Chondrogenic Differentiation of Defined Equine Mesenchymal Stem Cells Derived from Umbilical Cord Blood for Use in Cartilage Repair Therapy
Abstract
:1. Introduction
2. Results
2.1. Equine Umbilical Cord Blood-Derived Mesenchymal Stem Cells (eUCB-MSCs) Isolation
2.2. Growth Profiling and Cellular Senescence
2.3. MSCs Multipotential Capacity
2.4. eUCB-MSCs Surface Phenotype Expression
2.5. Expression Analysis of Chondrogenic Markers and Cartilage Non-Specific Genes in eUCB-MSCs in 3D Scaffolds
2.6. Effect of TGF-β3 in eUCB-MSCs Chondrogenesis
2.7. Protein Expression Analysis during eUCB-MSCs Commitment into Chondrocytes
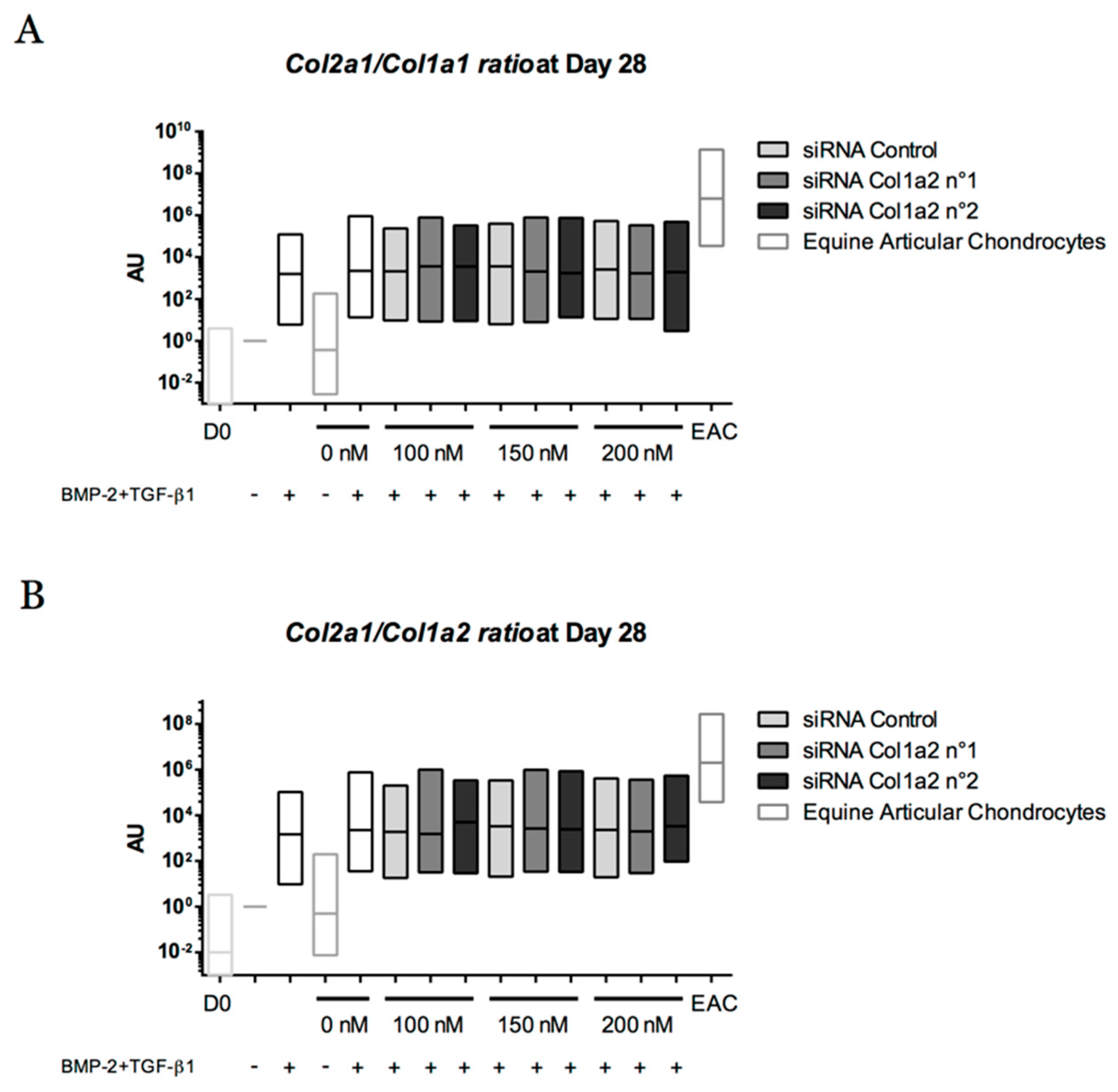
2.8. Gene Silencing Experiments to Overcome Type I Collagen Synthesis
3. Discussion
4. Material and Methods
4.1. eUCB-MSCs Isolation and Culture
4.2. Proliferation Capacity and Senescence Analysis
4.3. Immunophenotyping
4.4. Multilineage Capacity
4.5. Chondrogenic Differentiation in 3D Scaffolds
4.6. Gene Silencing Experiments
4.7. RNA Isolation and RT-qPCR
4.8. Western Blots
4.9. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McIlwraith, C.W.; Frisbie, D.D.; Kawcak, C.E. The horse as a model of naturally occurring osteoarthritis. Bone Jt. Res. 2012, 1, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, T.E. Fell-Muir lecture: Cartilage 2010—The known unknowns. Int. J. Exp. Pathol. 2010, 91, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.; Stöve, J. Collagens—Major component of the physiological cartilage matrix, major target of cartilage degeneration, major tool in cartilage repair. Adv. Drug Deliv. Rev. 2003, 55, 1569–1593. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.; Reid, S.; Morris, R. Profiling the New Zealand Thoroughbred racing industry. 2. Conditions interfering with training and racing. N. Z. Vet. J. 2005, 53, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.; Cook, J.; Gerwin, N.; Glasson, S.; Laverty, S.; Little, C.; McIlwraith, W.; Kraus, V. Histopathology atlas of animal model systems—Overview of guiding principles. Osteoarthr. Cartil. 2010, 18, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- McIlwraith, C.W.; Fortier, L.A.; Frisbie, D.D.; Nixon, A.J. Equine models of articular cartilage repair. Cartilage 2011, 2, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Nixon, A.J.; Begum, L.; Mohammed, H.O.; Huibregtse, B.; O’callaghan, M.M.; Matthews, G.L. Autologous chondrocyte implantation drives early chondrogenesis and organized repair in extensive full- and partial-thickness cartilage defects in an equine model. J. Orthop. Res. 2011, 29, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Benya, P.D.; Padilla, S.R.; Nimni, M.E. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell 1978, 15, 1313–1321. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Gorskaja, J.F.; Kulagina, N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976, 4, 267–274. [Google Scholar] [PubMed]
- Kobolak, J.; Dinnyes, A.; Memic, A.; Khademhosseini, A.; Mobasheri, A. Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro engineering of their niche. Methods 2015. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yang, B.; Tian, Y.; Jiao, H.; Zheng, W.; Wang, J.; Guan, F. Immunomodulatory effect of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells on lymphocytes. Cell. Immunol. 2011, 272, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Murata, D.; Miyakoshi, D.; Hatazoe, T.; Miura, N.; Tokunaga, S.; Fujiki, M.; Nakayama, K.; Misumi, K. Multipotency of equine mesenchymal stem cells derived from synovial fluid. Vet. J. 2014, 202, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ortved, K.F.; Nixon, A.J. Cell-based cartilage repair strategies in the horse. Vet. J. 2016, 208, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Tessier, L.; Bienzle, D.; Williams, L.B.; Koch, T.G. Phenotypic and Immunomodulatory Properties of Equine Cord Blood-Derived Mesenchymal Stromal Cells. PLoS ONE 2015, 10, e0122954. [Google Scholar] [CrossRef] [PubMed]
- De Schauwer, C.; Goossens, K.; Piepers, S.; Hoogewijs, M.K.; Govaere, J.L.J.; Smits, K.; Meyer, E.; Van Soom, A.; Van de Walle, G.R. Characterization and profiling of immunomodulatory genes of equine mesenchymal stromal cells from non-invasive sources. Stem Cell Res. Ther. 2014, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenderup, K.; Justesen, J.; Clausen, C.; Kassem, M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 2003, 33, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Bae, Y.K.; Kim, M.; Kwon, S.-J.; Jeon, H.B.; Choi, S.J.; Kim, S.W.; Yang, Y.S.; Oh, W.; Chang, J.W. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef] [PubMed]
- Perrier, E.; Ronzière, M.-C.; Bareille, R.; Pinzano, A.; Mallein-Gerin, F.; Freyria, A.-M. Analysis of collagen expression during chondrogenic induction of human bone marrow mesenchymal stem cells. Biotechnol. Lett. 2011, 33, 2091–2101. [Google Scholar] [CrossRef] [PubMed]
- Handorf, A.M.; Li, W.-J. Fibroblast growth factor-2 primes human mesenchymal stem cells for enhanced chondrogenesis. PLoS ONE 2011, 6, e22887. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, F.; He, X.; Xu, Y.; Yang, Z.; Chen, L.; Zhou, S.; Yang, Y.; Zhou, Z.; Sheng, W. Chondrogenic differentiation of umbilical cord-derived mesenchymal stem cells in type I collagen-hydrogel for cartilage engineering. Injury 2013, 44, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Mauck, R.L.; Yuan, X.; Tuan, R.S. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthr. Cartil. 2006, 14, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Freyria, A.-M.; Mallein-Gerin, F. Chondrocytes or adult stem cells for cartilage repair: The indisputable role of growth factors. Injury 2012, 43, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Kisiday, J.D.; Kopesky, P.W.; Evans, C.H.; Grodzinsky, A.J.; McIlwraith, C.W.; Frisbie, D.D. Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. J. Orthop. Res. 2008, 26, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Indrawattana, N.; Chen, G.; Tadokoro, M.; Shann, L.H.; Ohgushi, H.; Tateishi, T.; Tanaka, J.; Bunyaratvej, A. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem. Biophys. Res. Commun. 2004, 320, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Ronzière, M.C.; Perrier, E.; Mallein-Gerin, F.; Freyria, A.-M. Chondrogenic potential of bone marrow- and adipose tissue-derived adult human mesenchymal stem cells. Biomed. Mater. Eng. 2010, 20, 145–158. [Google Scholar] [PubMed]
- Murphy, M.K.; Huey, D.J.; Hu, J.C.; Athanasiou, K.A. TGF-β1, GDF-5, and BMP-2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow-derived stromal cells. Stem Cells 2014, 33, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Leduc, T.; Hervieu, M.; Legendre, F.; Bouyoucef, M.; Gruchy, N.; Poulain, L.; de Vienne, C.; Herlicoviez, M.; Demoor, M.; Galera, P. Chondrogenic commitment of human umbilical cord-blood derived mesenchymal stem cells in collagen matrices for cartilage engineering. Sci. Rep. 2016, 6, 32786. [Google Scholar] [CrossRef] [PubMed]
- Kafienah, W.; Mistry, S.; Dickinson, S.C.; Sims, T.J.; Learmonth, I.; Hollander, A.P. Three-dimensional cartilage tissue engineering using adult stem cells from osteoarthritis patients. Arthritis Rheumatol. 2007, 56, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Gardner, O.F.W.; Archer, C.W.; Alini, M.; Stoddart, M.J. Chondrogenesis of mesenchymal stem cells for cartilage tissue engineering. Histol. Histopathol. 2013, 28, 23–42. [Google Scholar] [PubMed]
- Cooke, M.E.; Allon, A.A.; Cheng, T.; Kuo, A.C.; Kim, H.T.; Vail, T.P.; Marcucio, R.S.; Schneider, R.A.; Lotz, J.C.; Alliston, T. Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy. Osteoarthr. Cartil. 2011, 19, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-G.; Park, S.-B.; Seo, M.-S.; Kim, H.-S.; Chae, J.-S.; Kang, K.-S. Characterization and clinical application of mesenchymal stem cells from equine umbilical cord blood. J. Vet. Sci. 2013, 14, 367–371. [Google Scholar] [CrossRef] [PubMed]
- McIlwraith, C.W.; Frisbie, D.D.; Rodkey, W.G.; Kisiday, J.D.; Werpy, N.M.; Kawcak, C.E.; Steadman, J.R. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 27, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Pigott, J.H.; Ishihara, A.; Wellman, M.L.; Russell, D.S.; Bertone, A.L. Investigation of the immune response to autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra-articular injection in horses. Vet. Immunol. Immunopathol. 2013, 156, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.B.; Koenig, J.B.; Black, B.; Gibson, T.W.G.; Sharif, S.; Koch, T.G. Equine allogeneic umbilical cord blood derived mesenchymal stromal cells reduce synovial fluid nucleated cell count and induce mild self-limiting inflammation when evaluated in an LPS induced synovitis model. Equine Vet. J. 2016, 48, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Wilke, M.M.; Nydam, D.V.; Nixon, A.J. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J. Orthop. Res. 2007, 25, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Hillmann, A.; Ahrberg, A.B.; Brehm, W.; Heller, S.; Josten, C.; Paebst, F.; Burk, J. Comparative characterization of human and equine mesenchymal stromal cells: A basis for translational studies in the equine model. Cell Transplant. 2016, 25, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Koch, T.G.; Heerkens, T.; Thomsen, P.D.; Betts, D.H. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 2007, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Koch, T.G.; Thomsen, P.D.; Betts, D.H. Improved isolation protocol for equine cord blood-derived mesenchymal stromal cells. Cytotherapy 2009, 11, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Bieback, K.; Kern, S.; Klüter, H.; Eichler, H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells 2004, 22, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hirai, M.; Cantero, S.; Ciubotariu, R.; Dobrila, L.; Hirsh, A.; Igura, K.; Satoh, H.; Yokomi, I.; Nishimura, T.; et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: Reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J. Cell. Biochem. 2011, 112, 1206–1218. [Google Scholar] [PubMed]
- Solchaga, L.A.; Penick, K.; Goldberg, V.M.; Caplan, A.I.; Welter, J.F. Fibroblast growth factor-2 enhances proliferation and delays loss of chondrogenic potential in human adult bone-marrow-derived mesenchymal stem cells. Tissue Eng. Part A 2010, 16, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Horn, P.; Castoldi, M.; Diehlmann, A.; Bork, S.; Saffrich, R.; Benes, V.; Blake, J.; Pfister, S.; Eckstein, V.; et al. Replicative senescence of mesenchymal stem cells: A continuous and organized process. PLoS ONE 2008, 3, e2213. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Barberini, D.J.; Freitas, N.P.P.; Magnoni, M.S.; Maia, L.; Listoni, A.J.; Heckler, M.C.; Sudano, M.J.; Golim, M.A.; da Cruz Landim-Alvarenga, F.; Amorim, R.M. Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: Immunophenotypic characterization and differentiation potential. Stem Cell Res. Ther. 2014, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- De Schauwer, C.; Piepers, S.; Van de Walle, G.R.; Demeyere, K.; Hoogewijs, M.K.; Govaere, J.L.J.; Braeckmans, K.; Van Soom, A.; Meyer, E. In search for cross-reactivity to immunophenotype equine mesenchymal stromal cells by multicolor flow cytometry. Cytom. Part A 2012, 81, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Vieira, N.M.; Brandalise, V.; Zucconi, E.; Secco, M.; Strauss, B.E.; Zatz, M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010, 19, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; Hack, A.; Weis-Klemm, M.; Conrad, S.; Treml, S.; Kohler, K.; Walliser, U.; Skutella, T.; Aicher, W.K. Evaluation of the osteogenic and chondrogenic differentiation capacities of equine adipose tissue-derived mesenchymal stem cells. Am. J. Vet. Res. 2010, 71, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Hackett, C.H.; Flaminio, M.J.B.F.; Fortier, L.A. Analysis of CD14 Expression levels in putative mesenchymal progenitor cells isolated from equine bone marrow. Stem Cells Dev. 2011, 20, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Iacono, E.; Merlo, B.; Romagnoli, N.; Rossi, B.; Ricci, F.; Spadari, A. Equine bone marrow and adipose tissue mesenchymal stem cells: cytofluorimetric characterization, in vitro differentiation, and clinical application. J. Equine Vet. Sci. 2015, 35, 130–140. [Google Scholar] [CrossRef]
- Kopesky, P.W.; Lee, H.Y.; Vanderploeg, E.J.; Kisiday, J.D.; Frisbie, D.D.; Plaas, A.H.K.; Ortiz, C.; Grodzinsky, A.J. Adult equine bone-marrow stromal cells produce a cartilage-like ECM mechanically superior to animal-matched adult chondrocytes. Matrix Biol. 2010, 29, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Co, C.; Vickaryous, M.K.; Koch, T.G. Membrane culture and reduced oxygen tension enhances cartilage matrix formation from equine cord blood mesenchymal stromal cells in vitro. Osteoarthr. Cartil. 2014, 22, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Ranera, B.; Remacha, A.R.; Álvarez-Arguedas, S.; Castiella, T.; Vázquez, F.J.; Romero, A.; Zaragoza, P.; Martín-Burriel, I.; Rodellar, C. Expansion under hypoxic conditions enhances the chondrogenic potential of equine bone marrow-derived mesenchymal stem cells. Vet. J. 2013, 195, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Kisiday, J.D.; Goodrich, L.R.; McIlwraith, C.W.; Frisbie, D.D. Effects of equine bone marrow aspirate volume on isolation, proliferation, and differentiation potential of mesenchymal stem cells. Am. J. Vet. Res. 2013, 74, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Duval, E.; Leclercq, S.; Elissalde, J.-M.; Demoor, M.; Galera, P.; Boumediene, K. Hypoxia-inducible factor 1α inhibits the fibroblast-like markers type I and type III collagen during hypoxia-induced chondrocyte redifferentiation. Arthritis Rheumatol. 2009, 60, 3038–3048. [Google Scholar] [CrossRef] [PubMed]
- Galera, P.; Rédini, F.; Vivien, D.; Bonaventure, J.; Penfornis, H.; Loyau, G.; Pujol, J.-P. Effect of transforming growth factor-beta 1 (TGF-beta 1) on matrix synthesis by monolayer cultures of rabbit articular chondrocytes during the dedifferentiation process. Exp. Cell Res. 1992, 200, 379–392. [Google Scholar] [CrossRef]
- Yang, B.; Guo, H.; Zhang, Y.; Chen, L.; Ying, D.; Dong, S. MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting SOX9. PLoS ONE 2011, 6, e21679. [Google Scholar] [CrossRef] [PubMed]
- Legendre, F.; Ollitrault, D.; Hervieu, M.; Baugé, C.; Maneix, L.; Goux, D.; Chajra, H.; Mallein-Gerin, F.; Boumediene, K.; Galera, P.; et al. Enhanced hyaline cartilage matrix synthesis in collagen sponge scaffolds by using siRNA to stabilize chondrocytes phenotype cultured with bone morphogenetic protein-2 under hypoxia. Tissue Eng. Part C Methods 2013, 19, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Aubert-Foucher, E.; Mayer, N.; Pasdeloup, M.; Pagnon, A.; Hartmann, D.; Mallein-Gerin, F. A unique tool to selectively detect the chondrogenic IIB form of human type II procollagen protein. Matrix Biol. 2014, 34, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Branly, T.; Contentin, R.; Desancé, M.; Jacquel, T.; Bertoni, L.; Jacquet, T.; Mallein-Gerin, F.; Denoix, J.-M.; Audigié, F.; Demoor, M.; et al. Improvement of the chondrocyte-specific phenotype upon equine bone marrow mesenchymal stem cell differentiation. Influence of culture time, transforming growth factors and type I collagen siRNAs on the differentiation index. Int. J. Mol. Sci. 2018, 19, 435. [Google Scholar] [CrossRef] [PubMed]





















| Antibodies | Isotype | Clone | Flurochrome | Source |
|---|---|---|---|---|
| Anti-CD29 | IgG1, κ | TS2/16 | APC (Allophycocyanin) | BioLegend (San Diego, CA, USA) |
| Anti-CD44 | IgG1, κ | J.173 | PE (Phycoerythrin) | IOTest (Brea, CA, USA) |
| Anti-CD45 | IgG2a | F10-89-4 | Pacific Blue® | AbD Serotec (Kidlington, UK) |
| Anti-CD73 | IgG1 | 10f1 | APC | Abcam (Cambridge, UK) |
| Anti-CD90 | IgM | DH24A | FITC (Fluorescein isothiocyanate) | InvestCare (London, UK) |
| Anti-CD105 | IgG1 | SN6 | FITC | Abcam (Cambridge, UK) |
| Anti-type II MHC | IgG1 | CVS20 | RPE | AbD Serotec (Kidlington, UK) |
| Goat anti-mouse IgG1 | IgG | Polyclonal | APC | Abcam (Cambridge, UK) |
| Goat anti-mouse IgM | IgG | Polyclonal | FITC | AbD Serotec (Kidlington, UK) |
| Genes | Primer Sequence (5′-3′) |
|---|---|
| β-Actin | Foward: AGGCACCAGGGCGTGAT |
| Reverse: CTCTTGCTCTGGGCCTCGT | |
| Col2a1 | Foward: GGCAATAGCAGGTTCACGTACA |
| Reverse: CGATAACAGTCTTGCCCCACTT | |
| Col1a1 | Foward: TGCCGTGACCTCAAGATGTG |
| Reverse: CGTCTCCATGTTGCAGAAGA | |
| Col9a1 | Foward: CCAAGAGGCCCAATCGACAT |
| Reverse: GGGGAAGTCCGTTATCCTGG | |
| HtrA1 | Foward: GGACTTCATGTTTCCCTCAA |
| Reverse: GTTCTGCTGAACAAGCAACA | |
| Acan | Foward: TGTCAACAACAATGCCCAAGAC |
| Reverse: CTTCTTCCGCCCAAAGGTCC | |
| Snorc | Foward: TTTACCAGCTCAGTCCTCGG |
| Reverse: CAGACAGAGAGCCATCCTGG | |
| Col10a1 | Foward: GCACCCCAGTAATGTACACCTATG |
| Reverse: GAGCCACACCTGGTCATTTTC | |
| Osteocalcin (Ostc or BGLAP gene) | Foward: AGAGTCTGGCAGAGGTGCAG |
| Reverse: TCGTCACAGTCTGGGTTGAG | |
| Mmp3 | Foward: GAGGAAATGAGGAACAAGCGG |
| Reverse : GAGGGAAACCCAGAGTGTGGA | |
| Mmp1 | Foward: CGAAGGGAACCCTCGGTGGGA |
| Reverse: TGGCCTGGTCCACATCTGCTC | |
| Mmp13 | Foward: TGAAGACCCGAACCCTAAACAT |
| Reverse: GAAGACTGGTGATGGCATCAAG | |
| Col11a1 | Foward: TTGCTGATGGGAAGTGGCAT |
| Reverse: GCTGCTTTGGGGTCACCTAT | |
| Runx2 | Foward: GCAGTTCCCAAGCATTTCAT |
| Reverse : CACTCTGGCTTTGGGAAGAG | |
| Col1a2 | Foward: CCAGAGTGGAGCAGCGGTTA |
| Reverse: GGGATGTTTTCAGGTTGAGCC | |
| p53 | Foward: CACCTGAGGTTGGCTCTGAC |
| Reverse: GCACAAACACGCACCTCAAA | |
| p21 | Foward: CTTGAAGTGGGCACAGCCTA |
| Reverse: AAGTGCAGAGGAAGCCAACA | |
| Ki67 | Foward: AAGCTGCACGTTCATGGAGA |
| Reverse: ACCCACAGTTCTTCCTCCGA | |
| Pcna | Foward: GCGTGAACCTCACCAGTATGT |
| Reverse: GCAAATTCGCCAGAAGGCAT | |
| Oct4 | Foward: AGTGAGAGGCAACCTGGAGA |
| Reverse: ATACCGGTCCCCCTGAGAAA | |
| Fgf1 | Foward: TTGTACGGCTCACAGACACC |
| Reverse: TTAGTCAGAGGAGACGGGCA | |
| Fgf2 | Foward: CAAACTACAACTTCAAGCAGAAGAGAGA |
| Reverse: CCAGTAACCTTCCATCTTCCTTCAT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desancé, M.; Contentin, R.; Bertoni, L.; Gomez-Leduc, T.; Branly, T.; Jacquet, S.; Betsch, J.-M.; Batho, A.; Legendre, F.; Audigié, F.; et al. Chondrogenic Differentiation of Defined Equine Mesenchymal Stem Cells Derived from Umbilical Cord Blood for Use in Cartilage Repair Therapy. Int. J. Mol. Sci. 2018, 19, 537. https://doi.org/10.3390/ijms19020537
Desancé M, Contentin R, Bertoni L, Gomez-Leduc T, Branly T, Jacquet S, Betsch J-M, Batho A, Legendre F, Audigié F, et al. Chondrogenic Differentiation of Defined Equine Mesenchymal Stem Cells Derived from Umbilical Cord Blood for Use in Cartilage Repair Therapy. International Journal of Molecular Sciences. 2018; 19(2):537. https://doi.org/10.3390/ijms19020537
Chicago/Turabian StyleDesancé, Mélanie, Romain Contentin, Lélia Bertoni, Tangni Gomez-Leduc, Thomas Branly, Sandrine Jacquet, Jean-Marc Betsch, Agnès Batho, Florence Legendre, Fabrice Audigié, and et al. 2018. "Chondrogenic Differentiation of Defined Equine Mesenchymal Stem Cells Derived from Umbilical Cord Blood for Use in Cartilage Repair Therapy" International Journal of Molecular Sciences 19, no. 2: 537. https://doi.org/10.3390/ijms19020537
APA StyleDesancé, M., Contentin, R., Bertoni, L., Gomez-Leduc, T., Branly, T., Jacquet, S., Betsch, J.-M., Batho, A., Legendre, F., Audigié, F., Galéra, P., & Demoor, M. (2018). Chondrogenic Differentiation of Defined Equine Mesenchymal Stem Cells Derived from Umbilical Cord Blood for Use in Cartilage Repair Therapy. International Journal of Molecular Sciences, 19(2), 537. https://doi.org/10.3390/ijms19020537





