Recent Updates on Treatment of Ocular Microbial Infections by Stem Cell Therapy: A Review
Abstract
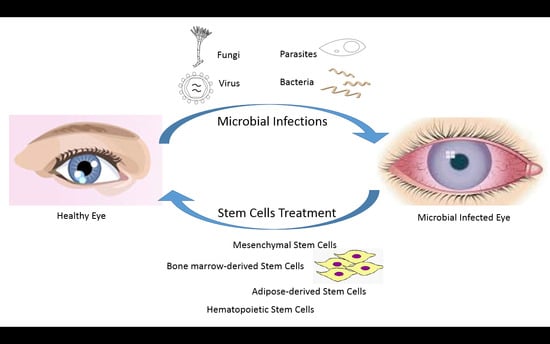
:1. Introduction
2. Challenges of Conventional Antimicrobial Treatments for Ocular Microbial Infections
2.1. Ocular Fungal Infections and the Challenges of Conventional Antifungal Treatment
2.2. Ocular Viral Infections and the Challenges of Conventional Antiviral Treatment
2.3. Ocular Parasitic Infections and the Challenges of Conventional Antiparasitic Treatment
2.4. Ocular Bacterial Infections and the Challenges of Conventional Antibiotics Treatment
3. Stem Cell Therapy
3.1. Direct Microbial Clearance by Stem Cells
3.2. Modulation of Tissue Remodeling by Stem Cells
3.3. Immunomodulatory Effects of Stem Cells
3.4. Tissue Replenishing Property of Stem Cells
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rowland, F.N.; Donovan, M.J.; Lindsay, M.; Weiss, W.I.; O’rourke, J.; Kreutzer, D.L. Demonstration of inflammatory mediator-induced inflammation and endothelial cell damage in the anterior segment of the eye. Am. J. Pathol. 1983, 110, 1–12. [Google Scholar] [PubMed]
- Garg, P. Fungal, Mycobacterial and Nocardia infections and the eye: An update. Eye 2012, 26, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.J.; Alvarado, J.A.; Weddell, J.E. Histology of the Human Eye: An Atlas and Textbook; WB Saunders Co.: Philadelphia, PA, USA, 1971; p. 35. [Google Scholar]
- Yu, D.Y.; Paula, K.Y.; Cringle, S.J.; Kang, M.H.; Su, E.N. Functional and morphological characteristics of the retinal and choroidal vasculature. Prog. Retin. Eye Res. 2014, 40, 53–93. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M. Fibrosis and diseases of the eye. J. Clin. Investig. 2007, 117, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.F.; Rai, M.K.; Lowder, C.Y.; Adal, K.A. Endogenous endophthalmitis: Case report and brief review. Am. Fam. Physician 1999, 60, 510–523. [Google Scholar] [PubMed]
- Breit, S.M.; Hariprasad, S.M.; Mieler, W.F.; Shah, G.K.; Mills, M.D.; Grand, M.G. Management of endogenous fungal endophthalmitis with voriconazole and caspofungin. Am. J. Ophthalmol. 2005, 139, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.P.; Kuo, H.K.; Tsai, S.H.; Chen, Y.J.; Kao, M.L. Acute retinal necrosis syndrome: Clinical manifestations and visual outcomes. Chang Gung Med. J. 2004, 27, 193–200. [Google Scholar] [PubMed]
- Egbert, P.R.; Pollard, R.B.; Gallagher, J.G.; Merigan, T.C. Cytomegalovirus retinitis in immunosuppressed hosts: II. Ocular manifestations. Ann. Intern. Med. 1980, 93, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Callegan, M.C.; Jett, B.D.; Hancock, L.E.; Gilmore, M.S. Role of hemolysin BL in the pathogenesis of extraintestinal Bacillus cereus infection as assessed using an endophthalmitis model. Infect. Immun. 1999, 67, 3357–3366. [Google Scholar] [PubMed]
- Leonardi, A. Vernal keratoconjunctivitis: Pathogenesis and treatment. Prog. Retin. Eye Res. 2002, 21, 319–339. [Google Scholar] [CrossRef]
- Vonk, A.G.; Netea, M.G.; van Krieken, J.H.; Iwakura, Y.; van der Meer, J.W.; Kullberg, B.J. Endogenous interleukin (IL)-1α and IL-1β are crucial for host defense against disseminated candidiasis. J. Infect. Dis. 2006, 193, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Van de Veerdonk, F.L.; Marijnissen, R.J.; Kullberg, B.J.; Koenen, H.J.; Cheng, S.C.; Joosten, I.; van den Berg, W.B.; Williams, D.L.; van der Meer, J.W.; Joosten, L.A.; et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe 2009, 5, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Doerr, H.W.; Cinatl, J. Human cytomegalovirus retinitis: Pathogenicity, immune evasion and persistence. Trends Microbiol. 2003, 11, 171–178. [Google Scholar] [CrossRef]
- Tosh, K.W.; Mittereder, L.; Bonne-Annee, S.; Hieny, S.; Nutman, T.B.; Singer, S.M.; Sher, A.; Jankovic, D. The IL-12 response of primary human dendritic cells and monocytes to Toxoplasma gondii is stimulated by phagocytosis of live parasites rather than host cell invasion. J. Immunol. 2016, 196, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Callegan, M.C.; Engelbert, M.; Parke, D.W.; Jett, B.D.; Gilmore, M.S. Bacterial endophthalmitis: Epidemiology, therapeutics and bacterium-host interactions. Clin. Microbiol. Rev. 2002, 15, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Robson, R.T.; Smith, D.J. Wounds and wound healing. In Essentials of Genereal Surgery; Lawrence, P.F., Bell, R.M., Dayton, M.T., Eds.; Williams and Wilkins: Philadelphia, PA, USA, 1992; pp. 119–125. [Google Scholar]
- Desmouliere, A.; Darby, I.A.; Gabbiani, G. Normal and pathologic soft tissue remodeling: Role of the myofibroblast, with special emphasis on liver and kidney fibrosis. Lab. Investig. 2003, 83, 1689–1707. [Google Scholar] [CrossRef] [PubMed]
- Pilling, D.; Fan, T.; Huang, D.; Kaul, B.; Gomer, R.H. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages and fibroblasts. PLoS ONE 2009, 4, e7475. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Pavesio, C.E.; Laidlaw, D.A.; Williamson, T.H.; Graham, E.M.; Stanford, M.R. Acute retinal necrosis: The effects of intravitreal foscarnet and virus type on outcome. Ophthalmology 2010, 117, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Schwetz, B.A. Safety of aristolochic acid. JAMA 2001, 285, 2705. [Google Scholar] [CrossRef] [PubMed]
- Kempen, J.H.; Min, Y.I.; Freeman, W.R.; Holland, G.N.; Friedberg, D.N.; Dieterich, D.T.; Jabs, D.A.; Studies of Ocular Complications of AIDS Research Group. Risk of immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis. Ophthalmology 2006, 113, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Azen, S.P.; Buley, A.; Torriani, F.; Cheng, L.; Chaidhawangul, S.; Ozerdem, U.; Scholz, B.; Freeman, W.R. Effect of anti-cytomegalovirus therapy on the incidence of immune recovery uveitis in AIDS patients with healed cytomegalovirus retinitis. Am. J. Ophthalmol. 2003, 136, 696–702. [Google Scholar] [CrossRef]
- Perron, M.; Harris, W.A. Retinal stem cells in vertebrates. Bioessays 2000, 22, 685–688. [Google Scholar] [CrossRef]
- Ahuja, A.K.; Dorn, J.D.; Caspi, A.; McMahon, M.J.; Dagnelie, G.; Stanga, P.; Humayun, M.S.; Greenberg, R.J.; Argus II Study Group. Blind subjects implanted with the Argus II retinal prosthesis are able to improve performance in a spatial-motor task. Br. J. Ophthalmol. 2011, 95, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.H.; Haitsma, J.J.; Dos Santos, C.C.; Deng, Y.; Lai, P.F.; Slutsky, A.S.; Liles, W.C.; Stewart, D.J. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am. J. Respir. Crit. Care Med. 2010, 182, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Fang, X.; Gupta, N.; Serikov, V.; Matthay, M.A. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc. Natl. Acad. Sci. USA 2009, 106, 16357–16362. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Feng, X.M.; Abbott, J.; Fang, X.H.; Hao, Q.; Monsel, A.; Qu, J.M.; Matthay, M.A.; Lee, J.W. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 2014, 32, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Krasnodembskaya, A.; Samarani, G.; Song, Y.; Zhuo, H.; Su, X.; Lee, J.W.; Gupta, N.; Petrini, M.; Matthay, M.A. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L1003–L1013. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.; Fortino, V.R.; Pelaez, D.; Cheung, H.S. Progress of mesenchymal stem cell therapy for neural and retinal diseases. World J. Stem Cells 2014, 6, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Vossmerbaeumer, U.; Ohnesorge, S.; Kuehl, S.; Haapalahti, M.; Kluter, H.; Jonas, J.B.; Thierse, H.J.; Bieback, K. Retinal pigment epithelial phenotype induced in human adipose tissue derived mesenchymal stromal cells. Cytotherapy 2009, 11, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Cui, L.; Qu, Z.; Lu, L.; Wang, F.; Wu, Y.; Zhang, J.; Gao, F.; Tian, H.; Xu, L.; et al. Subretinal transplantation of rat MSCs and erythropoietin gene modified rat MSCs for protecting and rescuing degenerative retina in rats. Curr. Mol. Med. 2013, 13, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Narendran, N.; Balasubramaniam, B.; Johnson, E.; Dick, A.; Mayer, E. Five-year retrospective review of guideline-based management of fungal endophthalmitis. Acta Ophthalmol. 2008, 86, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Stanescu, D.; Caspers-Velu, L.; Rozenberg, F.; Liesnard, C.; Gaudric, A.; Lehoang, P.; Bodaghi, B. Clinical characteristics of acute HSV-2 retinal necrosis. Am. J. Ophthalmol. 2004, 137, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Culbertson, W.W.; Blumenkranz, M.S.; Pepose, J.S.; Stewart, J.A.; Curtin, V.T. Varicella zoster virus is a cause of the acute retinal necrosis syndrome. Ophthalmology 1986, 93, 559–569. [Google Scholar] [CrossRef]
- Sorr, E.M. Meandering ocular toxocariasis. Retina 1984, 4, 90–96. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, G.R. Chemotherapy of toxoplasmosis and toxocariasis. Ocul. Ther. 1980, 51–57. [Google Scholar]
- Frazier, M.; Anderson, M.L.; Sophocleous, S. Treatment of ocular toxocariasis with albendezole: A case report. Optom. J. Am. Optom. Assoc. 2009, 80, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.Y.; Liu, P.Y.; Shi, Z.Y.; Lau, Y.J.; Hu, B.S.; Shyr, J.M.; Tsai, W.S.; Lin, Y.H. Endogenous endophthalmitis due to Escherichia coli: Case report and review. Clin. Infect. Dis. 1996, 22, 1107–1108. [Google Scholar] [CrossRef] [PubMed]
- Pollreisz, A.; Rafferty, B.; Kozarov, E.; Lalla, E. Klebsiella pneumoniae induces an inflammatory response in human retinal-pigmented epithelial cells. Biochem. Biophys. Res. Commun. 2012, 418, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.F.; Mangiaracine, A.B. Bacterial endophthalmitis after cataract extraction: A study of 22 infections in 20,000 operations. Arch. Ophthalmol. 1964, 72, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Montan, P.G.; Koranyi, G.; Setterquist, H.E.; Stridh, A.; Philipson, B.T.; Wiklund, K. Endophthalmitis after cataract surgery: Risk factors relating to technique and events of the operation and patient history: A retrospective case-control study. Ophthalmology 1998, 105, 2171–2177. [Google Scholar] [CrossRef]
- Forster, R.K. Experimental postoperative endophthalmitis. Trans. Am. Ophthalmol. Soc. 1992, 90, 505–559. [Google Scholar] [PubMed]
- Aaberg, T.M.; Flynn, H.W.; Schiffman, J.; Newton, J. Nosocomial acute-onset postoperative endophthalmitis survey: A 10-year review of incidence and outcomes. Ophthalmology 1998, 105, 1004–1010. [Google Scholar] [CrossRef]
- Endophthalmitis Vitrectomy Study Group. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch. Ophthalmol. 1995, 113, 1479–1496. [Google Scholar]
- Ferencz, J.R.; Assia, E.I.; Diamantstein, L.; Rubinstein, E. Vancomycin concentration in the vitreous after intravenous and intravitreal administration for postoperative endophthalmitis. Arch. Ophthalmol. 1999, 117, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Speaker, M.G.; Milch, F.A.; Shah, M.K.; Eisner, W.; Kreiswirth, B.N. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology 1991, 98, 639–650. [Google Scholar] [CrossRef]
- Bains, H.S.; Weinberg, D.V.; Feder, R.S.; Noskin, G.A. Postoperative vancomycin-resistant Enterococcus faecium endophthalmitis. Arch. Ophthalmol. 2007, 125, 1292–1293. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, N.; Li, X.; Zarbin, M.A. Post-traumatic Endophthalmitis. In Endophthalmitis; Springer International Publishing: Cham, Switzerland, 2016; pp. 151–170. [Google Scholar]
- Rishi, E.; Rishi, P.; Koundanya, V.V.; Sahu, C.; Roy, R.; Bhende, P.S. Post-traumatic endophthalmitis in 143 eyes of children and adolescents from India. Eye 2016, 30, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Hiraoka, T.; Kaji, Y.; Sakata, N.; Motoyama, Y.; Oshika, T. Successful treatment of endogenous Klebsiella pneumoniae endophthalmitis: A case report. Int. Ophthalmol. 2011, 31, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, M.J.; Wohl, L.G.; Sell, C.H. Metastatic bacterial endophthalmitis: A comtemporary reappraisal. Surv. Ophthalmol. 1986, 31, 81–101. [Google Scholar] [CrossRef]
- Esmaeli, B.; Holz, E.R.; Ahmadi, M.A.; Krathen, R.A.; Raad, I.I. Endogenous endophthalmitis secondary to vancomycin-resistant enterococci infection. Retina 2003, 23, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.R.; Devenyi, R.G.; Berger, A.R.; Dunn, W. Visual outcome following penetrating globe injuries with retained intraocular foreign bodies. Can. J. Ophthalmol. 1999, 34, 389–393. [Google Scholar] [PubMed]
- Maertzdorf, J.; Van Der Lelij, A.; Baarsma, G.S.; Osterhaus, A.D.; Verjans, G.M. Herpes simplex virus type 1 (HSV-1)–induced retinitis following herpes simplex encephalitis: Indications for brain-to-eye transmission of HSV-1. Ann. Neurol. 2001, 49, 104–106. [Google Scholar] [CrossRef]
- Yusuf, I.H.; Sipkova, Z.; Patel, S.; Benjamin, L. Neisseria meningitidis endogenous endophthalmitis with meningitis in an immunocompetent child. Ocul. Immunol. Inflamm. 2014, 22, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Jabs, D.A.; Studies of Ocular Complications of AIDS Research Group in collaboration with the AIDS Clinical Trials Group. Mortality in patients with the acquired immunodeficiency syndrome treated with either foscarnet or ganciclovir for cytomegalovirus retinitis. N. Engl. J. Med. 1992, 326, 213–220. [Google Scholar]
- Browning, D.J. Posterior segment manifestations of active ocular syphilis, their response to a neurosyphilis regimen of penicillin therapy and the influence of human immunodeficiency virus status on response. Ophthalmology 2000, 107, 2015–2023. [Google Scholar] [CrossRef]
- Pepose, J.S.; Holland, G.N.; Nestor, M.S.; Cochran, A.J.; Foos, R.Y. Acquired immune deficiency syndrome: Pathogenic mechanisms of ocular disease. Ophthalmology 1985, 92, 472–484. [Google Scholar] [CrossRef]
- Weledji, E.P. Intestinal colic. Int. J. Med. Biol. Front. 2014, 20, 231. [Google Scholar]
- Mannis, M.J.; Smolin, G. Natural defense mechanism of the ocular surface. In Ocular Infection and Immunity; Pepose, J.S., Holland, G.N., Wilhelmeus, K.R., Eds.; Mosby: St Louis, MO, USA, 1996; pp. 185–190. [Google Scholar]
- Tibbetts, M.D.; Shah, C.P.; Young, L.H.; Duker, J.S.; Maguire, J.I.; Morley, M.G. Treatment of acute retinal necrosis. Ophthalmology 2010, 117, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Karavellas, M.P.; Lowder, C.Y.; Macdonald, J.C.; Avila, C.P.; Freeman, W.R. Immune recovery vitritis associated with inactive cytomegalovirus retinitis: A new syndrome. Arch. Ophthalmol. 1998, 116, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Specht, C.S.; Mitchell, K.T.; Bauman, A.E.; Gupta, M. Ocular histoplasmosis with retinitis in a patient with Acquired Immune Deficiency Syndrome. Ophthalmology 1991, 98, 1356–1359. [Google Scholar] [CrossRef]
- Kuruvilla, A. Ocular tuberculosis. Lancet 2003, 361, 260–261. [Google Scholar] [CrossRef]
- Psomas, K.C.; Brun, M.; Causse, A.; Atoui, N.; Reynes, J.; Le Moing, V. Efficacy of ceftriaxone and doxycycline in the treatment of early syphilis. Méd. Mal. Infect. 2012, 42, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Muthiah, M.N.; Michaelides, M.; Child, C.S.; Mitchell, S.M. Acute retinal necrosis: A national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. Br. J. Ophthalmol. 2007, 91, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.E.; Martinez, J.A.; Murray, T.G.; Rubsamen, P.E.; Flynn, H.W.; Forster, R.K. Useful visual outcomes after treatment of Bacillus cereus endophthalmitis. Ophthalmology 1996, 103, 390–397. [Google Scholar] [CrossRef]
- Aguilar, H.E.; Meredith, T.A.; Drews, C.; Grossniklaus, H.; Sawant, A.D.; Gardner, S. Comparative treatment of experimental Staphylococcus aureus endophthalmitis. Am. J. Ophthalmol. 1996, 121, 310–317. [Google Scholar] [CrossRef]
- Kresloff, M.S.; Castellarin, A.A.; Zarbin, M.A. Endophthalmitis. Surv. Ophthalmol. 1998, 43, 193–224. [Google Scholar] [CrossRef]
- Mittra, R.A.; Mieler, W.F. Controversies in the management of open-globe injuries involving the posterior segment. Surv. Ophthalmol. 1999, 44, 215–225. [Google Scholar] [CrossRef]
- Streilein, J.W. Immunoregulatory mechanisms of the eye. Prog. Retin. Eye Res. 1999, 18, 357–370. [Google Scholar] [CrossRef]
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.A. Endothelial vesicles in the blood–brain barrier: Are they related to permeability? Cell. Mol. Neurobiol. 2000, 20, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.W.; Doft, B.H.; Kelsey, S.F.; Barza, M.; Wilson, L.A.; Barr, C.C.; Wisniewski, S.R.; Vine, A.K.; Blodi, B.A.; Elner, S.G.; et al. The endophthalmitis vitrectomy study: Relationship between clinical presentation and microbiologic spectrum. Ophthalmology 1997, 104, 261–272. [Google Scholar] [CrossRef]
- Okhravi, N.; Towler, H.M.; Hykin, P.; Matheson, M.; Lightman, S. Assessment of a standard treatment protocol on visual outcome following presumed bacterial endophthalmitis. Br. J. Ophthalmol. 1997, 81, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.E.; See, S.E.; Jones, L.V.; Stanford, M.S. Antibiotics versus control for toxoplasma retinochoroiditis. Cochrane Database Syst. Rev. 2002, 1, CD002218. [Google Scholar]
- Wiechens, B.; Neumann, D.; Grammer, J.B.; Pleyer, U.; Hedderich, J.; Duncker, G.I. Retinal toxicity of liposome-incorporated and free ofloxacin after intravitreal injection in rabbit eyes. Int. Ophthalmol. 1998, 22, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Lim, J.I. Aminoglycoside toxicity in the treatment of endophthalmitis. Arch. Ophthalmol. 1994, 112, 48–53. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, D.J.; Caspers-Velu, L.; Libert, J.; Shanks, E.; Schrooyen, M.; Hanninen, L.A.; Kenyon, K.R. Comparative toxicity of intravitreal aminoglycoside antibiotics. Am. J. Ophthalmol. 1985, 100, 264–275. [Google Scholar] [CrossRef]
- Baum, U.; Peyman, G.A.; Barza, M. Intravitreal administration of antibiotic in the treatment of bacterial endophthalmitis. III. Consensus. Surv. Ophthalmol. 1982, 26, 204–206. [Google Scholar] [CrossRef]
- Brod, R.D.; Flynn, H.W., Jr. Endophthalmitis: Current approaches to diagnosis and therapy. Curr. Opin. Infect. Dis. 1993, 6, 628–637. [Google Scholar] [CrossRef]
- Vahey, J.B.; Flynn, H.W. Results in the management of Bacillus endophthalmitis. Ophthalmic Surg. Lasers Imaging Retin. 1991, 22, 681–686. [Google Scholar]
- Edwards, J.E.; Lehrer, R.I.; Stiehm, E.R.; Fischer, T.J.; Young, L.S. Severe candidal infections: Clinical perspective, immune defense mechanisms and current concepts of therapy. Ann. Intern. Med. 1978, 89, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Medoff, G.; Kobayashi, G.S. Strategies in the treatment of systemic fungal infections. N. Engl. J. Med. 1980, 302, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Lalezari, J.P.; Stagg, R.J.; Kuppermann, B.D.; Holland, G.N.; Kramer, F.; Ives, D.V.; Youle, M.; Robinson, M.R.; Drew, W.L.; Jaffe, H.S. Intravenous cidofovir for peripheral cytomegalovirus retinitis in patients with AIDS: A randomized, controlled trial. Ann. Intern. Med. 1997, 126, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Pennesi, M.E.; Shah, K.; Qiao, X.; Hariprasad, S.M.; Mieler, W.F.; Wu, S.M.; Holz, E.R. Intravitreal voriconazole: An electroretinographic and histopathologic study. Arch. Ophthalmol. 2004, 122, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Hariprasad, S.M.; Mieler, W.F.; Lin, T.K.; Sponsel, W.E.; Graybill, J.R. Voriconazole in the treatment of fungal eye infections: A review of current literature. Br. J. Ophthalmol. 2008, 92, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Grillone, L.R.; Lanz, R. Fomivirsen. Drugs Today 2001, 37, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Aslanides, I.M.; De Souza, S.; Wong, D.T.; Giavedoni, L.R.; Altomare, F.; Detorakis, E.T.; Kymionis, G.D.; Pallikaris, I.G. Oral valacyclovir in the treatment of acute retinal necrosis syndrome. Retina 2002, 22, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.S.; Garabito, I.; Gutierrez, C.; Fortun, J. Famciclovir for the treatment of acute retinal necrosis (ARN) syndrome. Am. J. Ophthalmol. 1997, 123, 255–257. [Google Scholar] [CrossRef]
- Stürchler, D.; Schubarth, P.; Gualzata, M.; Gottstein, B.; Oettli, A. Thiabendazole vs. albendazole in treatment of toxocariasis: A clinical trial. Ann. Trop. Med. Parasitol. 1989, 83, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Soheilian, M.; Sadoughi, M.M.; Ghajarnia, M.; Dehghan, M.H.; Yazdani, S.; Behboudi, H.; Anisian, A.; Peyman, G.A. Prospective randomized trial of trimethoprim/sulfamethoxazole versus pyrimethamine and sulfadiazine in the treatment of ocular toxoplasmosis. Ophthalmology 2005, 112, 1876–1882. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Driessen, L.H.; Verbraak, F.D.; Suttorp-Schulten, M.S.; van Ruyven, R.L.; Klok, A.M.; Hoyng, C.B.; Rothova, A. A prospective, randomized trial of pyrimethamine and azithromycin vs pyrimethamine and sulfadiazine for the treatment of ocular toxoplasmosis. Am. J. Ophthalmol. 2002, 134, 34–40. [Google Scholar] [CrossRef]
- Rothova, A.; Meenken, C.; Buitenhuis, H.J.; Brinkman, C.J.; Baarsma, G.S.; Boen-Tan, T.N.; De Jong, P.T.; Klaassen-Broekema, N.; Schweitzer, C.M.; Timmerman, Z.; et al. Therapy for ocular toxoplasmosis. Am. J. Ophthalmol. 1993, 115, 517–523. [Google Scholar] [CrossRef]
- Han, D.P.; Wisniewski, S.R.; Wilson, L.A.; Barza, M.; Vine, A.K.; Doft, B.H.; Kelsey, S.F.; Endophthalmitis Vitrectomy Study Group. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am. J. Ophthalmol. 1996, 122, 1–7. [Google Scholar] [CrossRef]
- Kiss, S.; Damico, F.M.; Young, L.H. Ocular Manifestations and Treatment of Syphilis. Semin. Ophthalmol. 2009, 20, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Puech, C.; Gennai, S.; Pavese, P.; Pelloux, I.; Maurin, M.; Romanet, J.; Chiquet, C. Ocular manifestations of syphilis: Recent cases over a 2.5-year period. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Vrioni, G.; Levidiotou, S.; Matsiota-Bernard, P.; Marinis, E. Molecular characterization of Mycobacterium tuberculosis isolates presenting various drug susceptibility profiles from Greece using three DNA typing methods. J. Infect. 2004, 48, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Moyes, D.L.; Wilson, D.; Richardson, J.P.; Mogavero, S.; Tang, S.X.; Wernecke, J.; Höfs, S.; Gratacap, R.L.; Robbins, J.; Runglall, M.; et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 2016, 532, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Savani, D.V.; Perfect, J.R.; Cobo, L.M.; Durack, D.T. Penetration of new azole compounds into the eye and efficacy in experimental Candida endophthalmitis. Antimicrob. Agents Chemother. 1987, 31, 6–10. [Google Scholar] [CrossRef] [PubMed]
- O’Day, D.M.; Foulds, G.; Williams, T.E.; Robinson, R.D.; Allen, R.H.; Head, W.S. Ocular uptake of fluconazole following oral administration. Arch. Ophthalmol. 1990, 108, 1006–1008. [Google Scholar] [CrossRef] [PubMed]
- Barza, M. Treatment options for candidal endophthalmitis. Clin. Infect. Dis. 1998, 27, 1134–1136. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.A.; Zhang, J.; Ishimoto, S. Role of retinal vascular endothelial cells in development of CMV retinitis. Trans. Am. Ophthalmol. Soc. 1998, 96, 111–126. [Google Scholar] [PubMed]
- Cinatl, J.; Blaheta, R.; Bittoova, M.; Scholz, M.; Margraf, S.; Vogel, J.; Cinatl, J.; Doerr, H.W. Decreased neutrophil adhesion to human cytomegalovirus-infected retinal pigment epithelial cells is mediated by virus-induced up-regulation of fas ligand independent of neutrophil apoptosis. J. Immunol. 2000, 165, 4405–4413. [Google Scholar] [CrossRef] [PubMed]
- Holland, G.N. The progressive outer retinal necrosis syndrome. Int. Ophthalmol. 1994, 18, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Forster, D.J.; Dugel, P.U.; Frangieh, G.T.; Liggett, P.E.; Rao, N.A. Rapidly progressive outer retinal necrosis in the acquired immunodeficiency syndrome. Am. J. Ophthalmol. 1990, 110, 341–348. [Google Scholar] [CrossRef]
- Roig-Melo, E.A.; Macky, T.A.; Heredia-Elizondo, M.L.; Alfaro, D.V. Progressive outer retinal necrosis syndrome: Successful treatment with a new combination of antiviral drugs. Eur. J. Ophthalmol. 2001, 11, 200–202. [Google Scholar] [PubMed]
- Kim, S.J.; Equi, R.; Belair, M.L.; Fine, H.F.; Dunn, J.P. Long-term preservation of vision in progressive outer retinal necrosis treated with combination antiviral drugs and highly active antiretroviral therapy. Ocul. Immunol. Inflamm. 2007, 15, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.; Boivin, G. Resistance of herpes simplex viruses to nucleoside analogues: Mechanisms, prevalence and management. Antimicrob. Agents Chemother. 2011, 55, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Iwase, T.; Jo, Y.J.; Oveson, B.C. Effect of prophylactic 360 laser treatment for prevention of retinal detachment after phacovitrectomy: (Prophylactic 360 laser treatment for prevention of retinal detachment). BMC Ophthalmol. 2013, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.H.; Missotten, T.; Salzmann, J.; Lightman, S.L. Acute retinal necrosis: Features, management and outcomes. Ophthalmology 2007, 114, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Spencer, D.B.; Mochizuki, M. Therapy for acute retinal necrosis. Semin. Ophthalmol. 2008, 23, 285–290. [Google Scholar] [CrossRef] [PubMed]
- McDonald, H.R.; Lewis, H.; Kreiger, A.E.; Sidikaro, Y.; Heckenlively, J. Surgical management of retinal detachment associated with the acute retinal necrosis syndrome. Br. J. Ophthalmol. 1991, 75, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Jaccoma, E.H.; Conway, B.P.; Campochiaro, P.A. Cryotherapy causes extensive breakdown of the blood-retinal barrier: A comparison with argon laser photocoagulation. Arch. Ophthalmol. 1985, 103, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Baquera-Heredia, J.; Cruz-Reyes, A.; Flores-Gaxiola, A.; López-Pulido, G.; Díaz-Simental, E.; Valderrama-Valenzuela, L. Case report: Ocular gnathostomiasis in northwestern Mexico. Am. J. Trop. Med. Hyg. 2002, 66, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Driessen, L.E.; Berendschot, T.T.; Ongkosuwito, J.V.; Rothova, A. Ocular toxoplasmosis: Clinical features and prognosis of 154 patients. Ophthalmology 2002, 109, 869–878. [Google Scholar] [CrossRef]
- Shen, D.F.; Matteson, D.M.; Tuaillon, N.; Suedekum, B.K.; Buggage, R.R.; Chan, C.C. Involvement of apoptosis and interferon-gamma in murine toxoplasmosis. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2031–2036. [Google Scholar]
- Hu, M.S.; Schwartzman, J.D.; Yeaman, G.R.; Collins, J.; Seguin, R.; Khan, I.A.; Kasper, L.H. Fas-FasL interaction involved in pathogenesis of ocular toxoplasmosis in mice. Infect. Immun. 1999, 67, 928–935. [Google Scholar] [PubMed]
- Endophthalmitis Vitrectomy Study Group. Microbiologic factors and visual outcome in the Endophthalmitis Vitrectomy Study. Am. J. Ophthalmol. 1996, 122, 830–846. [Google Scholar]
- Ramadan, R.T.; Ramirez, R.; Novosad, B.D.; Callegan, M.C. Acute inflammation and loss of retinal architecture and function during experimental Bacillus endophthalmitis. Curr. Eye Res. 2006, 31, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Jett, B.D.; Jensen, H.G.; Nordquist, R.E.; Gilmore, M.S. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect. Immun. 1992, 60, 2445–2452. [Google Scholar] [PubMed]
- Ng, E.W.; Samiy, N.; Rubins, J.B.; Cousins, F.V.; Ruoff, K.L.; Baker, A.S.; D’Amico, D.J. Implication of pneumolysin as a virulence factor in Streptococcus pneumonia endophthalmitis. Retina 1997, 17, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Parkunan, S.M.; Astley, R.; Callegan, M.C. Role of TLR5 and flagella in Bacillus intraocular infection. PLoS ONE 2014, 9, e100543. [Google Scholar] [CrossRef] [PubMed]
- Flores-Díaz, M.; Monturiol-Gross, L.; Naylor, C.; Alape-Girón, A.; Flieger, A. Bacterial sphingomyelinases and phospholipases as virulence factors. Microbiol. Mol. Biol. Rev. 2016, 80, 597–628. [Google Scholar] [CrossRef] [PubMed]
- Jeßberger, N.; Dietrich, R.; Bock, S.; Didier, A.; Märtlbauer, E. Bacillus cereus enterotoxins act as major virulence factors and exhibit distinct cytotoxicity to different human cell lines. Toxicon 2014, 77, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Omer, H.; Alpha-Bazin, B.; Brunet, J.L.; Armengaud, J.; Duport, C. Proteomics identifies Bacillus cereus EntD as a pivotal protein for the production of numerous virulence factors. Front. Microbiol. 2015, 6, 1004. [Google Scholar] [CrossRef] [PubMed]
- Mingeot-Leclercq, M.P.; Tulkens, P.M. Aminoglycosides: Nephrotoxicity. Antimicrob. Agents Chemother. 1999, 43, 1003–1012. [Google Scholar] [PubMed]
- Xie, Y.; Xu, M.; Xiao, Y.; Liu, Z.; Jiang, C.; Kuang, X.; Wang, C.; Wu, H.; Peng, J.; Li, C.; et al. Treponema pallidum flagellin FlaA2 induces IL-6 secretion in THP-1 cells via the Toll-like receptor 2 signaling pathway. Mol. Immunol. 2017, 81, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Radolf, J.D.; Norgard, M.V.; Brandt, M.E.; Isaacs, R.D.; Thompson, P.A.; Beutler, B. Lipoproteins of Borrelia burgdorferi and Treponema pallidum activate cachectin/tumor necrosis factor synthesis. Analysis using a CAT reporter construct. J. Immunol. 1991, 147, 1968–1974. [Google Scholar] [PubMed]
- Garhyan, J.; Bhuyan, S.; Pulu, I.; Kalita, D.; Das, B.; Bhatnagar, R. Preclinical and clinical evidence of Mycobacterium tuberculosis persistence in the hypoxic niche of bone marrow mesenchymal stem cells after therapy. Am. J. Pathol. 2015, 185, 1924–1934. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.P.; Sakinah, S.; Sharmilah, K.; Hamat, R.A.; Sekawi, Z.; Higuchi, A.; Ling, M.P.; Nordin, S.A.; Benelli, G.; Kumar, S.S. Leptospirosis: Molecular trial path and immunopathogenesis correlated with dengue, malaria and mimetic hemorrhagic infections. Acta Trop. 2017, 176, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Cordero, A.; West, E.L.; Pearson, R.A.; Duran, Y.; Carvalho, L.S.; Chu, C.J.; Naeem, A.; Blackford, S.J.; Georgiadis, A.; Lakowski, J.; et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat. Biotechnol. 2013, 31, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Kumar, S.S.; Benelli, G.; Alarfaj, A.A.; Munusamy, M.A.; Umezawa, A.; Murugan, K. Stem cell therapies for reversing vision loss. Trends Biotechnol. 2017, 35, 1102–1117. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.S.; Leow, S.N.; Munisvaradass, R.; Koh, E.H.; Bastion, M.L.; Then, K.Y.; Kumar, S.; Mok, P.L. Revisiting the role of erythropoietin for treatment of ocular disorders. Eye 2016, 30, 1293–1309. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.L.; Kumar, S.; Mok, P.L. Cellular reparative mechanisms of mesenchymal stem cells for retinal diseases. Int. J. Mol. Sci. 2017, 18, 1406. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.P.; Higuchi, A.; Fanas, S.A.; Ling, M.P.; Neela, V.K.; Sunil, P.M.; Saraswathi, T.R.; Murugan, K.; Alarfaj, A.A.; Munusamy, M.A.; et al. Odontogenic epithelial stem cells: Hidden sources. Lab. Investig. 2015, 95, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.F.; Sandstedt, B.; Sørensen, O.; Weber, G.; Borregaard, N.; Ståhle-Bäckdahl, M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect. Immun. 1999, 67, 2561–2566. [Google Scholar]
- Nijnik, A.; Hancock, R.E. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr. Opin. Hematol. 2009, 16, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, S.B.; Marini, F.C.; Watson, K.; Zwezdaryk, K.J.; Dembinski, J.L.; LaMarca, H.L.; Tomchuck, S.L.; Zu Bentrup, K.H.; Danka, E.S.; Henkle, S.L.; et al. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3806–3811. [Google Scholar] [CrossRef] [PubMed]
- Gordon, Y.; Huang, L.; Romanowski, E.G.; Yates, K.A.; Proske, R.J.; McDermott, A.M. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr. Eye Res. 2005, 30, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Lo’pez-García, B.; Lee, P.; Yamasaki, K.; Gallo, R.L. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J. Investig. Dermatol. 2005, 125, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Cirioni, O.; Giacometti, A.; Ghiselli, R.; Bergnach, C.; Orlando, F.; Silvestri, C.; Mocchegiani, F.; Licci, A.; Skerlavaj, B.; Rocchi, M.; et al. LL-37 protects rats against lethal sepsis caused by gram-negative bacteria. Antimicrob. Agents Chemother. 2006, 50, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2–dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, E.; González, M.A.; Rico, L.; Büscher, D.; Delgado, M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 2009, 58, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Krasnodembskaya, A.; Song, Y.; Fang, X.; Gupta, N.; Serikov, V.; Lee, J.W.; Matthay, M.A. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 2010, 28, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, R.; Zanetti, M. Structural features and biological activities of the cathelicidinderived antimicrobial peptides. Biopolymers 2000, 55, 31–49. [Google Scholar] [CrossRef]
- Turner, J.; Cho, Y.; Dinh, N.N.; Waring, A.J.; Lehrer, R.I. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 1998, 42, 2206–2214. [Google Scholar] [PubMed]
- Brandenburg, L.O.; Varoga, D.; Nicolaeva, N.; Leib, S.L.; Podschun, R.; Wruck, C.J.; Wilms, H.; Lucius, R.; Pufe, T. Expression and regulation of antimicrobial peptide rCRAMP after bacterial infection in primary rat meningeal cells. J. Neuroimmunol. 2009, 217, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, L.O.; Varoga, D.; Nicolaeva, N.; Leib, S.L.; Wilms, H.; Podschun, R.; Wruck, C.J.; Schröder, J.M.; Pufe, T.; Lucius, R. Role of glial cells in the functional expression of the antimicrobial peptide LL-37/rCRAMP in meningitis. J. Neuropathol. Exp. Neurol. 2008, 67, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.J.; Bertoncello, I.; Borok, Z.; Kim, C.; Panoskaltsis-Mortari, A.; Reynolds, S.; Rojas, M.; Stripp, B.; Warburton, D.; Prockop, D.J. Stem cells and cell therapies in lung biology and lung diseases. Proc. Am. Thorac. Soc. 2011, 8, 223–272. [Google Scholar] [CrossRef] [PubMed]
- Meisel, R.; Brockers, S.; Heseler, K.; Degistirici, O.; Bülle, H.; Woite, C.; Stuhlsatz, S.; Schwippert, W.; Jäger, M.; Sorg, R.; et al. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase. Leukemia 2011, 25, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Maby-El Hajjami, H.; Ame-Thomas, P.; Pangault, C.; Tribut, O.; DeVos, J.; Jean, R.; Bescher, N.; Monvoisin, C.; Dulong, J.; Lamy, T.; et al. Functional alteration of the lymphoma stromal cell niche by the cytokine context: Role of indoleamine-2,3 dioxygenase. Cancer Res. 2009, 69, 3228–3237. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.; Kim, D.S.; Yang, Y.S.; Lee, J.K. Immunological properties of umbilical cord blood-derived mesenchymal stromal cells. Cell. Immunol. 2008, 251, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Bonfield, T.L.; Lennon, D.; Ghosh, S.K.; DiMarino, A.M.; Weinberg, A.; Caplan, A.I. Cell based therapy aides in infection and inflammation resolution in the murine model of cystic fibrosis lung disease. Stem Cell Discov. 2013, 3, 139–153. [Google Scholar] [CrossRef]
- Xu, J.; Woods, C.R.; Mora, A.L.; Joodi, R.; Brigham, K.L.; Iyer, S.; Rojas, M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L131–L141. [Google Scholar] [CrossRef] [PubMed]
- González, M.A.; Gonzalez-Rey, E.; Rico, L.; Büscher, D.; Delgado, M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheumatol. 2009, 60, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Bouffi, C.; Bony, C.; Courties, G.; Jorgensen, C.; Noel, D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS ONE 2010, 5, e14247. [Google Scholar] [CrossRef] [PubMed]
- González, M.A.; Gonzalez–Rey, E.; Rico, L.; Büscher, D.; Delgado, M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology 2009, 136, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Chen, J.; Cui, Y.; Lu, M.; Elias, S.B.; Mitchell, J.B.; Hammill, L.; Vanguri, P.; Chopp, M. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp. Neurol. 2005, 195, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Mou, S.; Wang, Q.; Shi, B.; Gu, L.; Ni, Z. Hepatocyte growth factor suppresses transforming growth factor-beta-1 and type III collagen in human primary renal fibroblasts. Kaohsiung J. Med. Sci. 2009, 25, 577–587. [Google Scholar] [CrossRef]
- Schievenbusch, S.; Strack, I.; Scheffler, M.; Wennhold, K.; Maurer, J.; Nischt, R.; Dienes, H.P.; Odenthal, M. Profiling of anti-fibrotic signaling by hepatocyte growth factor in renal fibroblasts. Biochem. Biophys. Res. Commun. 2009, 385, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, Y.; Higashi, K.; Kushida, M.; Hong, Y.Y.; Nakao, S.; Higashiyama, R.; Moro, T.; Itoh, J.; Mikami, T.; Kimura, T.; et al. Hepatocyte growth factor suppresses profibrogenic signal transduction via nuclear export of Smad3 with galectin-7. Gastroenterology 2008, 134, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Kanemura, H.; Iimuro, Y.; Takeuchi, M.; Ueki, T.; Hirano, T.; Horiguchi, K.; Asano, Y.; Fujimoto, J. Hepatocyte growth factor gene transfer with naked plasmid DNA ameliorates dimethylnitrosamine-induced liver fibrosis in rats. Hepatol. Res. 2008, 38, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Iso, Y.; Spees, J.L.; Serrano, C.; Bakondi, B.; Pochampally, R.; Song, Y.H.; Sobel, B.E.; Delafontaine, P.; Prockop, D.J. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem. Biophys. Res. Commun. 2007, 354, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, N.; Kangawa, K.; Itoh, T.; Iwase, T.; Murakami, S.; Miyahara, Y.; Fujii, T.; Uematsu, M.; Ohgushi, H.; Yamagishi, M.; et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation 2005, 112, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Bevan, D.; Gherardi, E.; Fan, T.P.; Edwards, D.; Warn, R. Diverse and potent activities of HGF/SF in skin wound repair. J. Pathol. 2004, 203, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Gille, J.; Khalik, M.; Konig, V.; Kaufmann, R. Hepatocyte growth factor/scatter factor (HGF/SF) induces vascular permeability factor (VPF/VEGF) expression by cultured keratinocytes. J. Investig. Dermatol. 1998, 111, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Reitamo, S.; Remitz, A.; Tamai, K.; Uitto, J. Interleukin-10 modulates type I collagen and matrix metalloprotease gene expression in cultured human skin fibroblasts. J. Clin. Investig. 1994, 94, 2489–2492. [Google Scholar] [CrossRef] [PubMed]
- Liechty, K.W.; Kim, H.B.; Adzick, N.S.; Crombleholme, T.M. Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J. Pediatr. Surg. 2000, 35, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.; Kozin, E.D.; Keswani, S.G.; Vaikunth, S.S.; Katz, A.B.; Zoltick, P.W.; Favata, M.; Radu, A.P.; Soslowsky, L.J.; Herlyn, M.; et al. Permissive environment in postnatal wounds induced by adenoviral-mediated overexpression of the anti-inflammatory cytokine interleukin-10 prevents scar formation. Wound Repair Regen. 2008, 16, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dai, C.; Liu, Y. A novel mechanism by which hepatocyte growth factor blocks tubular epithelial to mesenchymal transition. J. Am. Soc. Nephrol. 2005, 16, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wang, M.H.; Dong, Z.; Yang, T. Prostaglandin E2 is a potent inhibitor of epithelial-to-mesenchymal transition: Interaction with hepatocyte growth factor. Am. J. Physiol. Ren. Physiol. 2006, 291, F1323–F1331. [Google Scholar] [CrossRef] [PubMed]
- McAnulty, R.J. Fibroblasts and myofibroblasts: Their source, function and role in disease. Int. J. Biochem. Cell Biol. 2007, 39, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Matsumoto, K.; Mizuno, S.; Sawa, Y.; Matsuda, H. Hepatocyte growth factor prevents tissue fibrosis, remodeling and dysfunction in cardiomyopathic hamster hearts. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2131–H2139. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Minatoguchi, S.; Kosai, K.; Yuge, K.; Takahashi, T.; Arai, M.; Wang, N.; Misao, Y.; Lu, C.; Onogi, H.; et al. In vivo hepatocyte growth factor gene transfer reduces myocardial ischemia-reperfusion injury through its multiple actions. J. Card Fail. 2007, 13, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, S.; Zhang, Y.; Yu, B.; Xu, Y.; Guan, Z. Paracrine action mediate the antifibrotic effect of transplanted mesenchymal stem cells in a rat model of global heart failure. Mol. Biol. Rep. 2009, 36, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, J.J.; Cao, D.Y.; Li, X.; Zhang, L.Y.; He, Y.; Yue, S.Q.; Wang, D.S.; Dou, K.F. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World J. Gastroenterol. 2012, 18, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.A.; Atta, H.M.; Mahfouz, S.; Fouad, H.H.; Roshdy, N.K.; Ahmed, H.H.; Rashed, L.A.; Sabry, D.; Hassouna, A.A.; Hasan, N.M. Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clin. Biochem. 2007, 40, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Tu, C.T.; Hsiao, C.C.; Tsai, M.S.; Ho, C.M.; Cheng, N.C.; Hung, T.M.; Shih, D.T. Antifibrotic activity of human placental amnion membrane-derived CD34+ mesenchymal stem/progenitor cell transplantation in mice with thioacetamide-induced liver injury. Stem Cells Transl. Med. 2016, 5, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Xu, J.; Woods, C.R.; Mora, A.L.; Spears, W.; Roman, J.; Brigham, K.L. Bone marrow–derived mesenchymal stem cells in repair of the injured lung. Am. J. Respir. Cell Mol. Biol. 2005, 33, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Sedrakyan, S.; Da Sacco, S.; Milanesi, A.; Shiri, L.; Petrosyan, A.; Varimezova, R.; Warburton, D.; Lemley, K.V.; De Filippo, R.E.; Perin, L. Injection of amniotic fluid stem cells delays progression of renal fibrosis. J. Am. Soc. Nephrol. 2012, 23, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Semedo, P.; Correa-Costa, M.; Antonio Cenedeze, M.; Maria Avancini Costa Malheiros, D.; Antonia dos Reis, M.; Shimizu, M.H.; Seguro, A.C.; Pacheco-Silva, A.; Ĉamara, S.; Olsen, N. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells 2009, 27, 3063–3073. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, H.; Vanderbrink, B.A.; Campbell, M.T.; Hile, K.L.; Zhang, H.; Meldrum, D.R.; Meldrum, K.K. Arterially delivered mesenchymal stem cells prevent obstruction-induced renal fibrosis. J. Surg. Res. 2011, 168, e51–e59. [Google Scholar] [CrossRef] [PubMed]
- Tögel, F.; Cohen, A.; Zhang, P.; Yang, Y.; Hu, Z.; Westenfelder, C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2009, 18, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Franquesa, M.; Herrero, E.; Torras, J.; Ripoll, E.; Flaquer, M.; Goma, M.; Lloberas, N.; Anegon, I.; Cruzado, J.M.; Grinyó, J.M.; et al. Mesenchymal stem cell therapy prevents interstitial fibrosis and tubular atrophy in a rat kidney allograft model. Stem Cells Dev. 2012, 21, 3125–3135. [Google Scholar] [CrossRef] [PubMed]
- Yeghiazarians, Y.; Zhang, Y.; Prasad, M.; Shih, H.; Saini, S.A.; Takagawa, J.; Sievers, R.E.; Wong, M.L.; Kapasi, N.K.; Mirsky, R.; et al. Injection of bone marrow cell extract into infarcted hearts results in functional improvement comparable to intact cell therapy. Mol. Ther. 2009, 17, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Mias, C.; Lairez, O.; Trouche, E.; Roncalli, J.; Calise, D.; Seguelas, M.H.; Ordener, C.; Piercecchi-Marti, M.D.; Auge, N.; Salvayre, A.N.; et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells 2009, 27, 2734–2743. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, H.C.; Hatzistergos, K.E.; Oskouei, B.N.; Feigenbaum, G.S.; Rodriguez, J.E.; Valdes, D.; Pattany, P.M.; Zambrano, J.P.; Hu, Q.; McNiece, I.; et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc. Natl. Acad. Sci. USA 2009, 106, 14022–14027. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Zisa, D.; Suzuki, G.; Lee, T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: A noninvasive therapeutic regimen. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1888–H1897. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Miyoshi, S.; Ikegami, Y.; Hida, N.; Asada, H.; Togashi, I.; Suzuki, J.; Satake, M.; Nakamizo, H.; Tanaka, M.; et al. Xenografted human amniotic membrane–derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ. Res. 2010, 106, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Selmani, Z.; Naji, A.; Zidi, I.; Favier, B.; Gaiffe, E.; Obert, L.; Borg, C.; Saas, P.; Tiberghien, P.; Rouas-Freiss, N.; et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFoxp3+ regulatory T cells. Stem Cells 2008, 26, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, I.; Ringden, O.; Sundberg, B.; Le Blanc, K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation 2003, 76, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Nasef, A.; Zhang, Y.Z.; Mazurier, C.; Bouchet, S.; Bensidhoum, M.; Francois, S.; Gorin, N.C.; Lopez, M.; Thierry, D.; Fouillard, L.; et al. Selected Stro-1-enriched bone marrow stromal cells display a major suppressive effect on lymphocyte proliferation. Int. J. Lab. Hematol. 2009, 31, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.M.; Abdelrazik, H.; Becchetti, F.; Moretta, L. MSCs inhibit monocytederived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood 2009, 113, 6576–6583. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Krasnodembskaya, A.; Kapetanaki, M.; Mouded, M.; Tan, X.; Serikov, V.; Matthay, M.A. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 2012, 67, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Co, C.; Rojas, M. Mesenchymal stem cells and inflammatory lung diseases. Panminerva Medica 2009, 51, 5–16. [Google Scholar] [PubMed]
- Sordi, V.; Malosio, M.L.; Marchesi, F.; Mercalli, A.; Melzi, R.; Giordano, T.; Belmonte, N.; Ferrari, G.; Leone, B.E.; Bertuzzi, F.; et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood 2005, 106, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Honczarenko, M.; Le, Y.; Swierkowski, M.; Ghiran, I.; Glodek, A.; Silberstein, L.E. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 2005, 24, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Krampera, M.; Cosmi, L.; Angeli, R.; Pasini, A.; Liotta, F.; Andreini, A.; Santarlasci, V.; Mazzinghi, B.; Pizzolo, G.; Vinante, F.; et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 2006, 24, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Prevosto, C.; Zancolli, M.; Canevali, P.; Zocchi, M.R.; Poggi, A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica 2007, 92, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhang, Y.; Zhang, L.; Ren, G.; Shi, Y. The role of IL-6 in inhibition of lymphocyte apoptosis by mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2007, 361, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Raffaghello, L.; Bianchi, G.; Bertolotto, M.; Montecucco, F.; Busca, A.; Dallegri, F.; Ottonello, L.; Pistoia, V. Human mesenchymal stem cells inhibit neutrophil apoptosis: A model for neutrophil preservation in the bone marrow niche. Stem Cells 2008, 26, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Djouad, F.; Charbonnier, L.M.; Bouffi, C.; Louis-Plence, P.; Bony, C.; Apparailly, F.; Cantos, C.; Jorgensen, C.; Noel, D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells 2007, 25, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.X.; Zhang, Y.; Liu, B.; Zhang, S.X.; Wu, Y.; Yu, X.D.; Mao, N. Human mesenchymal stem cells inhibit differentiation and function of monocytederived dendritic cells. Blood 2005, 105, 4120–4126. [Google Scholar] [CrossRef] [PubMed]
- Haniffa, M.A.; Wang, X.N.; Holtick, U.; Rae, M.; Isaacs, J.D.; Dickinson, A.M.; Hilkens, C.M.; Collin, M.P. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J. Immunol. 2007, 179, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Jin, Z.; Liu, J.; Yu, S.; Cui, Q.; Yi, D. Mesenchymal stem cells might be used to induce tolerance in heart transplantation. Med. Hypotheses 2008, 70, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.J.; Kruisselbrink, A.B.; Lurvink, E.; Willemze, R.; Fibbe, W.E. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J. Immunol. 2006, 177, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V.; et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006, 107, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, P.A.; Perez, S.A.; Gritzapis, A.D.; Baxevanis, C.N.; Papamichail, M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 2006, 24, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Lamba, D.A.; Karl, M.O.; Ware, C.B.; Reh, T.A. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2006, 103, 12769–12774. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jiang, H.; Yang, H. In vitro culture of bone marrow mesenchymal stem cells in rats and differentiation into retinal neural-like cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2007, 27, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.M.; Dong, F.T.; Yu, W.H.; Gao, F. Differentiation of mesenchymal stem cell in the microenviroment of retinitis pigmentosa. Int. J. Ophthalmol. 2010, 3, 216–219. [Google Scholar] [PubMed]
- Arnhold, S.; Absenger, Y.; Klein, H.; Addicks, K.; Schraermeyer, U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.M.; Yen, M.L.; Liu, K.J.; Sytwu, H.K.; Yen, B.L. Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells. J. Biomed. Sci. 2011, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Zarzeczny, A.; Caulfield, T. Emerging ethical, legal and social issues associated with stem cell research & and the current role of the moral status of the embryo. Stem Cell Rev. Rep. 2009, 5, 96–101. [Google Scholar]
- Kawanabe, N.; Murata, S.; Murakami, K.; Ishihara, Y.; Hayano, S.; Kurosaka, H.; Kamioka, H.; Takano-Yamamoto, T.; Yamashiro, T. Isolation of multipotent stem cells in human periodontal ligament using stage-specific embryonic antigen-4. Differentiation 2010, 79, 74–83. [Google Scholar] [CrossRef] [PubMed]




| Microbial Infections | Species | Infections | Antimicrobial Treatments | Route of Administration | Duration of Administration | Reference |
|---|---|---|---|---|---|---|
| Fungal Infections | Candida albicans | chorioretinitis | caspofungin, micafungin, or anidulafungin | Intravenous or oral | Approximate 1 month | [7] |
| Aspergillus fumigatus | retinitis, invasive aspergillosis | voriconazole, or posaconazole | Intravenous or oral | - | [7,33,87,88] | |
| Cryptococcus neoformans | multifocal chorioretinitis | flucytosine and amphotericin B | Intravenous or oral | - | [84,85] | |
| Histoplasma capsulatum | histoplasmosis, retinitis | Laser cauterization | - | Repeated | [2,64] | |
| Viral Infections | CMV | retinitis | ganciclovir | Intravenous, intravitreous | >3 weeks | [21] |
| foscarnet | Intravenous | - | [57] | |||
| cidofovir | Intravenous | - | [62] | |||
| fomivirsen | Intravenous | - | [89] | |||
| VZV, HZV, HSV types 1 and 2 | ARN, PORN | acyclovir | Intravenous | 7–12 weeks | [62,67] | |
| foscarnet | Intravitreal | - | [20] | |||
| valaciclovir | Oral | - | [62,90] | |||
| famciclovir | Oral | - | [62,91] | |||
| Parasitic Infections | Toxocara canis, Toxocara cati | ocular toxocariasis | albendazole or thiabendazole | - | - | [37,38,92] |
| Toxoplasma gondii | ocular toxoplasmosis | pyrimethamine-sulfadiazine, trimethoprim-sulfamethoxazole or pyrimethamine-azithromycin | - | - | [93,94,95] | |
| Bacterial Infections | Enterococci, Streptococci, Bacilli, gram-negative bacteria | retinitis | vancomycin-amikacin or vancomycin-ceftazidime | - | - | [45,96] |
| Treponema pallidum | ocular syphilis | penicillin | Intravenous | 14 days | [58,97,98] | |
| ceftriaxone or doxycycline | Parenteral | 3 weeks | [66,98] | |||
| Mycobacterium tuberculosis | tubercular retinal vasculitis | isoniazid, rifampin and pyrazinamide, with or without ethambutol | - | Up to 9 months | [65] | |
| streptomycin, capreomycin, or quinolones | - | - | [99] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teh, S.W.; Mok, P.L.; Abd Rashid, M.; Bastion, M.-L.C.; Ibrahim, N.; Higuchi, A.; Murugan, K.; Mariappan, R.; Subbiah, S.K. Recent Updates on Treatment of Ocular Microbial Infections by Stem Cell Therapy: A Review. Int. J. Mol. Sci. 2018, 19, 558. https://doi.org/10.3390/ijms19020558
Teh SW, Mok PL, Abd Rashid M, Bastion M-LC, Ibrahim N, Higuchi A, Murugan K, Mariappan R, Subbiah SK. Recent Updates on Treatment of Ocular Microbial Infections by Stem Cell Therapy: A Review. International Journal of Molecular Sciences. 2018; 19(2):558. https://doi.org/10.3390/ijms19020558
Chicago/Turabian StyleTeh, Seoh Wei, Pooi Ling Mok, Munirah Abd Rashid, Mae-Lynn Catherine Bastion, Normala Ibrahim, Akon Higuchi, Kadarkarai Murugan, Rajan Mariappan, and Suresh Kumar Subbiah. 2018. "Recent Updates on Treatment of Ocular Microbial Infections by Stem Cell Therapy: A Review" International Journal of Molecular Sciences 19, no. 2: 558. https://doi.org/10.3390/ijms19020558
APA StyleTeh, S. W., Mok, P. L., Abd Rashid, M., Bastion, M.-L. C., Ibrahim, N., Higuchi, A., Murugan, K., Mariappan, R., & Subbiah, S. K. (2018). Recent Updates on Treatment of Ocular Microbial Infections by Stem Cell Therapy: A Review. International Journal of Molecular Sciences, 19(2), 558. https://doi.org/10.3390/ijms19020558