Intestinal Permeability and Cellular Antioxidant Activity of Phenolic Compounds from Mango (Mangifera indica cv. Ataulfo) Peels
Abstract
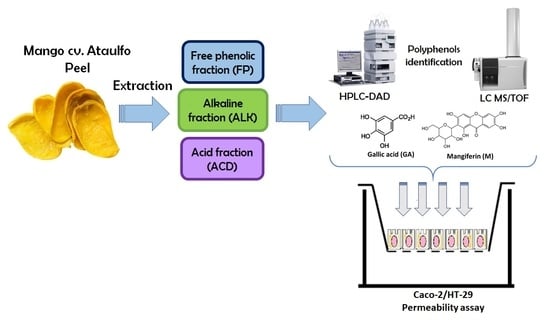
:1. Introduction
2. Results
2.1. Identification and Quantification of PCs in Mango cv. Ataulfo Peel Extracts
2.2. Cytotoxicity and Cellular Antioxidant Activity (CAA) of Mango cv. Ataulfo Peel Extracts
2.3. Intestinal Permeability Experiment
3. Discussion
3.1. Identification of PCs of Mango cv. Ataulfo Peel Extract
3.2. Cytotoxicity of Mango Peel Extract
3.3. Cellular Antioxidant Activity (CAA)
3.4. Instetinal Permeability Assay
4. Materials and Methods
4.1. Material and Methods
4.2. Chemicals and Reagents
4.3. Phenolics Extraction Procedure
4.4. High Performance Liquid Chromatography (HPLC) Analysis
4.5. Cell Culture
4.6. Cytotoxicity Assay
4.7. Cellular Antioxidant Activity (CAA) Assay
4.8. Caco-2/HT-29 Monolayer Permeability Assay
5. Statistical Analysis
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CAA | Cellular antioxidant activity |
| PC | Phenolic compounds |
| FP | Free phenolic fraction |
| ALK | Alkaline fraction |
| ACD | Acid fraction |
| GA | Gallic acid |
| M | Mangiferin |
| ROS | Reactive oxygen species |
| DFFH-DA | Dichloro-dihydro-fluorescein diacetate |
| AAPH | 2,2′-azobis-(2-methylpropionamidine) dihydrochloride |
| Papp | Apparent permeability coefficient |
| PappAP-BL | Apparent permeability coefficients from apical to basolateral direction |
References
- Do Socorro, M.R.; Alves, R.E.; de Brito, E.S.; Perez-Jimenez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Da Silva, L.M.R.; de Figueiredo, E.A.T.; Ricardo, N.M.P.S.; Vieira, I.G.P.; de Figuereido, R.W.; Brasil, I.M.; Gomes, C.L. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014, 143, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Res. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.A.; Perkins-Veazie, P. Influences of harvest date and location on the levels of β-carotene, ascorbic acid, total phenols, the in vitro antioxidant capacity, and phenolic profiles of five commercial varieties of mango (Mangifera indica L.). J. Agric. Food Chem. 2009, 57, 10825–10830. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Zavala, J.F.; Vega-Vega, V.; Rosas-Dominguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.A.; Siddiqui, W.; Dávila-Aviña, J.E.; González-Aguilar, G.A. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Yahia, E.M.; González-Aguilar, G.A. Identification and quantification of major phenolic compounds from mango (Manifera indica, cv. Ataulfo) fruit by HPLC-DAD-MS/MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem. 2012, 135, 105–111. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Ghafoor, K.; Al-Juhaimi, F.; Nyam, K.; Norulandi, N.A.N.; Sahena, F.; Omar, A.K.M. Mango (Mangifera indica L.) by-products and their valuable components: A review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef] [PubMed]
- López-Cobo, A.; Verado, V.; Diaz-de-Cerio, E.; Segura-Carretero, A.; Fernandez-Gutiérrez, A.; Gomez-Caravaca, A.M. Use of HPLC-and GC-QTOF to determine hydrophilic and lipophilic phenols in mango fruit (Mangifera indica L.) and its by-products. Food Res. Int. 2017, 100, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Blancas-Benitez, F.J.; Mercado-Mercado, G.; Quirós-Sauceda, A.E.; Montalvo-González, E.; González-Aguilar, G.A.; Sáyago-Ayerdi, S.G. Bioaccessibility of polyphenols associated with dietary fiber and in vitro kinetics release of polyphenols in Mexico ‘Ataulfo’ mango (Mangifera indica L.) by-products. Food Funct. 2015, 6, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Sauceda, A.E.; Chen, C.Y.O.; Blumberg, J.B.; Astiazaran-Garcia, H.; Wall-Medrano, A.; González-Aguilar, G.A. Processing ‘Ataulfo’ Mango into juice preserves the bioavailability and antioxidant capacity of its phenolic compounds. Nutrients 2017, 9, 1082. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 2012, 64, 280–289. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésey, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.E.; Zambrano, R.; Sepúlveda, B.; Simigiortis, M.J. Antioxidant properties and hyphenated HPLC-PDA-MS profiling of chilean Pica mango fruits (Mangifera indica L. cv. piqueño). Molecules 2013, 19, 438–458. [Google Scholar] [CrossRef] [PubMed]
- Geerkens, C.H.; Schweigger, R.M.; Steingass, H.; Boguhn, J.; Rodehutscord, M.; Carle, R. Influence of apple and citrus pectins, processed mango peels, a phenolic mango peel extract, and gallic acid as potential feed supplements on in vitro total gas production and rumen methanogenesis. J. Agric. Food Chem. 2013, 61, 5727–5737. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Caravaca, A.M.; Lopez-Cobo, A.; Verardo, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-q-TOF-MS as a powerful platform for the determination of phenolic and other polar compounds in the edible part of mango and its by-products (peel, seed, and seed husk). Electrophoresis 2016, 37, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Kapepula, P.M.; Kabamba Ngombe, N.; Tshisekedi Tshibangu, P.; Tsumbu, C.; Franck, T.; Mouithys-Mickalad, A.; Mumba, D.; Tshala-Katumbay, D.; Serteyn, D.; Tits, M.; et al. Comparison of metabolic profiles and bioactivities of the leaves of three edible Congolese Hibiscus species. Nat. Prod. Res. 2017, 31, 2885–2892. [Google Scholar] [CrossRef] [PubMed]
- Dorta, E.; González, M.; Lobo, G.; Sánchez-Moreno, C.; de Ancos, B. Screening of phenolic compounds in by-product extracts from mangoes (Mangifera indica L.) by HPLC-ESI-QTOF-MS and multivariate analysis for use as a food ingredient. Food Res. Int. 2014, 57, 51–60. [Google Scholar] [CrossRef]
- Schieber, A.; Ullrich, W.; Carle, R. Characterization of polyphenols in mango puree concentrate by HPLC with diode array and mass spectrometric detection. Innov. Food Sci. Emerg. Technol. 2000, 1, 161–166. [Google Scholar] [CrossRef]
- Heinrich, M.; Lorenz, P.; Daniels, R.; Stintzing, F.C.; Kammerer, D.R. Lipid and phenolic constituents from seeds of Hypericum perforatum L. and Hypericum tetrapterum Fr. and their antioxidant activity. Chem. Biodivers. 2017, 14, e1700100. [Google Scholar] [CrossRef] [PubMed]
- Sáyago-Ayerdi, S.G.; Moreno-Hernández, C.L.; Montalvo-González, E.; García-Magaña, M.L.; Mata-Montes de Oca, M.; Torres, J.L.; Perez-Jiménez, J. Mexican ‘Ataulfo’mango (Mangifera indica L.) as a source of hydrolyzable tannins. Analysis by MALDI-TOF/TOF MS. Food Res. Int. 2013, 51, 188–194. [Google Scholar] [CrossRef]
- Ajila, C.M.; Rao, U.P. Mango peel dietary fibre: Composition and associated bound phenolics. J. Funct. Foods 2013, 5, 444–450. [Google Scholar] [CrossRef]
- Ureña, C.; Mancipe, J.; Hernandez, J.; Castañeda, D.; Pombo, L.; Gomez, A.; Asea, A.; Florentino, S. Gallotannin-rich Caesalpinia spinosa fraction decrease the primary tumor and factors associated with poor prognosis in a murine breast cancer model. BMC Complement. Altern. Med. 2013, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, N.; Kim, H.; Krenek, K.; Talcott, S.T.; Mertens-Talcott, S.U. Mango polyphenolics suppressed tumor growth in breast cancer xenografts in mice: Role of the PI3K/AKT pathway and associated microRNAs. Nutr Res. 2015, 35, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Rengarajan, T.; Nandakumar, N.; Divya, H.; Nishigaki, I. Mangiferin in cancer chemoprevention and treatment: Pharmacokinetics and molecular targets. J. Recept. Signal Trasduct. Res. 2015, 35, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014, 8, 122–146. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.J.; Burd, R. Hormesis and synergy: Pathways and mechanisms of quercetin in cancer prevention and management. Nutr. Rev. 2010, 68, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Hodnick, W.F.; Milosavljevic, E.B.; Nelson, J.H.; Pardini, R.S. Relationships between redox potentials, inhibition of mitochondrial respiration, and production of oxygen radicals by flavonoids. Biochem. Pharmacol. 1988, 37, 2607–2611. [Google Scholar] [CrossRef]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ponce, M.T.; López-Biedma, A.; Sánchez-Quesada, C.; Casas, L.; Mantell, C.; Gaforio, J.J.; de la Ossa, E.M. Selective antitumoural action of pressurized mango leaf extracts against minimally and highly invasive breast cancer. Food Funct. 2017, 8, 3610–3620. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.M.; Liu, F.; Guo, X.; Fu, X.; Li, T.; Liu, R.H. Phytochemical composition, cellular antioxidant capacity and antiproliferative activity in mango (Mangifera indica L.) pulp and peel. Int. J. Food Sci. 2017, 52, 817–826. [Google Scholar] [CrossRef]
- Su, D.; Ti, H.; Zhang, R.; Zhang, M.; Wei, Z.; Deng, Y.; Guo, J. Structural elucidation and cellular antioxidant activity evaluation of major antioxidant phenolics in lychee pulp. Food Chem. 2014, 158, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Liu, R.H. Structure-activity relationships of flavonoids in the cellular antioxidant activity assay. J. Agric. Food Chem. 2008, 56, 8404–8411. [Google Scholar] [CrossRef] [PubMed]
- Tammela, P.; Laitinen, L.; Galkin, A.; Wennberg, T.; Heczko, R.; Vuorela, H.; Slotte, J.P.; Vuorela, P. Permeability characteristics and membrane affinity of flavonoids and alkyl gallates in Caco-2 cells and in phospholipid vesicles. Arch. Biochem. Biophys. 2004, 425, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Wu, L.F.; Zhao, H.J.; Liang, W.Y.; Chen, W.J.; Han, S.X.; Qi, Q.; Cui, Y.P.; Li, S.; Yang, G.H.; et al. Transport of corilagin, gallic acid, and ellagic acid from Fructus phyllanthi tannin fraction in Caco-2 cell monolayers. Evid. Based Complement. Altern. Med. 2016, 2016, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Luo, H.; Hong, C.; Ma, P.; Li, G.; Zhang, T.; Wu, T.; Ji, G. Phytic acid enhances the oral absorption of isorhamnetin, quercetin, and kaempferol in total flavones of Hippophae rhamnoides L. Fitoterapia 2014, 93, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Farrell, T.; Poquet, L.; Dionisi, F.; Barron, D.; Williamson, G. Characterization of hydroxycinnamic acid glucuronide and sulfate conjugates by HPLC–DAD–MS: Enhancing chromatographic quantification and application in Caco-2 cell metabolism. J. Pharm. Biomed. Anal. 2011, 55, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Kosińska-Cagnazzo, A.; Diering, S.; Prim, D.; Andlauer, W. Identification of bioaccessible and uptaken phenolic compounds from strawberry fruits in in vitro digestion/Caco-2 absorption model. Food Chem. 2015, 170, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.K.; Piwowarski, J.P. Ellagitannins, gallotannins and their metabolites-the contribution to the anti-inflammatory effect of food products and medicinal plants. Curr. Med. Chem. 2016, 23, 1–22. [Google Scholar] [CrossRef]
- Mattila, P.; Kumpulainen, J. Determination of free and total phenolic acids in plant-derived foods by HPLC with diode-array detection. J. Agric. Food Chem. 2002, 50, 3660–3667. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Estrada, B.A.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Chemopreventive effects of feruloyl putrescines from wastewater (Nejayote) of lime-cooked white maize (Zea mays). J. Cereal Sci. 2015, 64, 23–28. [Google Scholar] [CrossRef]
- López-Barrios, L.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A. Changes in antioxidant and antiinflammatory activity of black bean (Phaseolus vulgaris L.) protein isolates due to germination and enzymatic digestion. Food Chem. 2016, 203, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Ricardo, M.; Rodríguez-Rodríquez, C.; Gutiérrez-Uribe, J.A.; Cepeda-Cañedo, E.; Serna-Saldívar, S.O. Bioaccessibility, intestinal permeability and plasma stability of isorhamnetin glycosides from Opuntia ficus-indica (L.). Int. J. Mol. Sci. 2017, 18, 1816. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Kobayashi, S.; Shimizu, M. Transepithelial transport of p-coumaric acid and gallic acid in Caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2003, 67, 2317–2324. [Google Scholar] [CrossRef] [PubMed]





| Peak Number | UV Max | Accurate Mass | m/z (M–H)− | Molecular Formula | Tentative Identification | Concentration (µg/mg) | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| FP | ALK | ACD | |||||||
| 1 | 272 | 332.07 | 331 | C13H16O10 | Galloyl glycoside a | 0.81 | NQ | NQ | [16] |
| 2 | 270 | 170.02 | 169 | C7H6O5 | Gallic acid a | NQ | 271.46 | 184.56 | [18] |
| 3 | 282 | 344.07 | 343 | C14H16O10 | Galloyl quinic acid a | 3.24 | NQ | NQ | [19] |
| 4 | 280 | 370.05 | 369 | C15H14O11 | Caffeoyl hydroxycitric acid a | NQ | 10.71 | 6.82 | [19] |
| 5 | 275 | 322.03 | 321 | C14H10O9 | Digallic acid a | NQ | 40.29 | 23.22 | [18] |
| 6 | 238, 256, 318, 365 | 422.08 | 421 | C19H18O11 | Mangiferin b | 36.02 | NQ | 7.11 | [18] |
| 7 | 280 | 728.12 | 727 | C33H28O19 | Hyemaloside A a | 3.19 | NQ | NQ | [17] |
| 8 | 271 | 198.05 | 197 | C9H10O5 | Ethyl gallate a | NQ | 1.69 | 10.07 | [20] |
| 9 | 227, 309 | 164.05 | 163 | C9H8O3 | Coumaric acid a | NQ | 2.23 | 1.39 | [21] |
| 10 | 257, 352 | 464.09 | 463 | C21H20O12 | Quercetin hexoside I c | 0.54 | 0.56 | NQ | [10] |
| 11 | 241, 259, 317, 367 | 422.08 | 421 | C19H18O11 | Mangiferin isomer I a | 2.33 | 5.38 | NQ | [22] |
| 12 | 256, 351 | 464.09 | 463 | C21H20O12 | Quercetin hexoside II c | 0.23 | 0.37 | NQ | [16] |
| 13 | 280 | 336.05 | 335 | C15H12O9 | Methyl digallate ester a | 4.22 | NQ | NQ | [16] |
| 14 | 236, 261, 318, 369 | 422.08 | 421 | C19H18O11 | Mangiferin isomer II a | NQ | 0.71 | 0.07 | [22] |
| 15 | 279 | 1092.13 | 1091 | C48H36O30 | Hexagalloyl glucose a | 7.28 | NQ | NQ | [23] |
| 16 | 254, 370 | 302.04 | 301 | C15H10O7 | Quercetin c | NQ | 0.27 | 1.44 | [24] |
| Sum | 57.86 | 333.67 | 234.68 | ||||||
| Mango Extract | Cell Line | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caco-2 | HT-29 | Caco-2/HT-29 (75:25) | ||||||||||
| Free Phenolic | 135.03 | ± | 23.39 | Cb | 190.50 | ± | 11.45 | Ca | 135.84 | ± | 13.34 | Bb |
| Alkaline H | 246.46 | ± | 4.83 | Ba | 227.86 | ± | 3.32 | Bb | 157.38 | ± | 6.64 | Bc |
| Acid H. | 327.91 | ± | 12.89 | Aa | 261.05 | ± | 5.03 | Ab | 235.29 | ± | 3.84 | Ac |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco-Ordaz, R.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; González-Aguilar, G.A. Intestinal Permeability and Cellular Antioxidant Activity of Phenolic Compounds from Mango (Mangifera indica cv. Ataulfo) Peels. Int. J. Mol. Sci. 2018, 19, 514. https://doi.org/10.3390/ijms19020514
Pacheco-Ordaz R, Antunes-Ricardo M, Gutiérrez-Uribe JA, González-Aguilar GA. Intestinal Permeability and Cellular Antioxidant Activity of Phenolic Compounds from Mango (Mangifera indica cv. Ataulfo) Peels. International Journal of Molecular Sciences. 2018; 19(2):514. https://doi.org/10.3390/ijms19020514
Chicago/Turabian StylePacheco-Ordaz, Ramón, Marilena Antunes-Ricardo, Janet A. Gutiérrez-Uribe, and Gustavo A. González-Aguilar. 2018. "Intestinal Permeability and Cellular Antioxidant Activity of Phenolic Compounds from Mango (Mangifera indica cv. Ataulfo) Peels" International Journal of Molecular Sciences 19, no. 2: 514. https://doi.org/10.3390/ijms19020514
APA StylePacheco-Ordaz, R., Antunes-Ricardo, M., Gutiérrez-Uribe, J. A., & González-Aguilar, G. A. (2018). Intestinal Permeability and Cellular Antioxidant Activity of Phenolic Compounds from Mango (Mangifera indica cv. Ataulfo) Peels. International Journal of Molecular Sciences, 19(2), 514. https://doi.org/10.3390/ijms19020514