without β1 and β7 integrins. Blood 2006, 108, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Seguin, L.; Desgrosellier, J.S.; Weis, S.M.; Cheresh, D.A. Integrins and cancer: Regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015, 25, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, J.; Zheng, Y.; Pan, Y.; Zhang, K.; Chen, J. Distinct chemokine signaling regulates integrin ligand specificity to dictate tissue-specific lymphocyte homing. Dev. Cell 2014, 30, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Murakami, J.L.; Xu, B.; Franco, C.B.; Hu, X.; Galli, S.J.; Weissman, I.L.; Chen, C.C. Evidence that β7 Integrin Regulates Hematopoietic Stem Cell Homing and Engraftment Through Interaction with MAdCAM-1. Stem Cells Dev. 2016, 25, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Maric, G.; Annis, M.G.; Dong, Z.; Rose, A.A.; Ng, S.; Perkins, D.; MacDonald, P.A.; Ouellet, V.; Russo, C.; Siegel, P.M. GPNMB cooperates with neuropilin-1 to promote mammary tumor growth and engages integrin α5β1 for efficient breast cancer metastasis. Oncogene 2015, 34, 5494–5504. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, A.; Jalil, A.; Vergnon, I.; Le Maux Chansac, B.; Lazar, V.; Bismuth, G.; Chouaib, S.; Mami-Chouaib, F. αEβ7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J. Exp. Med. 2007, 204, 559–570. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, X.M.; Reichner, J.S. Neutrophil integrins and matrix ligands and NET release. Front. Immunol. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Doke, M.; Fukamachi, H.; Morisaki, H.; Arimoto, T.; Kataoka, H.; Kuwata, H. Nucleases from Prevotella intermedia can degrade neutrophil extracellular traps. Mol. Oral Microbiol. 2017, 32, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Liang, M.X.; Chen, Q. Production and stabilization of an integrin-binding moiety of complement component 3. Mol. Biol. 2015, 49, 723–727. [Google Scholar] [CrossRef]
- Lukacsi, S.; Nagy-Balo, Z.; Erdei, A.; Sandor, N.; Bajtay, Z. The role of CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in complement-mediated phagocytosis and podosome formation by human phagocytes. Immunol. Lett. 2017, 189, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bajic, G.; Andersen, G.R.; Christiansen, S.H.; Vorup-Jensen, T. The cationic peptide LL-37 binds Mac-1 (CD11b/CD18) with a low dissociation rate and promotes phagocytosis. Biochim. Biophys. Acta Proteins Proteom. 2016, 1864, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Podolnikova, N.P.; Podolnikov, A.V.; Haas, T.A.; Lishko, V.K.; Ugarova, T.P. Ligand recognition specificity of leukocyte integrin αMβ2 (Mac-1, CD11b/CD18) and its functional consequences. Biochemistry 2015, 54, 1408–1420. [Google Scholar] [CrossRef] [PubMed]
- Lishko, V.K.; Moreno, B.; Podolnikova, N.P.; Ugarova, T.P. Identification of Human Cathelicidin Peptide LL-37 as a Ligand for Macrophage Integrin αMβ2 (Mac-1, CD11b/CD18) that Promotes Phagocytosis by Opsonizing Bacteria. Res. Rep. Biochem. 2016, 2016, 39–55. [Google Scholar] [PubMed]
- Hase, K.; Murakami, M.; Iimura, M.; Cole, S.P.; Horibe, Y.; Ohtake, T.; Obonyo, M.; Gallo, R.L.; Eckmann, L.; Kagnoff, M.F. Expression of LL-37 by Human Gastric Epithelial Cells as a Potential Host Defense Mechanism Against Helicobacter pylori. Gastroenterology 2003, 125, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Dürr, U.H.N.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1408–1425. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, N.; Campana, L.; Gavina, M.; Covino, C.; de Metrio, M.; Panciroli, C.; Maiuri, L.; Maseri, A.; D’Angelo, A.; Bianchi, M.E.; et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 2014, 12, 2074–2088. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, Y.; Chilukuri, K.; Brady, O.A.; Boulos, M.I.; Kappes, J.C.; Galileo, D.S. L1 stimulation of human glioma cell motility correlates with FAK activation. J. Neurooncol. 2011, 105, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Burgett, M.E.; Lathia, J.D.; Roth, P.; Nowacki, A.S.; Galileo, D.S.; Pugacheva, E.; Huang, P.; Vasanji, A.; Li, M.; Byzova, T.; et al. Direct contact with perivascular tumor cells enhances integrin αvβ3 signaling and migration of endothelial cells. Oncotarget 2016, 7, 43852–43867. [Google Scholar] [CrossRef] [PubMed]
- Sundd, P.; Pospieszalska, M.K.; Ley, K. Neutrophil rolling at high shear: Flattening, catch bond behavior, tethers and slings. Mol. Immunol. 2013, 55, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Montresor, A.; Toffali, L.; Constantin, G.; Laudanna, C. Chemokines and the signaling modules regulating integrin affinity. Front. Immunol. 2012, 3, 127. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, J.; Wang, J.-H.; Springer, T.A. Distinct recognition of complement iC3b by integrins αXβ2 and αMβ2. Proc. Natl. Acad. Sci. USA 2017, 114, 3403–3408. [Google Scholar] [CrossRef] [PubMed]
- Kazzaz, N.M.; Sule, G.; Knight, J.S. Intercellular Interactions as Regulators of NETosis. Front. Immunol. 2016, 7, 453. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Rizo, V.; Martínez-Guzmán, M.A.; Iñiguez-Gutierrez, L.; García-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front. Immunol. 2017, 8, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Orlova, V.V.; Choi, E.Y.; Xie, C.; Chavakis, E.; Bierhaus, A.; Ihanus, E.; Ballantyne, C.M.; Gahmberg, C.G.; Bianchi, M.E.; Nawroth, P.P.; et al. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 2007, 26, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Gillenius, E.; Urban, C.F. The adhesive protein invasin of Yersinia pseudotuberculosis induces neutrophil extracellular traps via β1 integrins. Microbes Infect. 2015, 17, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Iommelli, F.; de Rosa, V.; Carriero, M.V.; Miceli, R.; Camerlingo, R.; di Minno, G.; del Vecchio, S. Integrin-dependent cell adhesion to neutrophil extracellular traps through engagement of fibronectin in neutrophil-like cells. PLoS ONE 2017, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; Bono, M.R.; Manjunath, N.; Weninger, W.; Cavanagh, L.L.; Rosemblatt, M.; Von Andrian, U.H. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature 2003, 424, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.M.; Collins, M.H.; Kemme, K.A.; Sherrill, J.D.; Wen, T.; Rochman, M.; Stucke, E.M.; Amin, L.; Tai, H.; Putnam, P.E.; et al. Cadherin 26 is an α integrin-binding epithelial receptor regulated during allergic inflammation. Mucosal Immunol. 2017, 10, 1190–1201. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, C.C. Musical chairs: In utero HCT via mobilization. Blood 2016, 128, 2378–2380. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.G.; Vrecenak, J.D.; Boelig, M.M.; Eissenberg, L.; Rettig, M.P.; Riley, J.S.; Holt, M.S.; Conner, M.A.; Loukogeorgakis, S.P.; Li, H.; et al. Enhanced in utero allogeneic engraftment in mice after mobilizing fetal HSCs by α4β1/7 inhibition. Blood 2016, 128, 2457–2461. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.A.; Gracieux, D.; Talib, M.; Tokarz, D.A.; Hensley, M.T.; Cores, J.; Vandergriff, A.; Tang, J.; de Andrade, J.B.; Dinh, P.U.; et al. Angiopellosis as an Alternative Mechanism of Cell Extravasation. Stem Cells 2017, 35, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Kiefel, H.; Pfeifer, M.; Bondong, S.; Hazin, J.; Altevogt, P. Linking L1CAM-mediated signaling to NF-κB activation. Trends Mol. Med. 2011, 17, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Voura, E.B.; Ramjeesingh, R.A.; Montgomery, A.M.; Siu, C.H. Involvement of integrin αvβ3 and cell adhesion molecule L1 in transendothelial migration of melanoma cells. Mol. Biol. Cell 2001, 12, 2699–2710. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, A.M.; Becker, J.C.; Siu, C.H.; Lemmon, V.P.; Cheresh, D.A.; Pancook, J.D.; Zhao, X.; Reisfeld, R.A. Human neural cell adhesion molecule L1 and rat homologue NILE are ligands for integrin αvβ3. J. Cell Biol. 1996, 132, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Spring, F.A.; Griffiths, R.E.; Mankelow, T.J.; Agnew, C.; Parsons, S.F.; Chasis, J.A.; Anstee, D.J. Tetraspanins CD81 and CD82 facilitate α4β1-mediated adhesion of human erythroblasts to vascular cell adhesion molecule-1. PLoS ONE 2013, 8, e62654. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C. What does virus evolution tell us about virus origins? J. Virol. 2011, 85, 5247–5251. [Google Scholar] [CrossRef] [PubMed]
- Stupack, D.G.; Cheresh, D.A. ECM remodeling regulates angiogenesis: Endothelial integrins look for new ligands. Sci. STKE 2002, 2002, PE7. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, D. Airway epithelial integrins: Why so many? Am. J. Respir. Cell Mol. Biol. 1998, 19, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.L.; Nemerow, G.R. Cell integrins: Commonly used receptors for diverse viral pathogens. Trends Microbiol. 2007, 15, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.; Walker, L.R.; Abdel-Raouf, U.M.; Desouky, S.A.; Montasser, A.K.; Akula, S.M. Beyond RGD: Virus interactions with integrins. Arch. Virol. 2015, 160, 2669–2681. [Google Scholar] [CrossRef] [PubMed]
- La Linn, M.; Eble, J.A.; Lübken, C.; Slade, R.W.; Heino, J.; Davies, J.; Suhrbier, A. An arthritogenic αvirus uses the α1β1 integrin collagen receptor. Virology 2005, 336, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Huhtala, M.; Pietiainen, V.; Kapyla, J.; Vuorinen, K.; Marjomaki, V.; Heino, J.; Johnson, M.S.; Hyypia, T.; Cheng, R.H. Structural and functional analysis of integrin α2I domain interaction with echovirus 1. J. Biol. Chem. 2004, 279, 11632–11638. [Google Scholar] [CrossRef] [PubMed]
- Marjomaki, V.; Turkki, P.; Huttunen, M. Infectious Entry Pathway of Enterovirus B Species. Viruses 2015, 7, 6387–6399. [Google Scholar] [CrossRef] [PubMed]
- Feire, A.L.; Roy, R.M.; Manley, K.; Compton, T. The glycoprotein B disintegrin-like domain binds β 1 integrin to mediate cytomegalovirus entry. J. Virol. 2010, 84, 10026–10037. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.L.; Takada, Y.; Coulson, B.S. Rotavirus spike protein VP5* binds α2β1 integrin on the cell surface and competes with virus for cell binding and infectivity. J. Gen. Virol. 2006, 87, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Coulson, B.S.; Londrigan, S.L.; Lee, D.J. Rotavirus contains integrin ligand sequences and a disintegrin-like domain that are implicated in virus entry into cells. Proc. Natl. Acad. Sci. USA 1997, 94, 5389–5394. [Google Scholar] [CrossRef] [PubMed]
- Akula, S.M.; Pramod, N.P.; Wang, F.Z.; Chandran, B. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 2002, 108, 407–419. [Google Scholar] [CrossRef]
- Salone, B.; Martina, Y.; Piersanti, S.; Cundari, E.; Cherubini, G.; Franqueville, L.; Failla, C.M.; Boulanger, P.; Saggio, I. Integrin α3β1 is an alternative cellular receptor for adenovirus serotype 5. J. Virol. 2003, 77, 13448–13454. [Google Scholar] [CrossRef] [PubMed]
- Delgui, L.; Ona, A.; Gutierrez, S.; Luque, D.; Navarro, A.; Caston, J.R.; Rodriguez, J.F. The capsid protein of infectious bursal disease virus contains a functional α4β 1 integrin ligand motif. Virology 2009, 386, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.; Blakemore, W.; Newman, J.W.; Knowles, N.J.; Mould, A.P.; Humphries, M.J.; King, A.M. Foot-and-mouth disease virus is a ligand for the high-affinity binding conformation of integrin α5β1: Influence of the leucine residue within the RGDL motif on selectivity of integrin binding. J. Gen. Virol. 2000, 81, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Tugizov, S.M.; Berline, J.W.; Palefsky, J.M. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat. Med. 2003, 9, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Davison, E.; Diaz, R.M.; Hart, I.R.; Santis, G.; Marshall, J.F. Integrin α5β1-mediated adenovirus infection is enhanced by the integrin-activating antibody TS2/16. J. Virol. 1997, 71, 6204–6207. [Google Scholar] [PubMed]
- Walker, L.R.; Hussein, H.A.M.; Akula, S.M. Disintegrin-like domain of glycoprotein B regulates Kaposi’s sarcoma-associated herpesvirus infection of cells. J. Gen. Virol. 2014, 95, 1770–1782. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Kamata, T.; Takada, Y.; Ruggeri, Z.M.; Nemerow, G.R. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J. Virol. 1996, 70, 4502–4508. [Google Scholar] [PubMed]
- Stanway, G.; Kalkkinen, N.; Roivainen, M.; Ghazi, F.; Khan, M.; Smyth, M.; Meurman, O.; Hyypia, T. Molecular and biological characteristics of echovirus 22, a representative of a new picornavirus group. J. Virol. 1994, 68, 8232–8238. [Google Scholar] [PubMed]
- Pulli, T.; Koivunen, E.; Hyypia, T. Cell-surface interactions of echovirus 22. J. Biol. Chem. 1997, 272, 21176–21180. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Brown, S.L.; Stupack, D.G.; Puente, X.S.; Cheresh, D.A.; Nemerow, G.R. Integrin αvβ1 is an adenovirus coreceptor. J. Virol. 2001, 75, 5405–5409. [Google Scholar] [CrossRef] [PubMed]
- Nelsen-Salz, B.; Eggers, H.J.; Zimmermann, H. Integrin αvβ3 (vitronectin receptor) is a candidate receptor for the virulent echovirus 9 strain Barty. J. Gen. Virol. 1999, 80, 2311–2313. [Google Scholar] [CrossRef] [PubMed]
- Roivainen, M.; Piirainen, L.; Hovi, T.; Virtanen, I.; Riikonen, T.; Heino, J.; Hyypia, T. Entry of coxsackievirus A9 into host cells: Specific interactions with αvβ 3 integrin, the vitronectin receptor. Virology 1994, 203, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Neff, S.; Sa-Carvalho, D.; Rieder, E.; Mason, P.W.; Blystone, S.D.; Brown, E.J.; Baxt, B. Foot-and-mouth disease virus virulent for cattle utilizes the integrin αvβ3 as its receptor. J. Virol. 1998, 72, 3587–3594. [Google Scholar] [PubMed]
- Fan, W.; Qian, P.; Wang, D.; Zhi, X.; Wei, Y.; Chen, H.; Li, X. Integrin αvβ3 promotes infection by Japanese encephalitis virus. Res. Vet. Sci. 2017, 111, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Garrigues, H.J.; Rubinchikova, Y.E.; Dipersio, C.M.; Rose, T.M. Integrin αVβ3 Binds to the RGD motif of glycoprotein B of Kaposi’s sarcoma-associated herpesvirus and functions as an RGD-dependent entry receptor. J. Virol. 2008, 82, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Matthys, V.S.; Gorbunova, E.E.; Gavrilovskaya, I.N.; Mackow, E.R. Andes virus recognition of human and Syrian hamster β3 integrins is determined by an L33P substitution in the PSI domain. J. Virol. 2010, 84, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Wickham, T.J.; Mathias, P.; Cheresh, D.A.; Nemerow, G.R. Integrins αvβ 3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 1993, 73, 309–319. [Google Scholar] [CrossRef]
- Zarate, S.; Romero, P.; Espinosa, R.; Arias, C.F.; Lopez, S. VP7 mediates the interaction of rotaviruses with integrin αvβ3 through a novel integrin-binding site. J. Virol. 2004, 78, 10839–10847. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, C.A.; Mendez, E.; Zarate, S.; Isa, P.; Lopez, S.; Arias, C.F. Integrin αvβ3 mediates rotavirus cell entry. Proc. Natl. Acad. Sci. USA 2000, 97, 14644–14649. [Google Scholar] [CrossRef] [PubMed]
- Gavrilovskaya, I.N.; Shepley, M.; Shaw, R.; Ginsberg, M.H.; Mackow, E.R. β3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl. Acad. Sci. USA 1998, 95, 7074–7079. [Google Scholar] [CrossRef] [PubMed]
- Veettil, M.V.; Sadagopan, S.; Sharma-Walia, N.; Wang, F.Z.; Raghu, H.; Varga, L.; Chandran, B. Kaposi’s Sarcoma-Associated Herpesvirus Forms a Multimolecular Complex of Integrins (V5, V3, and 31) and CD98-xCT during Infection of Human Dermal Microvascular Endothelial Cells, and CD98-xCT Is Essential for the Postentry Stage of Infection. J. Virol. 2008, 82, 12126–12144. [Google Scholar] [CrossRef] [PubMed]
- Chesnokova, L.S.; Hutt-Fletcher, L.M. Fusion of Epstein-Barr virus with epithelial cells can be triggered by αvβ5 in addition to αvβ6 and αvβ8, and integrin binding triggers a conformational change in glycoproteins gHgL. J. Virol. 2011, 85, 13214–13223. [Google Scholar] [CrossRef] [PubMed]
- Burman, A.; Clark, S.; Abrescia, N.G.; Fry, E.E.; Stuart, D.I.; Jackson, T. Specificity of the VP1 GH loop of Foot-and-Mouth Disease virus for αv integrins. J. Virol. 2006, 80, 9798–9810. [Google Scholar] [CrossRef] [PubMed]
- Berryman, S.; Clark, S.; Monaghan, P.; Jackson, T. Early events in integrin αvβ6-mediated cell entry of foot-and-mouth disease virus. J. Virol. 2005, 79, 8519–8534. [Google Scholar] [CrossRef] [PubMed]
- Gianni, T.; Salvioli, S.; Chesnokova, L.S.; Hutt-Fletcher, L.M.; Campadelli-Fiume, G. αvβ6- and αvβ8-integrins serve as interchangeable receptors for HSV gH/gL to promote endocytosis and activation of membrane fusion. PLoS Pathog. 2013, 9, e1003806. [Google Scholar] [CrossRef] [PubMed]
- Zell, R. Picornaviridae-the ever-growing virus family. Arch. Virol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Tuthill, T.J.; Groppelli, E.; Hogle, J.M.; Rowlands, D.J. Picornaviruses. Curr. Top. Microbiol. Immunol. 2010, 343, 43–89. [Google Scholar] [PubMed]
- Johnson, M.S.; Lu, N.; Denessiouk, K.; Heino, J.; Gullberg, D. Integrins during evolution: Evolutionary trees and model organisms. Biochim. Biophys. Acta 2009, 1788, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Emsley, J.; Knight, C.G.; Farndale, R.W.; Barnes, M.J.; Liddington, R.C. Structural basis of collagen recognition by integrin α2β1. Cell 2000, 101, 47–56. [Google Scholar] [CrossRef]
- Wary, K.K.; Mariotti, A.; Zurzolo, C.; Giancotti, F.G. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 1998, 94, 625–634. [Google Scholar] [CrossRef]
- Pietiainen, V.; Marjomaki, V.; Upla, P.; Pelkmans, L.; Helenius, A.; Hyypia, T. Echovirus 1 endocytosis into caveosomes requires lipid rafts, dynamin II, and signaling events. Mol. Biol. Cell 2004, 15, 4911–4925. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G. Caveolae and caveolins. Curr. Opin. Cell Biol. 1996, 8, 542–548. [Google Scholar] [CrossRef]
- Marjomaki, V.; Pietiainen, V.; Matilainen, H.; Upla, P.; Ivaska, J.; Nissinen, L.; Reunanen, H.; Huttunen, P.; Hyypia, T.; Heino, J. Internalization of echovirus 1 in caveolae. J. Virol. 2002, 76, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Bergelson, J.M.; Cunningham, J.A.; Droguett, G.; Kurt-Jones, E.A.; Krithivas, A.; Hong, J.S.; Horwitz, M.S.; Crowell, R.L.; Finberg, R.W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 1997, 275, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.S.; Higgs, S.; Horne, K.M.E.; Vanlandingham, D.L. Flavivirus-Mosquito interactions. Viruses 2014, 6, 4703–4730. [Google Scholar] [CrossRef] [PubMed]
- Unni, S.K.; Růžek, D.; Chhatbar, C.; Mishra, R.; Johri, M.K.; Singh, S.K. Japanese encephalitis virus: From genome to infectome. Microbes Infect. 2011, 13, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Luca, V.C.; AbiMansour, J.; Nelson, C.A.; Fremont, D.H. Crystal Structure of the Japanese Encephalitis Virus Envelope Protein. J. Virol. 2012, 86, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Laxminarayana, S.V.; Chandra, N.; Ravi, V.; Desai, A. Heat shock protein 70 on Neuro2a cells is a putative receptor for Japanese encephalitis virus. Virology 2009, 385, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.J.; Rajamanonmani, R.; Li, J.; Bhuvanakantham, R.; Lescar, J.; Ng, M.L. Inhibition of West Nile virus entry by using a recombinant domain III from the envelope glycoprotein. J. Gen. Virol. 2005, 86, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.J.H.; Ng, M.L. Interaction of West Nile virus with αVβ3 integrin mediates virus entry into cells. J. Biol. Chem. 2004, 279, 54533–54541. [Google Scholar] [CrossRef] [PubMed]
- Bogachek, M.V.; Zaitsev, B.N.; Sekatskii, S.K.; Protopopova, E.V.; Ternovoi, V.A.; Ivanova, A.V.; Kachko, A.V.; Ivanisenko, V.A.; Dietler, G.; Loktev, V.B. Characterization of glycoprotein E C-end of West Nile virus and evaluation of its interaction force with αVβ3 integrin as putative cellular receptor. Biochemistry 2010, 75, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lobigs, M. Substitutions at the putative receptor-binding site of an encephalitic flavivirus alter virulence and host cell tropism and reveal a role for glycosaminoglycans in entry. J. Virol. 2000, 74, 8867–8875. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.-W.; Lin, C.-F.; Lu, Y.-T.; Lei, H.-Y.; Anderson, R.; Lin, Y.-S. Endothelial cell surface expression of protein disulfide isomerase activates β1 and β3 integrins and facilitates dengue virus infection. J. Cell. Biochem. 2012, 113, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- van der Most, R.G.; Corver, J.; Strauss, J.H. Mutagenesis of the RGD motif in the yellow fever virus 17D envelope protein. Virology 1999, 265, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Feire, A.L.; Koss, H.; Compton, T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc. Natl. Acad. Sci. USA 2004, 101, 15470–15475. [Google Scholar] [CrossRef] [PubMed]
- Assuncao-Miranda, I.; Cruz-Oliveira, C.; Da Poian, A.T. Molecular mechanisms involved in the pathogenesis of αvirus-induced arthritis. BioMed Res. Int. 2013, 2013, 973516. [Google Scholar] [CrossRef] [PubMed]
- Mangel, W.F.; San Martin, C. Structure, function and dynamics in adenovirus maturation. Viruses 2014, 6, 4536–4570. [Google Scholar] [CrossRef] [PubMed]
- Nemerow, G.R.; Stewart, P.L. Role of αv integrins in adenovirus cell entry and gene delivery. Microbiol. Mol. Biol. Rev. MMBR 1999, 63, 725–734. [Google Scholar] [PubMed]
- Wickham, T.J.; Filardo, E.J.; Cheresh, D.A.; Nemerow, G.R. Integrin αvβ5 selectively promotes adenovirus mediated cell membrane permeabilization. J. Cell Biol. 1994, 127, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Wickham, T.J. Targeting adenovirus. Gene Ther. 2000, 7, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Harfe, B.; Freimuth, P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J. Virol. 1993, 67, 5198–5205. [Google Scholar] [PubMed]
- Miyamoto, S.; Akiyama, S.K.; Yamada, K.M. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science 1995, 267, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Stupack, D.; Klemke, R.; Cheresh, D.A.; Nemerow, G.R. Adenovirus endocytosis via αv integrins requires phosphoinositide-3-OH kinase. J. Virol. 1998, 72, 2055–2061. [Google Scholar] [PubMed]
- Li, E.; Stupack, D.; Bokoch, G.M.; Nemerow, G.R. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J. Virol. 1998, 72, 8806–8812. [Google Scholar] [PubMed]
- Schmaljohn, C.; Hjelle, B. Hantaviruses: A global disease problem. Emerg. Infect. Dis. 1997, 3, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Bondu, V.; Wu, C.; Cao, W.; Simons, P.C.; Gillette, J.; Zhu, J.; Erb, L.; Zhang, X.F.; Buranda, T. Low-affinity binding in cis to P2Y2R mediates force-dependent integrin activation during hantavirus infection. Mol. Biol. Cell 2017, 28, 2887–2903. [Google Scholar] [CrossRef] [PubMed]
- Gavrilovskaya, I.N.; Peresleni, T.; Geimonen, E.; Mackow, E.R. Pathogenic hantaviruses selectively inhibit β3 integrin directed endothelial cell migration. Arch. Virol. 2002, 147, 1913–1931. [Google Scholar] [CrossRef] [PubMed]
- Gavrilovskaya, I.N.; Gorbunova, E.E.; Mackow, N.A.; Mackow, E.R. Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. J. Virol. 2008, 82, 5797–5806. [Google Scholar] [CrossRef] [PubMed]
- Bottcher, B.; Kiselev, N.A.; Stel’Mashchuk, V.Y.; Perevozchikova, N.A.; Borisov, A.V.; Crowther, R.A. Three-dimensional structure of infectious bursal disease virus determined by electron cryomicroscopy. J. Virol. 1997, 71, 325–330. [Google Scholar] [PubMed]
- Ye, C.; Han, X.; Yu, Z.; Zhang, E.; Wang, L.; Liu, H. Infectious Bursal Disease Virus Activates c-Src To Promote α4β1 Integrin-Dependent Viral Entry by Modulating the Downstream Akt-RhoA GTPase-Actin Rearrangement Cascade. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; Parashar, U.D. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000–2013. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 62, S96–S105. [Google Scholar] [CrossRef] [PubMed]
- Yeager, M.; Dryden, K.A.; Olson, N.H.; Greenberg, H.B.; Baker, T.S. Three-dimensional structure of rhesus rotavirus by cryoelectron microscopy and image reconstruction. J. Cell Biol. 1990, 110, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Maginnis, M.S.; Forrest, J.C.; Kopecky-Bromberg, S.A.; Dickeson, S.K.; Santoro, S.A.; Zutter, M.M.; Nemerow, G.R.; Bergelson, J.M.; Dermody, T.S. Β1 integrin mediates internalization of mammalian reovirus. J. Virol. 2006, 80, 2760–2770. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, C. Applications of snake venom components to modulate integrin activities in cell-matrix interactions. Int. J. Biochem. Cell Biol. 2013, 45, 1974–1986. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-F.; Hsu, C.-C.; Kuo, Y.-J. Anti-thrombotic agents derived from snake venom proteins. Thromb. J. 2016, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Musial, J.; Niewiarowski, S.; Rucinski, B.; Stewart, G.J.; Cook, J.J.; Williams, J.A.; Edmunds, L.H., Jr. Inhibition of platelet adhesion to surfaces of extracorporeal circuits by disintegrins. RGD-containing peptides from viper venoms. Circulation 1990, 82, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.M.; Marcinkiewicz, C. Non-RGD-containing snake venom disintegrins, functional and structural relations. Toxicon 2011, 58, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J. The continuing saga of snake venom disintegrins. Toxicon 2013, 62, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, C.; Lobb, R.R.; Marcinkiewicz, M.M.; Daniel, J.L.; Smith, J.B.; Dangelmaier, C.; Weinreb, P.H.; Beacham, D.A.; Niewiarowski, S. Isolation and characterization of EMS16, a C-lectin type protein from Echis multisquamatus venom, a potent and selective inhibitor of the α2β1 integrin. Biochemistry 2000, 39, 9859–9867. [Google Scholar] [CrossRef] [PubMed]
- Horii, K.; Okuda, D.; Morita, T.; Mizuno, H. Crystal structure of EMS16 in complex with the integrin α2-I domain. J. Mol. Biol. 2004, 341, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, H.; Ortega, N.; Plouet, J. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration, and survival through integrin ligation. FASEB J. 2003, 17, 1520–1522. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.A.; Bruckner, P.; Mayer, U. Vipera lebetina venom contains two disintegrins inhibiting laminin-binding β1 integrins. J. Biol. Chem. 2003, 278, 26488–26496. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Yamasaki, M.; Hirai, K.; Matsubara, T.; Nomura, T.; Sato, F.; Mimata, H. Angiopoietin-like protein 2 induces androgen-independent and malignant behavior in human prostate cancer cells. Oncol. Rep. 2015, 33, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Yugami, M.; Odagiri, H.; Endo, M.; Tsutsuki, H.; Fujii, S.; Kadomatsu, T.; Masuda, T.; Miyata, K.; Terada, K.; Tanoue, H.; et al. Mice Deficient in Angiopoietin-like Protein 2 (Angptl2) Gene Show Increased Susceptibility to Bacterial Infection Due to Attenuated Macrophage Activity. J. Biol. Chem. 2016, 291, 18843–18852. [Google Scholar] [CrossRef] [PubMed]
- Vlahakis, N.E.; Young, B.A.; Atakilit, A.; Hawkridge, A.E.; Issaka, R.B.; Boudreau, N.; Sheppard, D. Integrin α9β1 directly binds to vascular endothelial growth factor (VEGF)-A and contributes to VEGF-A-induced angiogenesis. J. Biol. Chem. 2007, 282, 15187–15196. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Lansing, L.; Merillon, J.M.; Davis, F.B.; Tang, H.Y.; Shih, A.; Vitrac, X.; Krisa, S.; Keating, T.; Cao, H.J.; et al. Integrin αVβ3 contains a receptor site for resveratrol. FASEB J. 2006, 20, 1742–1744. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Tang, H.-Y.; Keating, T.; Wu, Y.-H.; Shih, A.; Hammond, D.; Sun, M.; Hercbergs, A.; Davis, F.B.; Davis, P.J. Resveratrol is pro-apoptotic and thyroid hormone is anti-apoptotic in glioma cells: Both actions are integrin and ERK mediated. Carcinogenesis 2008, 29, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Belleri, M.; Ribatti, D.; Savio, M.; Stivala, L.A.; Forti, L.; Tanghetti, E.; Alessi, P.; Coltrini, D.; Bugatti, A.; Mitola, S.; et al. αvβ3 Integrin-dependent antiangiogenic activity of resveratrol stereoisomers. Mol. Cancer Ther. 2008, 7, 3761–3770. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Cody, V.; Davis, F.B.; Hercbergs, A.A.; Luidens, M.K.; Mousa, S.A.; Davis, P.J. Identification and functions of the plasma membrane receptor for thyroid hormone analogues. Discov. Med. 2011, 11, 337–347. [Google Scholar] [PubMed]
- Cayrol, F.; Flaqué, M.C.D.; Fernando, T.; Yang, S.N.; Sterle, H.A.; Bolontrade, M.; Amorós, M.; Isse, B.; Farías, R.N.; Ahn, H.; et al. Integrin αvβ3 acting as membrane receptor for thyroid hormones mediates angiogenesis in malignant T cells. Blood 2015, 125, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Bergh, J.J.; Lin, H.Y.; Lansing, L.; Mohamed, S.N.; Davis, F.B.; Mousa, S.; Davis, P.J. Integrin αVβ3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 2005, 146, 2864–2871. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Sun, M.; Lin, C.; Tang, H.Y.; London, D.; Shih, A.; Davis, F.B.; Davis, P.J. Androgen-induced human breast cancer cell proliferation is mediated by discrete mechanisms in estrogen receptor-α-positive and -negative breast cancer cells. J. Steroid Biochem. Mol. Biol. 2009, 113, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.-T.; Yang, S.-H.; Chang, T.-C.; Changou, C.A.; Lai, H.-Y.; Fu, E.; Huangfu, W.-C.; Davis, P.J.; Lin, H.-Y.; Liu, L.F. Mechanisms of dihydrotestosterone action on resveratrol- induced anti-proliferation in breast cancer cells with different ERα status. Oncotarget 2015, 6, 35866–35879. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Rao, J.; Zha, X.L.; Xu, H. Angiopoietin-like 3 induces podocyte f-actin rearrangement through integrin α v β 3 /FAK/PI3K pathway-mediated rac1 Activation. BioMed Res. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Gomez Perdiguero, E.; Liabotis-Fontugne, A.; Durand, M.l.; Faye, C.M.; Ricard-Blum, S.; Simonutti, M.; Augustin, S.B.; Robb, B.M.; Paques, M.; Valenzuela, D.M.; et al. ANGPTL4-αvβ3 interaction counteracts hypoxia-induced vascular permeability by modulating Src signalling downstream of vascular endothelial growth factor receptor 2. J. Pathol. 2016, 240, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Clement, L.C.; Macé, C.; Avila-Casado, C.; Joles, J.A.; Kersten, S.; Chugh, S.S. Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat. Med. 2014, 20, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Worthington, J.J.; Klementowicz, J.E.; Travis, M.A. TGFβ: A sleeping giant awoken by integrins. Trends Biochem. Sci. 2011, 36, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhao, B.; Iacob, R.E.; Zhu, J.; Koksal, A.C.; Lu, C.; Engen, J.R.; Springer, T.A. Force interacts with macromolecular structure in activation of TGF-β. Nature 2017, 542, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Juárez, P.; Comas, I.; González-Candelas, F.; Calvete, J.J. Evolution of snake venom disintegrins by positive Darwinian selection. Mol. Biol. Evol. 2008, 25, 2391–2407. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.H.F.; Iemma, M.R.C.; Ferreira, L.L.; Faria, J.P.; Oliva, M.L.V.; Zingali, R.B.; Niewiarowski, S.; Selistre-de-Araujo, H.S. The Disintegrin-like Domain of the Snake Venom Metalloprotease Alternagin Inhibits α2β1 Integrin-Mediated Cell Adhesion. Arch. Biochem. Biophys. 2000, 384, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Kamiguti, A.S.; Hay, C.R.; Zuzel, M. Inhibition of collagen-induced platelet aggregation as the result of cleavage of α 2 β 1-integrin by the snake venom metalloproteinase jararhagin. Biochem. J. 1996, 320, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, P.; Calvete, J.J.; Eble, J.A.; Lazarovici, P.; Marcinkiewicz, C. Identification of inhibitors of α2β1 integrin, members of C-lectin type proteins, in Echis sochureki venom. Toxicol. Appl. Pharmacol. 2013, 269, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Pilorget, A.; Conesa, M.; Sarray, S.; Michaud-Levesque, J.; Daoud, S.; Kim, K.S.; Demeule, M.; Marvaldi, J.; El Ayeb, M.; Marrakchi, N.; et al. Lebectin, a Macrovipera lebetina venom-derived C-type lectin, inhibits angiogenesis both in vitro and in vivo. J. Cell. Physiol. 2007, 211, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Momic, T.; Cohen, G.; Reich, R.; Arlinghaus, F.T.; Eble, J.A.; Marcinkiewicz, C.; Lazarovici, P. Vixapatin (VP12), a C-type lectin-protein from Vipera xantina palestinae venom: Characterization as a novel anti-angiogenic compound. Toxins 2012, 4, 862–877. [Google Scholar] [CrossRef] [PubMed]
- Rosenow, F.; Ossig, R.; Thormeyer, D.; Gasmann, P.; Schlüter, K.; Brunner, G.; Haier, J.; Eble, J.A. Antimetastatic Integrin as Inhibitors of Snake Venoms. Neoplasia 2008, 10, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Frankel, G.; Lider, O.; Hershkoviz, R.; Mould, A.P.; Kachalsky, S.G.; Candy, D.C.; Cahalon, L.; Humphries, M.J.; Dougan, G. The cell-binding domain of intimin from enteropathogenic Escherichia coli binds to β1 integrins. J. Biol. Chem. 1996, 271, 20359–20364. [Google Scholar] [CrossRef] [PubMed]
- Watarai, M.; Funato, S.; Sasakawa, C. Interaction of Ipa proteins of Shigella flexneri with α5β1 integrin promotes entry of the bacteria into mammalian cells. J. Exp. Med. 1996, 183, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Claus, S.; Relman, D.A. Bordetella pertussis filamentous hemagglutinin interacts with a leukocyte signal transduction complex and stimulates bacterial adherence to monocyte CR3 (CD11b/CD18). J. Exp. Med. 1994, 180, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Tilly, K.; Rosa, P.A.; Stewart, P.E. Biology of Infection with Borrelia burgdorferi. Infect. Dis. Clin. N. Am. 2008, 22, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Hyde, J.A. Borrelia burgdorferi Keeps Moving and Carries on: A Review of Borrelial Dissemination and Invasion. Front. Immunol. 2017, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Caine, J.A.; Coburn, J. Multifunctional and Redundant Roles of Borrelia burgdorferi Outer Surface Proteins in Tissue Adhesion, Colonization, and Complement Evasion. Front. Immunol. 2016, 7, 442. [Google Scholar] [CrossRef] [PubMed]
- Coburn, J.; Magoun, L.; Bodary, S.C.; Leong, J.M. Integrins αvβ3 and α5β1 mediate attachment of lyme disease spirochetes to human cells. Infect. Immun. 1998, 66, 1946–1952. [Google Scholar] [PubMed]
- Coburn, J.; Leong, J.M.; Erban, J.K. Integrin α IIb β 3 mediates binding of the Lyme disease agent Borrelia burgdorferi to human platelets. Proc. Natl. Acad. Sci. USA 1993, 90, 7059–7063. [Google Scholar] [CrossRef] [PubMed]
- Coburn, J.; Chege, W.; Magoun, L.; Bodary, S.C.; Leong, J.M. Characterization of a candidate Borrelia burgdorferi β3-chain integrin ligand identified using a phage display library. Mol. Microbiol. 1999, 34, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Defoe, G.; Coburn, J. Delineation of Borrelia burgdorferi p66 sequences required for integrin αIIbβ3 recognition. Infect. Immun. 2001, 69, 3455–3459. [Google Scholar] [CrossRef] [PubMed]
- Ristow, L.C.; Bonde, M.; Lin, Y.P.; Sato, H.; Curtis, M.; Wesley, E.; Hahn, B.L.; Fang, J.; Wilcox, D.A.; Leong, J.M.; et al. Integrin binding by Borrelia burgdorferi P66 facilitates dissemination but is not required for infectivity. Cell. Microbiol. 2015, 17, 1021–1036. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Ristow, L.C.; Shi, M.; Mukherjee, P.; Caine, J.A.; Lee, W.Y.; Kubes, P.; Coburn, J.; Chaconas, G. Intravital Imaging of Vascular Transmigration by the Lyme Spirochete: Requirement for the Integrin Binding Residues of the B. burgdorferi P66 Protein. PLoS Pathog. 2015, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wood, E.; Tamborero, S.; Mingarro, I.; Esteve-Gassent, M.D. BB0172, a Borrelia burgdorferi outer membrane protein that binds integrin α3β1. J. Bacteriol. 2013, 195, 3320–3330. [Google Scholar] [CrossRef] [PubMed]
- Behera, A.K.; Durand, E.; Cugini, C.; Antonara, S.; Bourassa, L.; Hildebrand, E.; Hu, L.T.; Coburn, J. Borrelia burgdorferi BBB07 interaction with integrin α3β1 stimulates production of pro-inflammatory mediators in primary human chondrocytes. Cell. Microbiol. 2008, 10, 320–331. [Google Scholar] [PubMed]
- Song, J.; Zhang, X.; Buscher, K.; Wang, Y.; Wang, H.; di Russo, J.; Li, L.; Lutke-Enking, S.; Zarbock, A.; Stadtmann, A.; et al. Endothelial Basement Membrane Laminin 511 Contributes to Endothelial Junctional Tightness and Thereby Inhibits Leukocyte Transmigration. Cell Rep. 2017, 18, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef] [PubMed]
- Saber, T.; Ghonaim, M.M.; Yousef, A.R.; Khalifa, A.; Al Qurashi, H.; Shaqhan, M.; Samaha, M. Association of Helicobacter pylori cagA Gene with Gastric Cancer and Peptic Ulcer in Saudi Patients. J. Microbiol. Biotechnol. 2015, 25, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Parsonnet, J.; Friedman, G.D.; Orentreich, N.; Vogelman, H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacterpylori infection. Gut 1997, 40, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Wallden, K.; Rivera-Calzada, A.; Waksman, G. Type IV secretion systems: Versatility and diversity in function. Cell. Microbiol. 2010, 12, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Terradot, L.; Waksman, G. Architecture of the Helicobacter pylori Cag-type IV secretion system. FEBS J. 2011, 278, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Conradi, J.; Tegtmeyer, N.; Woźna, M.; Wissbrock, M.; Michalek, C.; Gagell, C.; Cover, T.L.; Frank, R.; Sewald, N.; Backert, S. An RGD helper sequence in CagL of Helicobacter pylori assists in interactions with integrins and injection of CagA. Front. Cell. Infect. Microbiol. 2012, 2, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiedemann, T.; Hofbaur, S.; Tegtmeyer, N.; Huber, S.; Sewald, N.; Wessler, S.; Backert, S.; Rieder, G. Helicobacter pylori CagL dependent induction of gastrin expression via a novel v 5-integrin-integrin linked kinase signalling complex. Gut 2012, 61, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Kwok, T.; Zabler, D.; Urman, S.; Rohde, M.; Hartig, R.; Wessler, S.; Misselwitz, R.; Berger, J.; Sewald, N.; König, W.; et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature 2007, 449, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Conradi, J.; Huber, S.; Gaus, K.; Mertink, F.; Royo Gracia, S.; Strijowski, U.; Backert, S.; Sewald, N. Cyclic RGD peptides interfere with binding of the Helicobacter pylori protein CagL to integrins αvβ3 and α5β1. Amino Acids 2012, 43, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Barden, S.; Niemann, H.H. Adhesion of several cell lines to helicobacter pylori CagL Is mediated by integrin αvβ6 via an rgdlxxl motif. J. Mol. Biol. 2015, 427, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Bönig, T.; Olbermann, P.; Bats, S.H.; Fischer, W.; Josenhans, C.; Blaser, M.J.; Atherton, J.C.; Kusters, J.G.; Vliet, A.H.V.; Kuipers, E.J.; et al. Systematic site-directed mutagenesis of the Helicobacter pylori CagL protein of the Cag type IV secretion system identifies novel functional domains. Sci. Rep. 2016, 6, 38101. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.; Tegtmeyer, N.; Brandt, S.; Yamaoka, Y.; de Poire, E.; Sgouras, D.; Wessler, S.; Torres, J.; Smolka, A.; Backert, S. c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains. J. Clin. Investig. 2012, 122, 1553–1566. [Google Scholar] [CrossRef] [PubMed]
- Tohidpour, A. CagA-mediated pathogenesis of Helicobacter pylori. Microb. Pathogen. 2016, 93, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Tertti, R.; Skurnik, M.; Vartio, T.; Kuusela, P. Adhesion protein YadA of Yersinia species mediates binding of bacteria to fibronectin. Infect. Immun. 1992, 60, 3021–3024. [Google Scholar] [PubMed]
- El Tahir, Y.; Skurnik, M. YadA, the multifaceted Yersinia adhesin. Int. J. Med. Microbiol. IJMM 2001, 291, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Isberg, R.R.; Leong, J.M. Multiple β1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell 1990, 60, 861–871. [Google Scholar] [CrossRef]
- Hamzaoui, N.; Kerneis, S.; Caliot, E.; Pringault, E. Expression and distribution of β1 integrins in in vitro-induced M cells: implications for Yersinia adhesion to Peyer’s patch epithelium. Cell. Microbiol. 2004, 6, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Schulte, R.; Kerneis, S.; Klinke, S.; Bartels, H.; Preger, S.; Kraehenbuhl, J.P.; Pringault, E.; Autenrieth, I.B. Translocation of Yersinia entrocolitica across reconstituted intestinal epithelial monolayers is triggered by Yersinia invasin binding to β1 integrins apically expressed on M-like cells. Cell. Microbiol. 2000, 2, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A.; Hirst, B.H.; Jepson, M.A. M-cell surface β1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer’s patch M cells. Infect. Immun. 1998, 66, 1237–1243. [Google Scholar] [PubMed]
- Leong, J.M.; Morrissey, P.E.; Marra, A.; Isberg, R.R. An aspartate residue of the Yersinia pseudotuberculosis invasin protein that is critical for integrin binding. EMBO J. 1995, 14, 422–431. [Google Scholar] [PubMed]
- Hamburger, Z.A.; Brown, M.S.; Isberg, R.R.; Bjorkman, P.J. Integrin-Binding Protein Crystal Structure of Invasin: A Bacterial Crystal Structure of Invasin: A Bacterial Integrin-Binding Protein. Science 1999, 286, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, A.; Jones, B.F.; Harrison, L.M.; Chadderdon, R.C.; Cappello, M. Isolation and molecular cloning of a secreted hookworm platelet inhibitor from adult Ancylostoma caninum. Mol. Biochem. Parasitol. 2003, 129, 167–177. [Google Scholar] [CrossRef]
- Chadderdon, R.C.; Cappello, M. The hookworm platelet inhibitor: Functional blockade of integrins GPIIb/IIIa (αIIbβ3) and GPIa/IIa (α2β1) inhibits platelet aggregation and adhesion in vitro. J. Infect. Dis. 1999, 179, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.L.; Henzel, W.J.; Nevins, B.; Stults, J.T.; Lazarus, R.A. Decorsin. A potent glycoprotein IIb-IIIa antagonist and platelet aggregation inhibitor from the leech Macrobdella decora. J. Biol. Chem. 1990, 265, 10143–10147. [Google Scholar] [PubMed]
- Zhang, Z.; Gao, L.; Shen, C.; Rong, M.; Yan, X.; Lai, R. A potent anti-thrombosis peptide (vasotab TY) from horsefly salivary glands. Int. J. Biochem. Cell. Biol. 2014, 54, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Xu, X.; An, S.; Liu, H.; Yang, X.; Andersen, J.F.; Wang, Y.; Tokumasu, F.; Ribeiro, J.M.; Francischetti, I.M.; et al. A novel family of RGD-containing disintegrins (Tablysin-15) from the salivary gland of the horsefly Tabanus yao targets αIIbβ3 or αVβ3 and inhibits platelet aggregation and angiogenesis. Thromb. Haemost. 2011, 105, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, J.; Endris, R.; Connolly, T.M. Disagregin is a fibrinogen receptor antagonist lacking the Arg-Gly-Asp sequence from the tick, Ornithodoros moubata. J. Biol. Chem. 1994, 269, 6702–6708. [Google Scholar] [PubMed]
- Tang, J.; Fang, Y.; Han, Y.; Bai, X.; Yan, X.; Zhang, Y.; Lai, R.; Zhang, Z. YY-39, a tick anti-thrombosis peptide containing RGD domain. Peptides 2015, 68, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Coons, L.B.; Taylor, D.B.; Stevens, S.E., Jr.; Gartner, T.K. Variabilin, a novel RGD-containing antagonist of glycoprotein IIb-IIIa and platelet aggregation inhibitor from the hard tick Dermacentor variabilis. J. Biol. Chem. 1996, 271, 17785–17790. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, L.; Moreau, F.; Cornick, S.; Chadee, K. The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive Entamoeba histolytica via Activation of α5β1 Integrin at the Macrophage-Amebae Intercellular Junction. PLoS Pathog. 2015, 11, e1004887. [Google Scholar] [CrossRef] [PubMed]
- Muchowski, P.J.; Zhang, L.; Chang, E.R.; Soule, H.R.; Plow, E.F.; Moyle, M. Functional interaction between the integrin antagonist neutrophil inhibitory factor and the I domain of CD11b/CD18. J. Biol. Chem. 1994, 269, 26419–26423. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Mortimer, L.; Chadee, K. Entamoeba histolytica cysteine proteinase 5 binds integrin on colonic cells and stimulates NFkappaB-mediated pro-inflammatory responses. J. Biol. Chem. 2010, 285, 35497–35504. [Google Scholar] [CrossRef] [PubMed]
- Cornick, S.; Moreau, F.; Chadee, K. Entamoeba histolytica Cysteine Proteinase 5 Evokes Mucin Exocytosis from Colonic Goblet Cells via αvβ3 Integrin. PLoS Pathog. 2016, 12, e1005579. [Google Scholar] [CrossRef] [PubMed]
- Kucik, C.J.; Martin, G.L.; Sortor, B.V. Common intestinal parasites. Am. Fam. Physician 2004, 69, 1161–1168. [Google Scholar] [PubMed]
- Gunther, J.; Shafir, S.; Bristow, B.; Sorvillo, F. Short report: Amebiasis-related mortality among United States residents, 1990–2007. Am. J. Trop. Med. Hyg. 2011, 85, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.; Park, S.J.; Yong, T.S.; Im, K.I.; Shin, M.H. Involvement of β 2-integrin in ROS-mediated neutrophil apoptosis induced by Entamoeba histolytica. Microbes Infect. 2007, 9, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Pillai, D.R.; Kain, K.C. Entamoeba histolytica: Identification of a distinct β2 integrin-like molecule with a potential role in cellular adherence. Exp. Parasitol. 2005, 109, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, K.; Hernandez-Ramirez, V.I.; Rosales-Encina, J.L.; Mondragon, R.; Garibay-Cerdenares, O.L.; Flores-Robles, D.; Javier-Reyna, R.; Pertuz, S.; Talamas-Rohana, P. Physical, structural, and functional properties of the β1 integrin-like fibronectin receptor (β1EhFNR) in Entamoeba histolytica. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2009, 9, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Francischetti, I.M.; Ribeiro, J.M.; Andersen, J.F. The structure of hookworm platelet inhibitor (HPI), a CAP superfamily member from Ancylostoma caninum. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2015, 71, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Moyle, M.; Foster, D.L.; McGrath, D.E.; Brown, S.M.; Laroche, Y.; de Meutter, J.; Stanssens, P.; Bogowitz, C.A.; Fried, V.A.; Ely, J.A.; et al. A hookworm glycoprotein that inhibits neutrophil function is a ligand of the integrin CD11b/CD18. J. Biol. Chem. 1994, 269, 10008–10015. [Google Scholar] [PubMed]
- Assumpcao, T.C.F.; Ribeiro, J.M.C.; Francischetti, I.M.B. Disintegrins from hematophagous sources. Toxins 2012, 4, 296–322. [Google Scholar] [CrossRef] [PubMed]
- Fremont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Tseng, S.H.; Lin, S.M.; Chen, J.C.; Su, Y.H.; Huang, H.Y.; Chen, C.K.; Lin, P.Y.; Chen, Y. Resveratrol suppresses the angiogenesis and tumor growth of gliomas in rats. Clin. Cancer Res. 2004, 10, 2190–2202. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer Molecular Mechanisms of Resveratrol. Front. Nutr. 2016, 3, 82–83. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.T.; Hsieh, M.T.; Yang, S.H.; Tsai, P.W.; Wang, S.H.; Wang, C.C.; Lee, Y.S.; Cheng, G.Y.; HuangFu, W.C.; London, D.; et al. Anti-proliferative and gene expression actions of resveratrol in breast cancer cells in vitro. Oncotarget 2014, 5, 12891–12907. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Goel, A.; Shakibaei, M. Resveratrol Regulates Colorectal Cancer Cell Invasion by Modulation of Focal Adhesion Molecules. Nutrients 2017, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, S.J.; Vasko-Moser, J.; Miller, W.H.; Lark, M.W.; Gowen, M.; Stroup, G. Rapid inhibition of thyroxine-induced bone resorption in the rat by an orally active vitronectin receptor antagonist. J. Pharmacol. Exp. Therap. 2002, 302, 205–211. [Google Scholar] [CrossRef]
- Lin, H.Y.; Sun, M.; Tang, H.Y.; Lin, C.; Luidens, M.K.; Mousa, S.A.; Incerpi, S.; Drusano, G.L.; Davis, F.B.; Davis, P.J. L-Thyroxine vs. 3,5,3’-triiodo-l-thyronine and cell proliferation: Activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Am. J. Physiol. Cell Physiol. 2009, 296, C980–C991. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G. Angiopoietin-like proteins: A comprehensive look. Front. Endocrinol. 2014, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, X.; Tian, R.; Wei, W.; Hu, W.; Chen, X.; Han, W.; Chen, H.; Gong, Y. Angiopoietin-related growth factor (AGF) supports adhesion, spreading, and migration of keratinocytes, fibroblasts, and endothelial cells through interaction with RGD-binding integrins. Biochem. Biophys. Res. Commun. 2006, 347, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, X.; Zhai, Y.; Shen, Q.; Sun, L.; Feng, C.; Rao, J.; Liu, H.; Zha, X.; Guo, M.; et al. A novel role of angiopoietin-like-3 associated with podocyte injury. Pediatr. Res. 2015, 77, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Mu, Z.; Dabovic, B.; Jurukovski, V.; Yu, D.; Sung, J.; Xiong, X.; Munger, J.S. Absence of integrin-mediated TGFβ1 activation in vivo recapitulates the phenotype of TGFβ1-null mice. J. Cell Biol. 2007, 176, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Katsumoto, T.R.; Violette, S.M.; Sheppard, D. Blocking TGFβ via Inhibition of the αvβ6 Integrin: A Possible Therapy for Systemic Sclerosis Interstitial Lung Disease. Int. J. Rheumatol. 2011, 2011, 208219. [Google Scholar] [CrossRef] [PubMed]
- Munger, J.S.; Huang, X.; Kawakatsu, H.; Griffiths, M.J.; Dalton, S.L.; Wu, J.; Pittet, J.F.; Kaminski, N.; Garat, C.; Matthay, M.A.; et al. The integrin αvβ6 binds and activates latent TGF β 1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999, 96, 319–328. [Google Scholar] [CrossRef]
- Puthawala, K.; Hadjiangelis, N.; Jacoby, S.C.; Bayongan, E.; Zhao, Z.; Yang, Z.; Devitt, M.L.; Horan, G.S.; Weinreb, P.H.; Lukashev, M.E.; et al. Inhibition of integrin αvβ6, an activator of latent transforming growth factor-β, prevents radiation-induced lung fibrosis. Am. J. Respir. Crit. Care Med. 2008, 177, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Horan, G.S.; Wood, S.; Ona, V.; Li, D.J.; Lukashev, M.E.; Weinreb, P.H.; Simon, K.J.; Hahm, K.; Allaire, N.E.; Rinaldi, N.J.; et al. Partial inhibition of integrin αvβ6 prevents pulmonary fibrosis without exacerbating inflammation. Am. J. Respir. Crit. Care Med. 2008, 177, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.A.; Brekken, R.A. The VEGF family in cancer and antibody-based strategies for their inhibition. mAbs 2010, 2, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Hicklin, D.J.; Ellis, L.M. Antiangiogenic therapy—evolving view based on clinical trial results. Nat. Rev. Clin. Oncol. 2012, 9, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Vlahakis, N.E.; Young, B.A.; Atakilit, A.; Sheppard, D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin α9β1. J. Biol. Chem. 2005, 280, 4544–4552. [Google Scholar] [CrossRef] [PubMed]
- Temming, K.; Schiffelers, R.M.; Molema, G.; Kok, R.J. RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer Chemother. 2005, 8, 381–402. [Google Scholar] [CrossRef] [PubMed]
- Marelli, U.K.; Rechenmacher, F.; Sobahi, T.R.; Mas-Moruno, C.; Kessler, H. Tumor Targeting via Integrin Ligands. Front. Oncol. 2013, 3, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garanger, E.; Boturyn, D.; Dumy, P. Tumor targeting with RGD peptide ligands-design of new molecular conjugates for imaging and therapy of cancers. Anti-Cancer Agents Med. Chem. 2007, 7, 552–558. [Google Scholar] [CrossRef]
- Wen, A.M.; Steinmetz, N.F. Design of virus-based nanomaterials for medicine, biotechnology, and energy. Chem. Soc. Rev. 2016, 45, 4074–4126. [Google Scholar] [CrossRef] [PubMed]
- Hovlid, M.L.; Steinmetz, N.F.; Laufer, B.; Lau, J.L.; Kuzelka, J.; Wang, Q.; Hyypia, T.; Nemerow, G.R.; Kessler, H.; Manchester, M.; et al. Guiding plant virus particles to integrin-displaying cells. Nanoscale 2012, 4, 3698–3705. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.H.; Ruegsegger, M.A.; Murugesan, G.; Kottke-Marchant, K.; Marchant, R.E. Extracellular matrix-like surfactant polymers containing arginine-glycine-aspartic acid (RGD) peptides. Macromol. Biosci. 2004, 4, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.X.; Ng, S.R.; Khoo, S.Y.; Zheng, X.; Chen, P.; Li, C.M. RGD-peptide functionalized graphene biomimetic live-cell sensor for real-time detection of nitric oxide molecules. ACS Nano 2012, 6, 6944–6951. [Google Scholar] [CrossRef] [PubMed]
- Stephanopoulos, N.; Freeman, R.; North, H.A.; Sur, S.; Jeong, S.J.; Tantakitti, F.; Kessler, J.A.; Stupp, S.I. Bioactive DNA-peptide nanotubes enhance the differentiation of neural stem cells into neurons. Nano Lett. 2015, 15, 603–609. [Google Scholar] [CrossRef] [PubMed]



| Integrin Dimers | Common Name | Non-ECM Ligand | Function of Interaction [Key Refs] | |
|---|---|---|---|---|
| α4β1 | VLA-4 | Very late antigen-4 | MAdCAM1 VCAM1 AM-B | Leukocyte adhesion [10,11,12] Leukocyte adhesion [10,11,12] Erythrocyte differentiation [13,14,15] Cancer cell metastasis [16] Leukocyte transmigration [11] |
| α4β7 | LPAM | Lymphocyte Peyer’s patch adhesion molecule | MAdCAM1 | T-lymphocyte homing [17] HSC homing to bone marrow [18] |
| α5β1 | Fibronectin receptor | Fibronectin receptor | Glycoprotein NMB | Cancer cell growth, metastasis [19] |
| αEβ7 | E-cadherin | Cytotoxic T cell targeting of tumor cells [20] | ||
| αLβ2 | LFA-1 | Lymphocyte function associated antigen-1 | ICAM1, 2, 3 JAM-A | Leukocyte adhesion [10,11] Leukocyte transmigration [11] |
| αMβ2 | Mac-1/CR3 | Macrophage antigen-1/Complement receptor-3 | ICAM1 β-glucan Complement C3 LL-37 JAM-C HMGB1 | Leukocyte adhesion [10,11] NETosis [21,22] Phagocytosis [23,24] Bacterial opsonization [25,26,27,28,29] Leukocyte transmigration [11] NETosis [30] |
| αVβ3 | Vitronectin receptor | Vitronectin receptor | L1CAM | Cancer cell metastasis [31,32] |
| αXβ2 | CR4/CD11c/CD18 | Complement receptor-4 | Complement C3 | Phagocytosis [23,24] |
| Integrin | Virus Name [Key Refs] |
|---|---|
| α1β1 | Ross River virus [55] |
| α2β1 | Echovirus 1 [56,57] Cytomegalovirus [58] Rotavirus [59,60] |
| α3β1 | Kaposi’s sarcoma-associated herpesvirus [61] Adenovirus [62] |
| α4β1 | Infectious bursal disease virus [63] Rotatvirus [60] |
| α5β1 | Foot-and-mouth disease virus [64] Epstein-Barr virus [65] Adenovirus [66] |
| α6β1 | Cytomegalovirus [58] |
| α9β1 | Kaposi’s sarcoma-associated herpesvirus [67] |
| αMβ2 | Adenovirus [68] |
| αVβ1 | Echovirus 22 [69,70] Adenovirus [71] |
| αVβ3 | Echovirus 9 [72] Coxsackievirus A9 [73] Foot-and-mouth disease virus [74] Japanese encephalitis virus [75] Kaposi’s sarcoma-associated herpesvirus [76] Cytomegalovirus [58] Andes virus [77] Adenovirus [78] Rotavirus [79,80] Sin Nombre virus [81] |
| αVβ5 | Kaposi’s sarcoma-associated herpesvirus [82] Adenovirus [78] Epstein-Barr virus [83] |
| αVβ6 | Coxsackievirus A9 [73] Foot-and-mouth disease virus [84,85] Epstein-Barr virus [83] Herpes simplex virus [86] |
| αVβ8 | Epstein-Barr virus [83] Herpes simplex virus [86] |
| αXβ2 | Rotavirus [60] |
| αIIbβ3 | Sin Nombre virus [81] |
| Integrin | Non-ECM Ligand | Function [Key Refs] |
|---|---|---|
| α1β1 | KTS/RTS disintegrins | Block cell adhesion [128,129] |
| α2β1 | EMS16 CLP | Block adhesion to collagen [130,131] |
| α3β1 | VEGF Disintegrin Lebein 1/2 | Cell adhesion [132] Block cell adhesion [133] |
| α4β1 | MLD disintegrins | Block cell adhesion [128] |
| α4β7 | MLD disintegrins | Block cell adhesion [128] |
| α5β1 | ANGPTL2 | Cancer cell migration/proliferation [134] Macrophage pro-inflammatory response [135] |
| α6β1 | Disintegrin Lebein 1/2 | Block cell adhesion [133] |
| α7β1 | Disintegrin Lebein 1/2 | Block cell adhesion [133] |
| α9β1 | VEGF-A, -C, -D MLD disintegrins | Endothelial adhesion & lymphangiogenesis [136] Block cell adhesion [128] |
| αVβ3 | Resveratrol Thyroid hormones (T3/T4) DHT ANGPTL3 ANGPTL4 VEGF | Anti-angiogenesis [137,138,139] Cell proliferation/angiogenesis [140,141,142] Cancer cell proliferation [143,144] Podocyte motility [145] Enhanced endothelial junctions [146] Endothelial cell adhesion [132] |
| αVβ5 | ANGPTL4 | Reduce proteinuria [147] |
| αVβ6 | Pro-TGFβ | TGFβ activation [148,149] |
| Integrin | Species | Binding Protein [Key Refs] |
|---|---|---|
| α2β1 | Ancylostoma caninum | Hookworm platelet inhibitor (HPI) [193,194] |
| αIIbβ3 | Ancylostoma caninum Macrobdella decora Tabanus yao Ornithodoros moubata Ixodes pacificus Dermacentor variabilis | Hookworm platelet inhibitor (HPI) [193,194] Decorsin [195] Vasotab TY [196] Tablysin-15 [197] Disagregin [198] YY-39 [199] Variabilin [200] |
| α3β1 | Borrelia burgdorfori Yersinia | BBB07, BB0172 [170] Invasin [187,188] |
| α4β1 | Escherichia coli Yersinia | Intimin [157] Invasin [187,188] |
| α5β1 | Helicobacter pylori Escherichia coli Shigella flexneri Entamoeba histolytica Yersinia | CagL [177,179] Intimin [157] Ipa B, C, D [158] EhCP5 [201] Invasin [187] |
| α6β1 | Yersinia | Invasin [187,188] |
| αMβ2 | Bordetella pertussis Ancylostoma caninum | Filamentous hemagglutinin protein [159] Neutrophil inhibitor factor (NIF) [202] |
| αVβ1 | Yersinia | Invasin [187] |
| αVβ3 | Borrelia burgdorfori Helicobacter pylori Entamoeba histolytica | P66 [165] CagL [177] EhCP5 [203,204] |
| αVβ5 | Helicobacter pylori | CagL [178,180] |
| αVβ6 | Helicobacter pylori | CagL [181] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
LaFoya, B.; Munroe, J.A.; Miyamoto, A.; Detweiler, M.A.; Crow, J.J.; Gazdik, T.; Albig, A.R. Beyond the Matrix: The Many Non-ECM Ligands for Integrins. Int. J. Mol. Sci. 2018, 19, 449. https://doi.org/10.3390/ijms19020449
LaFoya B, Munroe JA, Miyamoto A, Detweiler MA, Crow JJ, Gazdik T, Albig AR. Beyond the Matrix: The Many Non-ECM Ligands for Integrins. International Journal of Molecular Sciences. 2018; 19(2):449. https://doi.org/10.3390/ijms19020449
Chicago/Turabian StyleLaFoya, Bryce, Jordan A. Munroe, Alison Miyamoto, Michael A. Detweiler, Jacob J. Crow, Tana Gazdik, and Allan R. Albig. 2018. "Beyond the Matrix: The Many Non-ECM Ligands for Integrins" International Journal of Molecular Sciences 19, no. 2: 449. https://doi.org/10.3390/ijms19020449
APA StyleLaFoya, B., Munroe, J. A., Miyamoto, A., Detweiler, M. A., Crow, J. J., Gazdik, T., & Albig, A. R. (2018). Beyond the Matrix: The Many Non-ECM Ligands for Integrins. International Journal of Molecular Sciences, 19(2), 449. https://doi.org/10.3390/ijms19020449