Evaluation of Anti-Metastatic Potential of the Combination of Fisetin with Paclitaxel on A549 Non-Small Cell Lung Cancer Cells
Abstract
:1. Introduction
2. Results
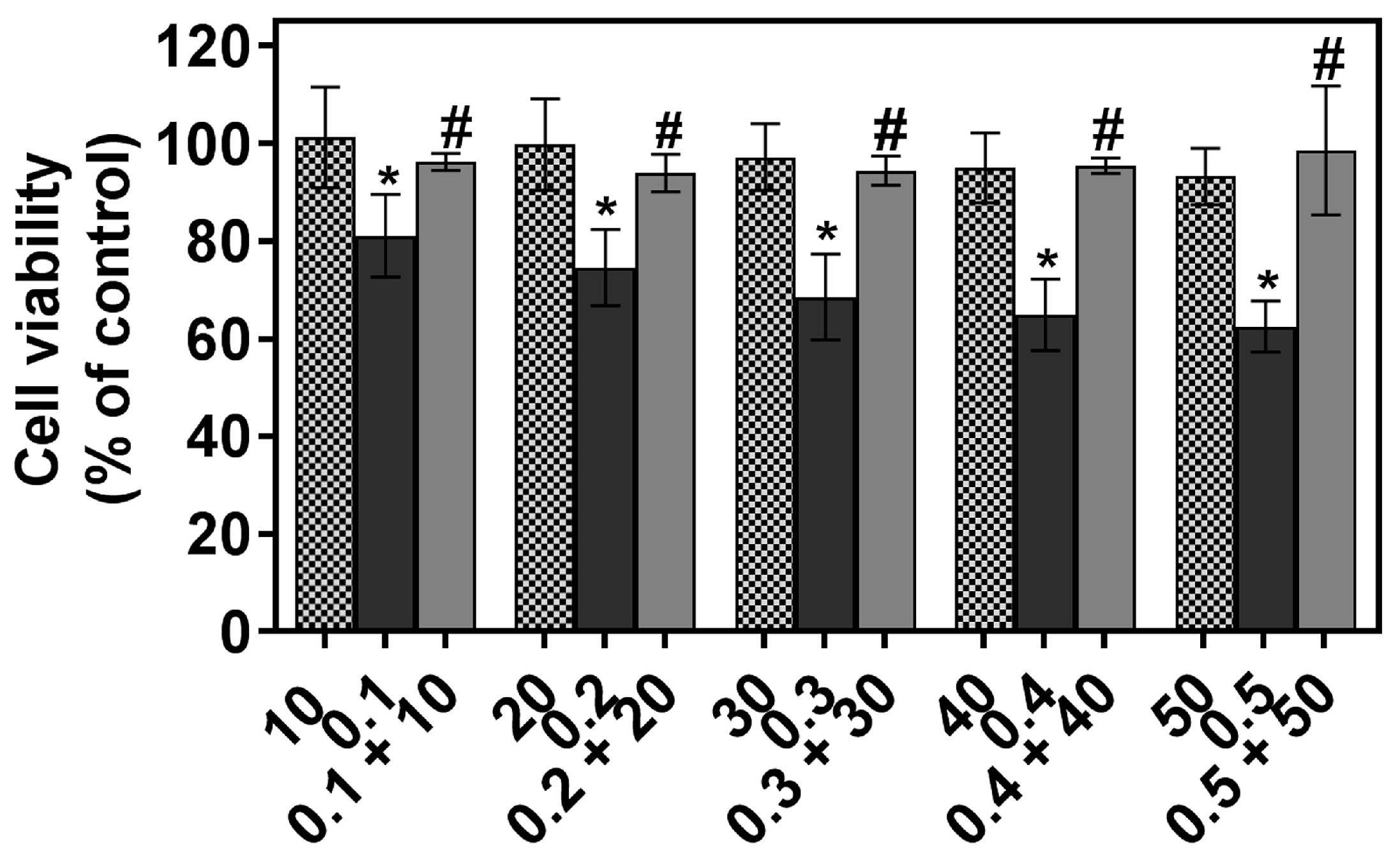
2.1. The Individual and Combined Effect of FIS and PTX on Normal Lung MRC-5 Cells
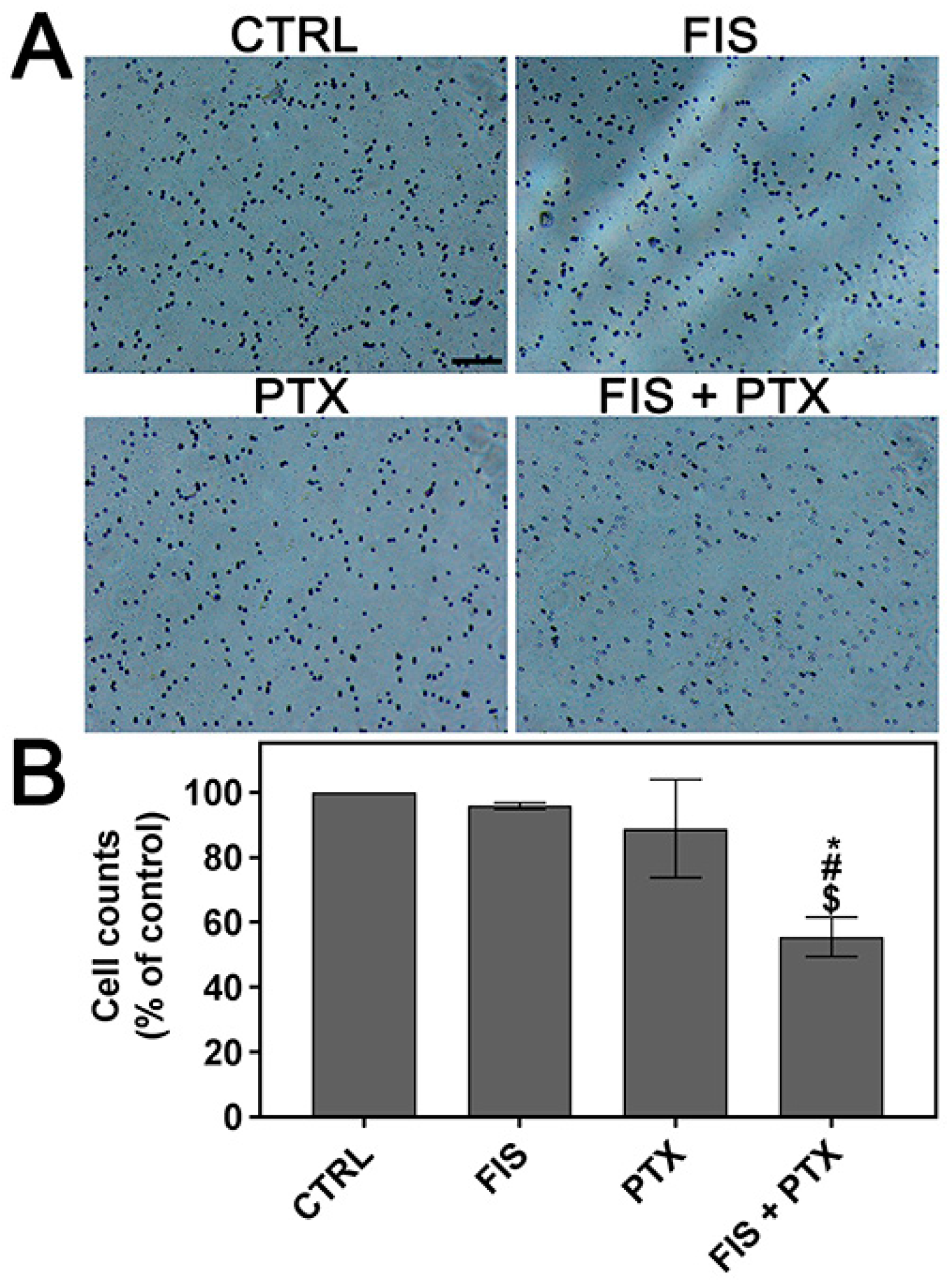
2.2. The Individual and Combined Effect of FIS and PTX on the Migration and Invasion of A549 Cells
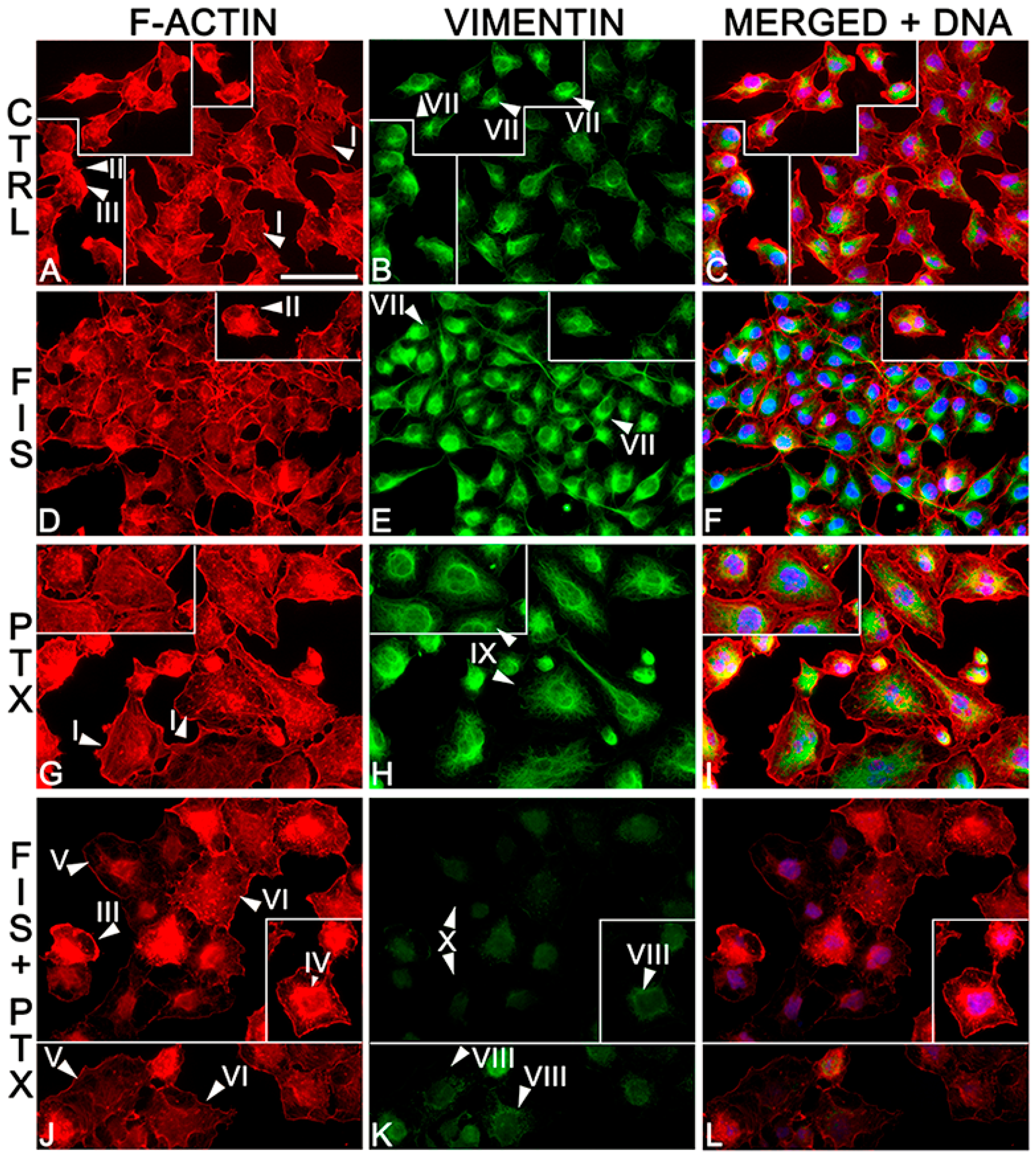
2.3. The Individual and Combined Effect of FIS and PTX on Actin and Vimentin Filament Network in A549 Cells
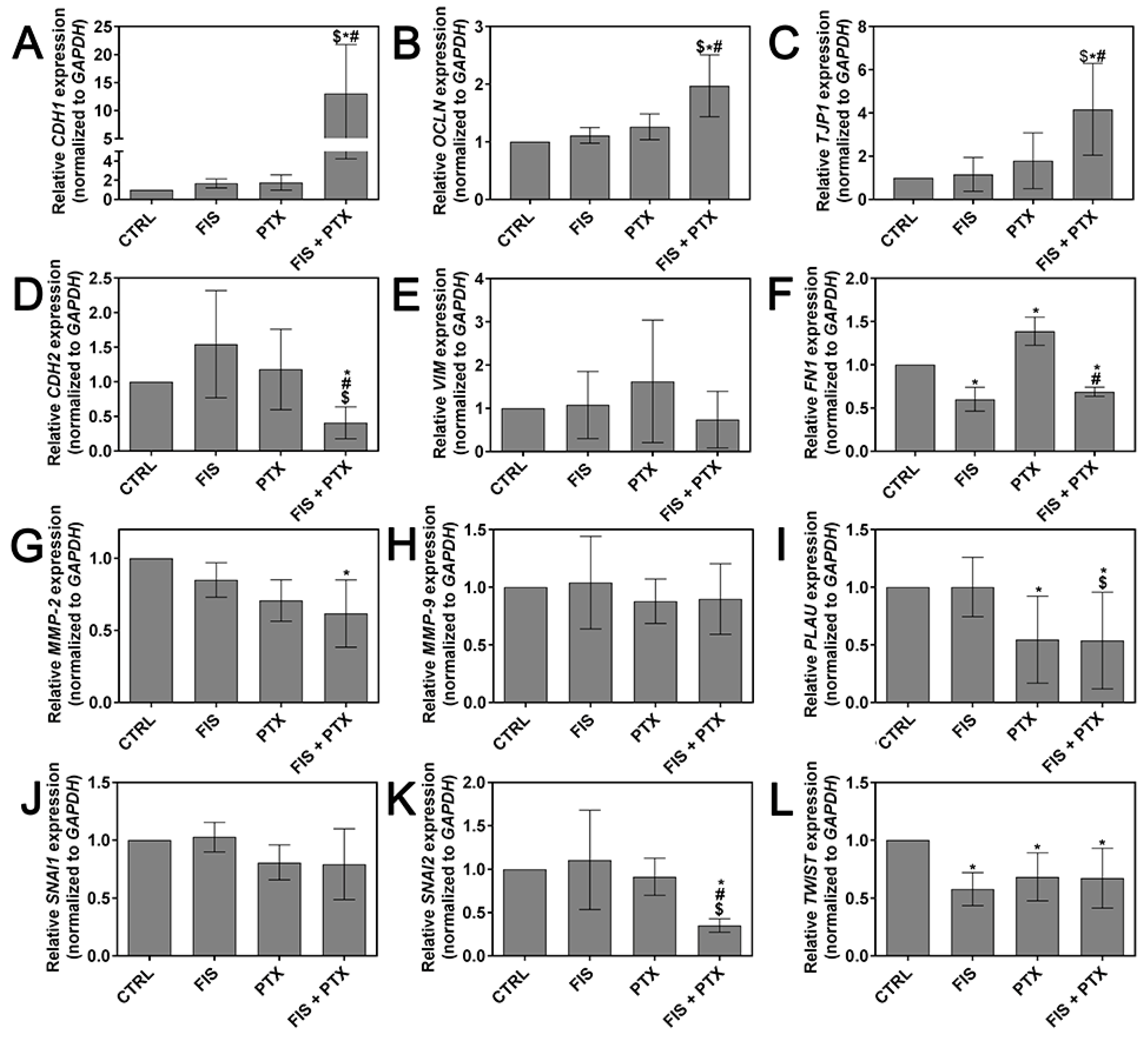
2.4. The Individual and Combined Effect of FIS and PTX on the Expression Levels of Metastasis-Related Genes
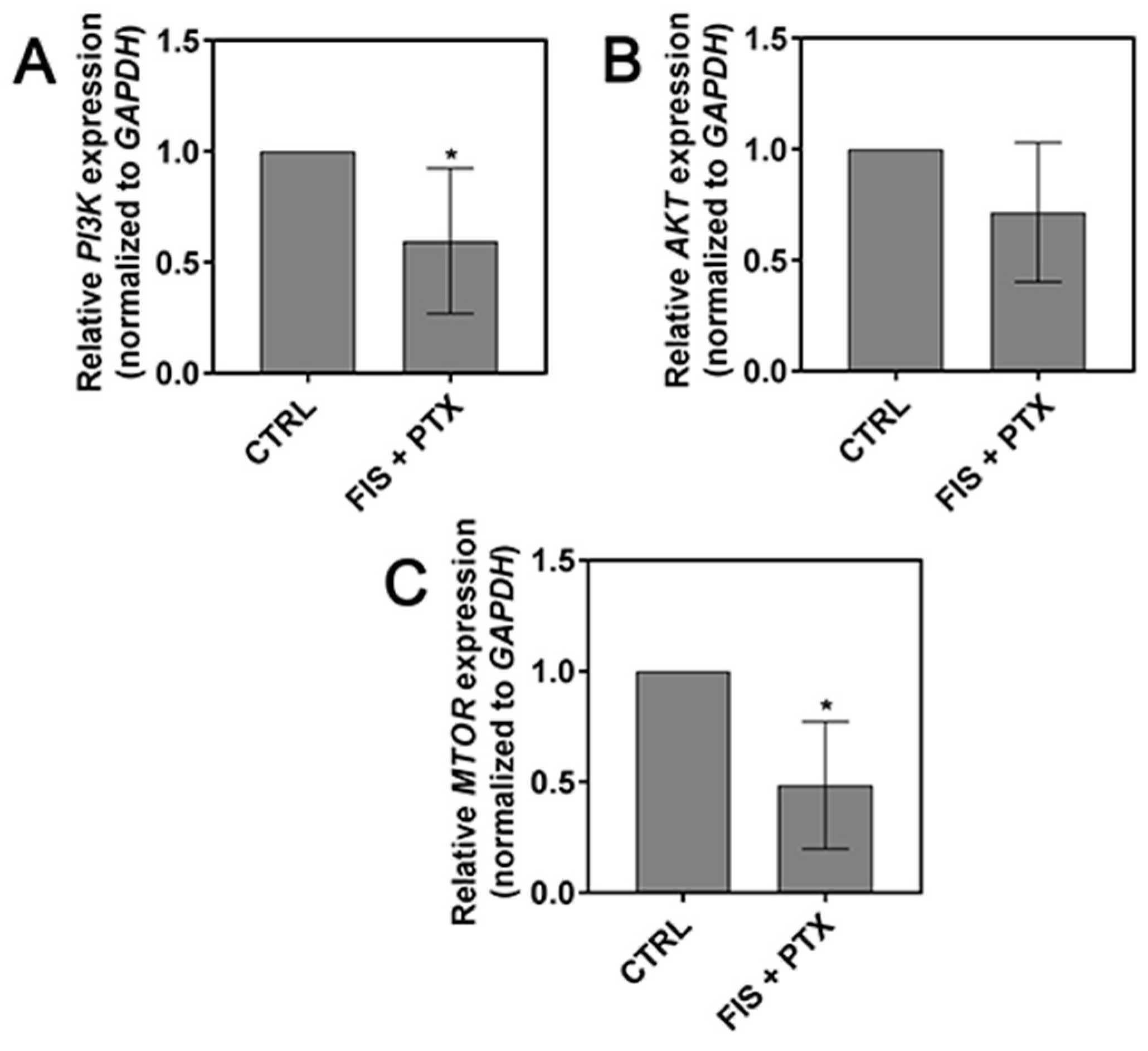
2.5. The Effect of FIS in the Combination with PTX on the PI3K, AKT and mTOR Expression Level
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Treatment
4.3. MTT Assay
4.4. Fluorescence of Cytoskeletal Proteins
4.5. Scratch Wound-Healing Assay
4.6. Cell Invasion Assay
4.7. Quantitative Real-Time PCR Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| FIS | fisetin |
| PTX | paclitaxel |
| NSCLC | non-small cell lung cancer |
| EMT | epithelial-to-mesenchymal transition |
References
- Levin, E.G. Cancer Therapy through Control of Cell Migration. Curr. Cancer Drug Targets 2005, 5, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Thaiparambil, J.T.; Bender, L.; Ganesh, T.; Kline, E.; Patel, P.; Liu, Y.; Tighiouart, M.; Vertino, P.M.; Harvey, R.D.; Garcia, A.; et al. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int. J. Cancer 2011, 129, 2744–2755. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A.; Ye, L.; Sanders, A.J.; Lane, J.; Jiang, W.G. Cancer Invasion and Metastasis: Molecular and Cellular Perspective. In Metastatic Cancer: Clinical and Biological Perspectives; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A Dietary antioxidant for health promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Khusro, F.H.; Mustafa Adhami, V.; Suh, Y.; Mukhtar, H. Dual inhibition of phosphatidylinositol 3-kinase/Akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int. J. Cancer 2012, 130, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Syed, D.N.; Mukhtar, H. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis 2008, 29, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.Q.; Venkateswaran, V.; Viswanathan, L.; Teahan, S.J.; Fleshner, N.E.; Klotz, L.H. Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2006, 9, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheng, Y.; Qu, W.; Sun, Y.; Wang, Z.; Wang, H.; Tian, B. Fisetin, a dietary flavonoid, induces cell cycle arrest and apoptosis through activation of P53 and inhibition of NF-Kappa B pathways in bladder cancer cells. Basic Clin. Pharmacol. Toxicol. 2011, 108, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Rengarajan, T.; Yaacob, N.S. The flavonoid fisetin as an anticancer agent targeting the growth signaling pathways. Eur. J. Pharmacol. 2016, 789, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.S.; Shen, K.H.; Huang, J.S.; Ko, S.C.; Shih, Y.W. Antimetastatic potential of fisetin involves inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2/9 expressions in prostate cancer PC-3 cells. Mol. Cell. Biochem. 2010, 333, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.H.; Hsieh, S.C.; Yu, Y.L.; Huang, M.H.; Huang, Y.C.; Hsieh, Y.H. Fisetin inhibits migration and invasion of human cervical cancer cells by down-regulating urokinase plasminogen activator expression through suppressing the p38 MAPK-dependent NF-κB signaling pathway. PLoS ONE 2013, 8, e71983. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Hsieh, Y.H.; Hwang, J.M.; Jan, H.J.; Hsieh, S.C.; Lin, S.H.; Lai, C.Y. Fisetin suppresses ADAM9 expression and inhibits invasion of glioma cancer cells through increased phosphorylation of ERK1/2. Tumour Biol. 2015, 36, 3407–3415. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.C.; Shih, Y.W.; Chao, C.H.; Lee, X.Y.; Chiang, T.A. Involvement of the ERK signaling pathway in fisetin reduces invasion and migration in the human lung cancer cell line A549. J. Agric. Food Chem. 2009, 57, 8933–8941. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhao, Y.; Chen, J.; Shao, S.; Zhang, X. Fisetin inhibits migration, invasion and epithelial-mesenchymal transition of LMP1-positive nasopharyngeal carcinoma cells. Mol. Med. Rep. 2014, 9, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, E.; Adhami, V.M.; Sechi, M.; Mukhtar, H. Dietary flavonoid fisetin binds to β-tubulin and disrupts microtubule dynamics in prostate cancer cells. Cancer Lett. 2015, 28, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Touil, Y.S.; Fellous, A.; Scherman, D.; Chabot, G.G. Flavonoid-induced morphological modifications of endothelial cells through microtubule stabilization. Nutr. Cancer 2009, 61, 310–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, H.C.; Diamond, A.C.; Strickland, L.R.; Kappes, J.C.; Katiyar, S.K.; Elmets, C.A.; Athar, M.; Afaq, F. Fisetin, a dietary flavonoid, augments the anti-invasive and anti-metastatic potential of sorafenib in melanoma. Oncotarget 2015, 7, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, E.; Adhami, V.M.; Siddiqui, I.A.; Verma, A.K.; Mukhtar, H. Fisetin enhances chemotherapeutic effect of cabazitaxel against human prostate cancer cells. Mol. Cancer Ther. 2016, 15, 2863–2874. [Google Scholar] [CrossRef] [PubMed]
- Barbuti, A.M.; Chen, Z.S. Paclitaxel through the ages of anticancer therapy: Exploring its role in chemoresistance and radiation therapy. Cancers 2015, 7, 2360–2371. [Google Scholar] [CrossRef] [PubMed]
- Volk-Draper, L.; Hall, K.; Griggs, C.; Rajput, S.; Kohio, P.; DeNardo, D.; Ran, S. Paclitaxel therapy promotes breast cancer metastasis in a TLR4-dependent manner. Cancer Res. 2014, 74, 5421–5434. [Google Scholar] [CrossRef] [PubMed]
- Gingis-Velitski, S.; Loven, D.; Benayoun, L.; Munster, M.; Bril, R.; Voloshin, T.; Alishekevitz, D.; Bertolini, F.; Shaked, Y. Host response to short-term, single-agent chemotherapy induces matrix metalloproteinase-9 expression and accelerates metastasis in mice. Cancer Res. 2011, 71, 6986–6996. [Google Scholar] [CrossRef] [PubMed]
- Larzabal, L.; El-Nikhely, N.; Redrado, M.; Seeger, W.; Savai, R.; Calvo, A. Differential effects of drugs targeting cancer stem cell (CSC) and non-CSC populations on lung primary tumors and metastasis. PLoS ONE 2013, 8, e79798. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S.; Takada, Y.; Banerjee, S.; Newman, R.A.; Bueso-Ramos, C.E.; Price, J.E. Curcumin suppresses the paclitaxel-induced nuclear factor-κB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin. Cancer Res. 2005, 11, 7490–7498. [Google Scholar] [CrossRef] [PubMed]
- Volk, L.D.; Flister, M.J.; Chihade, D.; Desai, N.; Trieu, V.; Ran, S. Synergy of Nab-paclitaxel and Bevacizumab in eradicating large orthotopic breast tumors and preexisting metastases. Neoplasia 2011, 13, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, H.; Shibata, K.; Terauchi, M.; Yamashita, M.; Ino, K.; Nawa, A.; Kikkawa, F. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int. J. Oncol. 2007, 31, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Yang, G.Y.; Medina, D.J.; Vassil, A.D.; Liao, J.; Hait, W.N. Treatment of multidrug resistant (MDR1) murine leukemia with P-glycoprotein substrates accelerates the course of the disease. Biochem. Biophys. Res. Commun. 1999, 266, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Daenen, L.G.M.; Roodhart, J.M.L.; van Amersfoort, M.; Dehnad, M.; Roessingh, W.; Ulfman, L.H.; Derksen, P.W.B.; Voest, E.E. Chemotherapy enhances metastasis formation via VEGFR-1-expressing endothelial cells. Cancer Res. 2011, 71, 6976–6985. [Google Scholar] [CrossRef] [PubMed]
- Quintavalle, M.; Elia, L.; Price, J.H.; Heynen-Genel, S.; Courtneidge, S.A. A cell-based high-content screening assay reveals activators and inhibitors of cancer cell invasion. Sci. Signal. 2011, 4, ra49. [Google Scholar] [CrossRef] [PubMed]
- Camara, O.; Rengsberger, M.; Egbe, A.; Koch, A.; Gajda, M.; Hammer, U.; Jörke, C.; Rabenstein, C.; Untch, M.; Pachmann, K. The relevance of circulating epithelial tumor cells (CETC) for therapy monitoring during neoadjuvant (primary systemic) chemotherapy in breast cancer. Ann. Oncol. 2007, 18, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Brennan, D.J.; Rexhepaj, E.; Ruffell, B.; Shiao, S.L.; Madden, S.F.; Gallagher, W.M.; Wadhwani, N.; Keil, S.D.; Junaid, S.A.; et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011, 1, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, L.; Mendoza, T.R.; Reuben, J.M.; Martinez, M.M.; Willey, J.S.; Lara, J.; Syed, A.; Fritsche, H.A.; Bruera, E.; Booser, D.; et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine 2004, 25, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Tanei, T.; Morimoto, K.; Shimazu, K.; Kim, S.J.; Tanji, Y.; Taguchi, T.; Tamaki, Y.; Noguchi, S. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin. Cancer Res. 2009, 15, 4234–4241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, S.; Yen, Y.; Brown, J.; Ta, J.Q.; Le, A.D. A subpopulation of CD133(+) cancer stem-like cells characterized in human oral squamous cell carcinoma confer resistance to chemotherapy. Cancer Lett. 2010, 289, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.V.; Tresoldi, I.; Modesti, A.; Bei, R. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klimaszewska-Wisniewska, A.; Halas-Wisniewska, M.; Tadrowski, T.; Gagat, M.; Grzanka, D.; Grzanka, A. Paclitaxel and the Dietary Flavonoid Fisetin: A Synergistic Combination That Induces Mitotic Catastrophe and Autophagic Cell Death in A549 Non-Small Cell Lung Cancer Cells. Cancer Cell Int. 2016, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, D.; Kurisu, S.; Takenawa, T. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 2005, 96, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Van Zijl, F.; Krupitza, G.; Mikulits, W. Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutat. Res. 2011, 728, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Leduc, C.; Etienne-Manneville, S. Intermediate filaments in cell migration and invasion: The unusual suspects. Curr. Opin. Cell Biol. 2015, 32, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.F.; Sahai, E. The actin cytoskeleton in cancer cell motility. Clin. Exp. Metastasis 2009, 26, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Huang, S. Role of mTOR signaling in tumor cell motility, invasion and metastasis. Curr. Protein Pept. Sci. 2011, 12, 30–42. [Google Scholar] [PubMed]
- Khan, M.I.; Adhami, V.M.; Lall, R.K.; Sechi, M.; Joshi, D.C.; Haidar, O.M.; Syed, D.N.; Siddiqui, I.A.; Chiu, S.-Y.; Mukhtar, H. YB-1 expression promotes epithelial-to-mesenchymal transition in prostate cancer that is inhibited by a small molecule fisetin. Oncotarget 2014, 5, 2462–2474. [Google Scholar] [CrossRef] [PubMed]
- Carmel, R.J.; Brown, J.M. The effect of cyclophosphamide and other drugs on the incidence of pulmonary metastases in mice. Cancer Res. 1977, 37, 145–151. [Google Scholar] [PubMed]
- Park, S.I.; Liao, J.; Berry, J.E.; Li, X.; Koh, A.J.; Michalski, M.E.; Eber, M.R.; Soki, F.N.; Sadler, D.; Sud, S.; et al. Cyclophosphamide creates a receptive microenvironment for prostate cancer skeletal metastasis. Cancer Res. 2012, 72, 2522–2532. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Yang, M.; Hayashi, K.; Jiang, P.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Moossa, A.R.; Bouvet, M.; Hoffman, R.M. Induction of cancer metastasis by cyclophosphamide pretreatment of host mice: An opposite effect of chemotherapy. Cancer Res. 2008, 68, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.S.; Müller, P.M.; Bajorat, J.; Schroeder, A.; Giaisi, M.; Amin, E.; Ahmadian, M.R.; Rocks, O.; Köhler, R.; Krammer, P.H.; Li-Weber, M. The anticancer phytochemical rocaglamide inhibits Rho GTPase activity and cancer cell migration. Oncotarget 2016, 7, 51908–51921. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Eliaz, I.; Sliva, D. Suppression of growth and invasive behavior of human prostate cancer cells by ProstaCaidTM: Mechanism of activity. Int. J. Oncol. 2011, 38, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Gandalovičová, A.; Rosel, D.; Fernandes, M.; Veselý, P.; Heneberg, P.; Čermák, V.; Petruželka, L.; Kumar, S.; Sanz-Moreno, V.; Brábek, J. Migrastatics-anti-metastatic and anti-invasion drugs: Promises and challenges. Trends Cancer 2017, 3, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Huttenlocher, A. Cell polarization mechanisms during directed cell migration. Nat. Cell Biol. 2005, 7, 336–337. [Google Scholar] [CrossRef] [PubMed]
- Lorch, J.H.; Thomas, T.O.; Schmoll, H.J. Bortezomib inhibits cell-cell adhesion and cell migration and enhances epidermal growth factor receptor inhibitor-induced cell death in squamous cell cancer. Cancer Res. 2007, 67, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Hayot, C.; Debeir, O.; Van Ham, P.; Van Damme, M.; Kiss, R.; Decaestecker, C. Characterization of the activities of actin-affecting drugs on tumor cell migration. Toxicol. Appl. Pharmacol. 2006, 211, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Szajnik, M.; Szczepanski, M.J.; Czystowska, M.; Elishaev, E.; Mandapathil, M.; Nowak-Markwitz, E.; Spaczynski, M.; Whiteside, T.L. TLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancer. Oncogene 2009, 28, 4353–4363. [Google Scholar] [CrossRef] [PubMed]
- Dauphin, M.; Barbe, C.; Lemaire, S.; Nawrocki-Raby, B.; Lagonotte, E.; Delepine, G.; Birembaut, P.; Gilles, C.; Polette, M. Vimentin expression predicts the occurrence of metastases in non-small cell lung carcinomas. Lung Cancer 2013, 81, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell. Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef] [PubMed]
- Shia, C.S.; Tsai, S.Y.; Kuo, S.C.; Hou, Y.C.; Chao, P.D.L. Metabolism and pharmacokinetics of 3,3′,4′,7-tetrahydroxyflavone (fisetin), 5-hydroxyflavone, and 7-hydroxyflavone and antihemolysis effects of fisetin and its serum metabolites. J. Agric. Food Chem. 2009, 57, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wang, J.; Wu, Q.; Qian, J.; Yang, C.; Bo, P. Genistein inhibits the growth and regulates the migration and invasion abilities of melanoma cells via the FAK/Paxillin and MAPK pathways. Oncotarget 2017, 8, 21674–21691. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.D.; Kalvala, A.K.; Koneru, M.; Kumar, J.M.; Kuncha, M.; Rachamalla, S.S.; Sistla, R. Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF-ΚB activation and antioxidant defence. PLoS ONE 2014, 9, e105070. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, J.; Liu, L.; Sharma, S.; Dong, Q. Quercetin potentiates Doxorubicin mediated antitumor effects against liver cancer through P53/Bcl-Xl. PLoS ONE 2012, 7, e51764. [Google Scholar] [CrossRef] [PubMed]
- Olayinka, E.T.; Ore, A.; Ola, O.S.; Adeyemo, O.A. Protective effect of quercetin on melphalan-induced oxidative stress and impaired renal and hepatic functions in rat. Chemother. Res. Pract. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Klimaszewska-Wiśniewska, A.; Hałas-Wiśniewska, M.; Izdebska, M.; Gagat, M.; Grzanka, A.; Grzanka, D. Antiproliferative and antimetastatic action of quercetin on A549 non-small cell lung cancer cells through its effect on the cytoskeleton. Acta Histochem. 2017, 119, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Głodek, A.; Jankowska, A. CGB activates ERK and AKT kinases in cancer cells via LHCGR-independent mechanism. Tumour Biol. 2014, 35, 5467–5479. [Google Scholar] [CrossRef] [PubMed]
- Toiyama, Y.; Yasuda, H.; Saigusa, S. Increased expression of Slug and Vimentin as novel predictive biomarkers for lymph node metastasis and poor prognosis in colorectal cancer. Carcinogenesis 2013, 34, 2548–2557. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Tsai, P.H.; Kandaswami, C.C. Effects of dietary flavonoids, luteolin, and quercetin on the reversal of epithelial-mesenchymal transition in A431 epidermal cancer cells. Cancer Sci. 2011, 102, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Goossens, K.; Van Soom, A.; Van Zeveren, A.; Favoreel, H.; Peelman, L.J. Quantification of fibronectin 1 (FN1) splice variants, including two novel ones, and analysis of integrins as candidate FN1 receptors in bovine preimplantation embryos. BMC Dev. Biol. 2009, 9, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Chen, Y.; Han, J.; Meng, Q.; Xi, Q.; Wu, G.; Zhang, B. DCAF4L2 promotes colorectal cancer invasion and metastasis via mediating degradation of NFκb negative regulator PPM1B. Am. J. Transl. Res. 2016, 8, 405–418. [Google Scholar] [PubMed]
- Song, Q.; Song, J.; Wang, Q.; Ma, Y.; Sun, N.; Ma, J.; Chen, Q.; Xia, G.; Huo, Y.; Yang, L.; Li, B. miR-548d-3p/TP53BP2 axis regulates the proliferation and apoptosis of breast cancer cells. Cancer Med. 2016, 5, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Liu, Q.; Mao, F.; Wu, J.; Lei, T. TNF-α-induced VEGF and MMP-9 expression promotes hemorrhagic transformation in pituitary adenomas. Int. J. Mol. Sci. 2011, 12, 4165–4179. [Google Scholar] [CrossRef] [PubMed]
- Dokladny, K.; Ye, D.; Kennedy, J.C.; Moseley, P.L.; Ma, T.Y. Cellular and molecular mechanisms of heat stress-induced up-regulation of occludin protein expression: Regulatory role of heat shock factor-1. Am. J. Pathol. 2008, 172, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.P.; Costa, P.M.; Morais, C.M.; Lopes, I.R.; Cardoso, A.M.; Cardoso, A.L.; Mano, M.; Jurado, A.S.; Pedroso de Lima, M.C. High-throughput screening uncovers miRNAs enhancing glioblastoma cell susceptibility to tyrosine kinase inhibitors. Hum. Mol. Genet. 2017, 26, 4375–4387. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Cao, B.; Wu, J. Protease-activated receptor 2 enhances renal cell carcinoma cell invasion and migration via PI3K/AKT signaling pathway. Exp. Mol. Pathol. 2015, 98, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.J.; Russell, R.C.; Roche, O.; Burry, T.N.; Fish, J.E.; Chow, V.W.; Kim, W.Y.; Saravanan, A.; Maynard, M.A.; Gervais, M.L.; et al. VHL promotes E2 box-dependent E-cadherin transcription by HIF-mediated regulation of SIP1 and SNAIL. Mol. Cell. Biol. 2007, 27, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, D.; Han, S.; Gao, P.; Liu, C.; Li, J.; Pan, X. Fibulin-3 promotes osteosarcoma invasion and metastasis by inducing epithelial to mesenchymal transition and activating the Wnt/β-catenin signaling pathway. Sci. Rep. 2017, 7, 6215. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Z.; Zhang, J.J.; Gao, C.C.; Zhao, M.; Liu, S.Y.; Gao, G.M.; Zheng, Z.H. Expression of autophagy related genes mTOR, Becline-1, LC3 and p62 in the peripheral blood mononuclear cells of systemic lupus erythematosus. Am. J. Clin. Exp. Immunol. 2017, 6, 1–8. [Google Scholar] [PubMed]
- Zhan, J.; Niu, M.; Wang, P.; Zhu, X.; Li, S.; Song, J.; He, H.; Wang, Y.; Xue, L.; Fang, W.; et al. Elevated HOXB9 expression promotes differentiation and predicts a favourable outcome in colon adenocarcinoma patients. Br. J. Cancer. 2014, 111, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Hauck, S.M.; Kinkl, N.; Deeg, C.A.; Swiatek-de Lange, M.; Schöffmann, S.; Ueffing, M. GDNF family ligands trigger indirect neuroprotective signaling in retinal glial cells. Mol. Cell. Biol. 2006, 26, 2746–2757. [Google Scholar] [CrossRef] [PubMed]

| Genes | Primer Sequences | References |
|---|---|---|
| AKT | F 5′-GGCTATTGTGAAGGAGGGTTG-3′ | [60] |
| R 5′-TCCTTGTAGCCAATGAAGGTG-3′ | ||
| CDH1 | F 5′-GCCGAGAGCTACACGTTCAC-3′ | [61] |
| R 5′-ACTTTGAATCGGGTGTCGAG-3′ | ||
| CDH2 | F 5′-GCTTCTGGTGAAATCGCATTA-3′ | [62] |
| R 5′-AGTCTCTCTTCTGCCTTTGTAG-3′ | ||
| FN1 | F 5′-ACTGCCCACTCCTACAACCA-3′ | [63] |
| R 5′-TCTGCGAACACCACTCCA-3′ | ||
| GAPDH | F 5′-ACAACTTTGGTATCGTGGAAGG-3′ | [64] |
| R 5′-GCCATCACGCCACAGTTTC-3′ | ||
| MMP-2 | F 5′-GATACCCCTTTGACGGTAAGGA-3′ | [65] |
| R 5′-CCTTCTCCCAAGGTCCATAGC-3′ | ||
| MMP-9 | F 5′-CCCTGGAGACCTGAGAACCA-3′ | [66] |
| R 5′-CCCGAGTGTAACCATAGCGG-3′ | ||
| OCLN | F 5′-CCCCATCTGACTATGTGGAAAGA-3′ | [67] |
| R 5′-AAAACCGCTTGTCATTCACTTTG-3′ | ||
| PI3K | F 5′-AGTAGGCAACCGTGAAGAAAAG-3′ | [68] |
| R 5′-GAGGTGAATTGAGGTCCCTAAGA-3′ | ||
| PLAU | F 5′-GGGAATGGTCACTTTTACCGAG-3′ | [69] |
| R 5′-GGGCATGGTACGTTTGCTG-3′ | ||
| SNAI1 | F 5′-TTCAACTGCAAATACTGCAACAAG-3′ | [70] |
| R 5′-CGTGTGGCTTCGGATGTG-3′ | ||
| SNAI2 | F 5′-TGTGACAAGGAATATGTGAGCC-3′ | [71] |
| R 5′-TGAGCCCTCAGATTTGACCTG-3′ | ||
| MTOR | F 5′-TCACATTACCCCCTTCACCA-3′ | [72] |
| R 5′-TCAGCGAGTTCTTGCTATTCC-3′ | ||
| TJP1 | F 5′-ACCAGTAAGTCGTCCTGATCC-3′ | [64] |
| R 5′-TCGGCCAAATCTTCTCACTCC-3′ | ||
| TWIST | F 5′-GTCCGCAGTCTTACGAGGAG-3′ | [73] |
| R 5′-GCTTGAGGGTCTGAATCTTGCT-3′ | ||
| VIM | F 5′-GTTTCCAAGCCTGACCTCAC-3′ | [74] |
| R 5′-GCTTCAACGGCAAAGTTCTC-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimaszewska-Wiśniewska, A.; Hałas-Wiśniewska, M.; Grzanka, A.; Grzanka, D. Evaluation of Anti-Metastatic Potential of the Combination of Fisetin with Paclitaxel on A549 Non-Small Cell Lung Cancer Cells. Int. J. Mol. Sci. 2018, 19, 661. https://doi.org/10.3390/ijms19030661
Klimaszewska-Wiśniewska A, Hałas-Wiśniewska M, Grzanka A, Grzanka D. Evaluation of Anti-Metastatic Potential of the Combination of Fisetin with Paclitaxel on A549 Non-Small Cell Lung Cancer Cells. International Journal of Molecular Sciences. 2018; 19(3):661. https://doi.org/10.3390/ijms19030661
Chicago/Turabian StyleKlimaszewska-Wiśniewska, Anna, Marta Hałas-Wiśniewska, Alina Grzanka, and Dariusz Grzanka. 2018. "Evaluation of Anti-Metastatic Potential of the Combination of Fisetin with Paclitaxel on A549 Non-Small Cell Lung Cancer Cells" International Journal of Molecular Sciences 19, no. 3: 661. https://doi.org/10.3390/ijms19030661
APA StyleKlimaszewska-Wiśniewska, A., Hałas-Wiśniewska, M., Grzanka, A., & Grzanka, D. (2018). Evaluation of Anti-Metastatic Potential of the Combination of Fisetin with Paclitaxel on A549 Non-Small Cell Lung Cancer Cells. International Journal of Molecular Sciences, 19(3), 661. https://doi.org/10.3390/ijms19030661






