Metabolic Reprogramming in Chloroplasts under Heat Stress in Plants
Abstract
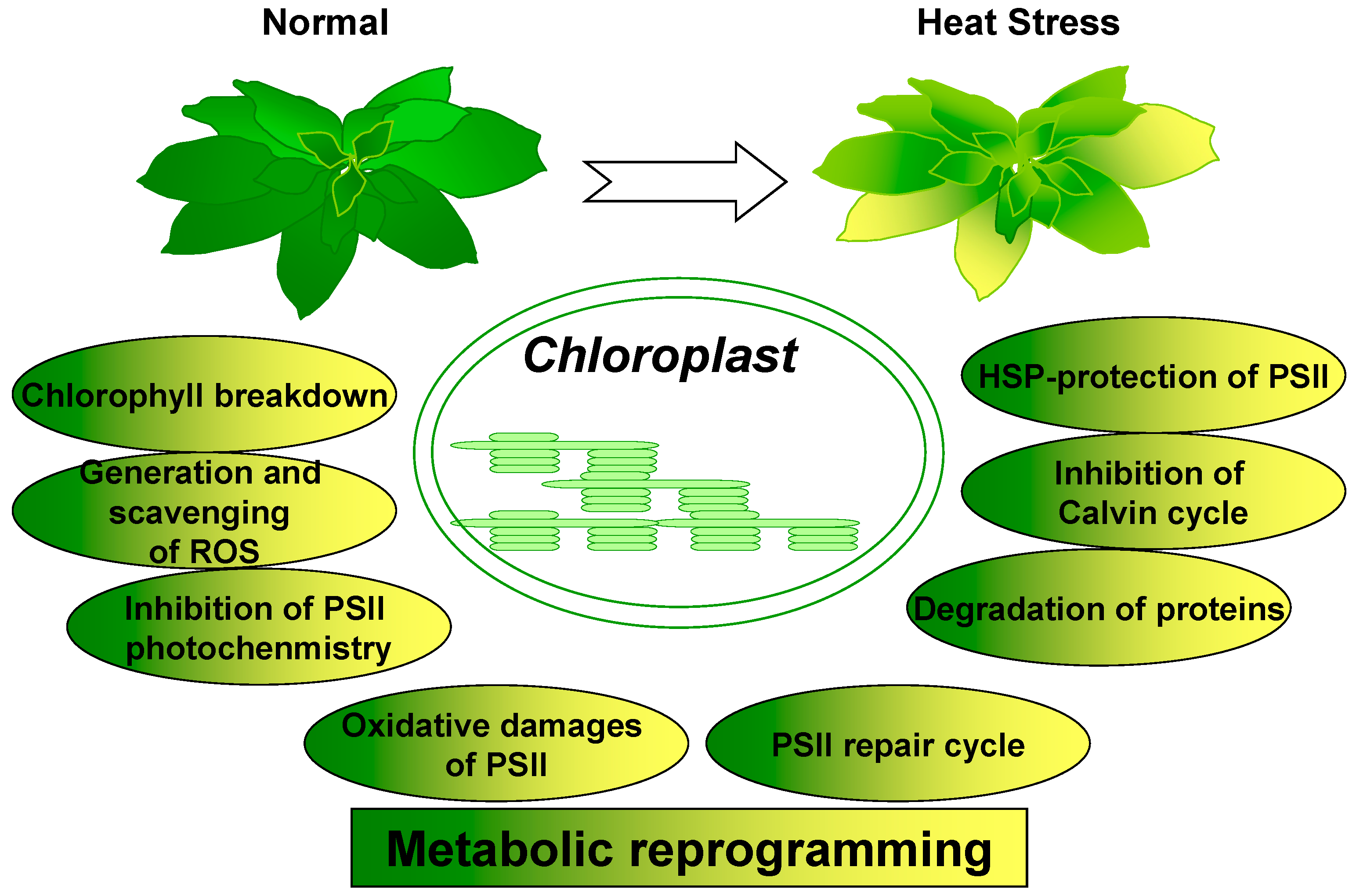
:1. Introduction
2. Chlorophyll Breakdown under Heat Stress
3. Generation and Homeostasis of ROS in Chloroplasts under Heat Stress
4. Turnover of PSII Core Subunits and PSII Protection under Heat Stress
5. Effects of Heat Stress on Metabolic Flux through the Calvin-Benson-Bassham Cycle
6. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 1-MCP | 1-methylcyclopropene |
| 1O2 | singlet state of oxygen |
| 3O2 | triplet state of oxygen |
| ACD1 | Accelerated cell death |
| APX | ascorbate peroxidase |
| AsA | Ascorbate |
| ASH | ascorbic acid |
| ATP | adenosine 5′-triphosphate |
| CAM | crassulacean acid metabolism |
| CAT | catalase |
| Chl | Chlorophyll |
| Chl a | Chlorophyll a |
| Chlase | chlorophyllase |
| CLD1 | CHLOROPHYLL DEPHYTYLASE1 |
| CLHs | Chlorophyllase genes |
| CPAS | 3-cyclopropyl-1-enyl-propanoic acid sodium salt |
| cpn60 beta | beta-subunit of chaperonin-60 |
| Deg/HtrA | high temperature requirement A |
| DHAR | dehydroascorbate reductase |
| FtsH | filamentation temperature sensitive H |
| Fv/Fm | variable fluorescence to maximum fluorescence |
| GPX | glutathione peroxidase |
| GR | glutathione reductase |
| GSH | glutathione |
| GST | glutathione-S-transferase |
| GUN5 | genomes uncoupled 5 |
| H2O2 | hydrogen peroxide |
| HSPs | heat shock proteins |
| ipt | isopentenyl transferase |
| LeCDJ1 | tomato (Lycopersicon esculentum) chloroplast-targeted DnaJ protein |
| LHCPII | light harvesting chlorophyll a/b-protein complex II |
| MDA | malondialdehyde |
| MDHAR | monodehydroascorbate reductase |
| NAD(P)H | nicotinamide adenine dinucleotide phosphate (NADP) |
| NOL | NYC1-LIKE |
| NYC1 | NON-YELLOW COLORING1 |
| O2•− | superoxide anion radical |
| OEC | oxygen evolving complex |
| OH | hydroxyl radicals |
| PAO | pheide a oxygenase |
| pFCC | primary fluorescent chlorophyll catabolite |
| pheide | pheophorbide |
| POD | peroxidase |
| PPH | pheophytinase |
| PPH2 | PHEOPHORBIDASE 2 |
| PsbO | PHOTOSYSTEM II SUBUNIT O |
| PSII | Photosystem II |
| QTL | quantitative trait loci |
| RCA | Rubisco activase |
| RCAL | RCA large isoform |
| RCAS | RCA small isoform |
| RCCR | Red chl catabolite reductase |
| ROS | Reactive oxygen species |
| Rubisco | Ribulose-1,5-bisphosphate carboxylase/oxygenase |
| RuBP | Ribulose-1,5-bisphosphate |
| SGR | STAY-GREEN |
| SID | senescence-induced degradation |
| SOD | superoxide dismutase |
| ZR | zeatin riboside |
References
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.; Bjorkman, O. Photosynthetic Response and Adaptation to Temperature in Higher-Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Yu, H.-D.; Yang, X.-F.; Chen, S.-T.; Wang, Y.-T.; Li, J.-K.; Shen, Q.; Liu, X.-L.; Guo, F.-Q. Downregulation of Chloroplast RPS1 Negatively Modulates Nuclear Heat-Responsive Expression of HsfA2 and Its Target Genes in Arabidopsis. PLoS Genet. 2012, 8, e1002669. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-T.; He, N.-Y.; Chen, J.-H.; Guo, F.-Q. Identification of core subunits of photosystem II as action sites of HSP21, which is activated by the GUN5-mediated retrograde pathway in Arabidopsis. Plant J. 2017, 89, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.-Z.; Guo, F.-Q. Chloroplast Retrograde Regulation of Heat Stress Responses in Plants. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M. Characterization of Thermal-Damage to the Photosynthetic Electron-Transport System in Potato Leaves. Plant Sci. 1993, 94, 19–33. [Google Scholar] [CrossRef]
- Sharkey, T.D. Effects of moderate heat stress on photosynthesis: Importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ. 2005, 28, 269–277. [Google Scholar] [CrossRef]
- Yamada, M.; Hidaka, T.; Fukamachi, H. Heat tolerance in leaves of tropical fruit crops as measured by chlorophyll fluorescence. Sci. Hortic. 1996, 67, 39–48. [Google Scholar] [CrossRef]
- Wise, R.R.; Olson, A.J.; Schrader, S.M.; Sharkey, T.D. Electron transport is the functional limitation of photosynthesis in field-grown Pima cotton plants at high temperature. Plant Cell Environ. 2004, 27, 717–724. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Schrader, S.M. High temperature stress. In Physiology and Molecular Biology of Stress Tolerance in Plants; Springer: New York, NY, USA, 2006; pp. 101–129. [Google Scholar]
- Havaux, M.; Tardy, F. Temperature-dependent adjustment of the thermal stability of photosystem II in vivo: Possible involvement of xanthophyll-cycle pigments. Planta 1996, 198, 324–333. [Google Scholar] [CrossRef]
- Klimov, V.V.; Baranov, S.V.; Allakhverdiev, S.I. Bicarbonate protects the donor side of photosystem II against photoinhibition and thermoinactivation. FEBS Lett. 1997, 418, 243–246. [Google Scholar] [CrossRef]
- Yamane, Y.; Kashino, Y.; Koike, H.; Satoh, K. Effects of high temperatures on the photosynthetic systems in spinach: Oxygen-evolving activities, fluorescence characteristics and the denaturation process. Photosynth. Res. 1998, 57, 51–59. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Relationship between the heat tolerance of photosynthesis and the thermal stability of Rubisco activase in plants from contrasting thermal environments. Plant Physiol. 2004, 134, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Gounaris, K.; Brain, A.R.R.; Quinn, P.J.; Williams, W.P. Structural Reorganization of Chloroplast Thylakoid Membranes in Response to Heat-Stress. Biochim. Biophys. Acta 1984, 766, 198–208. [Google Scholar] [CrossRef]
- Semenova, G.A. Structural reorganization of thylakoid systems in response to heat treatment. Photosynthetica 2004, 42, 521–527. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Aminaka, R.; Yoshioka, M.; Khatoon, M.; Komayama, K.; Takenaka, D.; Yamashita, A.; Nijo, N.; Inagawa, K.; Morita, N.; et al. Quality control of photosystem II: Impact of light and heat stresses. Photosynth. Res. 2008, 98, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Vani, B.; Saradhi, P.P.; Mohanty, P. Alteration in chloroplast structure and thylakoid membrane composition due to in vivo heat treatment of rice seedlings: Correlation with the functional changes. J. Plant Physiol. 2001, 158, 583–592. [Google Scholar] [CrossRef]
- Kmiecik, P.; Leonardelli, M.; Teige, M. Novel connections in plant organellar signalling link different stress responses and signalling pathways. J. Exp. Bot. 2016, 67, 3793–3807. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Hortensteiner, S. Stay-green regulates chlorophyll and chlorophyll-binding protein degradation during senescence. Trends Plant Sci. 2009, 14, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Hortensteiner, S. Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Hortensteiner, S.; Feller, U. Nitrogen metabolism and remobilization during senescence. J. Exp. Bot. 2002, 53, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, S.; Schellenberg, M.; Matile, P. Cleavage of Chlorophyll-Porphyrin—Requirement for Reduced Ferredoxin and Oxygen. Plant Physiol. 1994, 105, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Ohta, H.; Okawa, K.; Iwamatsu, A.; Shimada, H.; Masuda, T.; Takamiya, K. Cloning of chlorophyllase, the key enzyme in chlorophyll degradation: Finding of a lipase motif and the induction by methyl jasmonate. Proc. Natl. Acad. Sci. USA 1999, 96, 15362–15367. [Google Scholar] [CrossRef] [PubMed]
- Schenk, N.; Schelbert, S.; Kanwischer, M.; Goldschmidt, E.E.; Doermann, P.; Hoertensteiner, S. The chlorophyllases AtCLH1 and AtCLH2 are not essential for senescence-related chlorophyll breakdown in Arabidopsis thaliana. FEBS Lett. 2007, 581, 5517–5525. [Google Scholar] [CrossRef] [PubMed]
- Schelbert, S.; Aubry, S.; Burla, B.; Agne, B.; Kessler, F.; Krupinska, K.; Hoertensteiner, S. Pheophytin Pheophorbide Hydrolase (Pheophytinase) Is Involved in Chlorophyll Breakdown during Leaf Senescence in Arabidopsis. Plant Cell 2009, 21, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Rodoni, S.; Muhlecker, W.; Anderl, M.; Krautler, B.; Moser, D.; Thomas, H.; Matile, P.; Hortensteiner, S. Chlorophyll breakdown in senescent chloroplasts—Cleavage of pheophorbide a in two enzymic steps. Plant Physiol. 1997, 115, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Wuthrich, K.L.; Bovet, L.; Hunziker, P.E.; Donnison, I.S.; Hortensteiner, S. Molecular cloning, functional expression and characterisation of RCC reductase involved in chlorophyll catabolism. Plant J. 2000, 21, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Pruzinska, A.; Tanner, G.; Anders, I.; Roca, M.; Hortensteiner, S. Chlorophyll breakdown: Pheophorbide a oxygenase is a Rieske-type iron-sulfur protein, encoded by the accelerated cell death 1 gene. Proc. Natl. Acad. Sci. USA 2003, 100, 15259–15264. [Google Scholar] [CrossRef] [PubMed]
- Pruzinska, A.; Anders, I.; Aubry, S.; Schenk, N.; Tapernoux-Luthi, E.; Muller, T.; Krautler, B.; Hortensteiner, S. In vivo participation of red chlorophyll catabolite reductase in chlorophyll breakdown. Plant Cell 2007, 19, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Hinder, B.; Schellenberg, M.; Rodon, S.; Ginsburg, S.; Vogt, E.; Martinoia, E.; Matile, P.; Hortensteiner, S. How plants dispose of chlorophyll catabolites—Directly energized uptake of tetrapyrrolic breakdown products into isolated vacuoles. J. Biol. Chem. 1996, 271, 27233–27236. [Google Scholar] [CrossRef] [PubMed]
- Tommasini, R.; Vogt, E.; Fromenteau, M.; Hortensteiner, S.; Matile, P.; Amrhein, N.; Martinoia, E. An ABC-transporter of Arabidopsis thaliana has both glutathione-conjugate and chlorophyll catabolite transport activity. Plant J. 1998, 13, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, M.; Ito, H.; Morita, R.; Iida, S.; Sato, Y.; Fujimoto, M.; Kawasaki, S.; Tanaka, R.; Hirochika, H.; Nishimura, M.; et al. Rice NON-YELLOW COLORING1 is involved in light-harvesting complex II and grana degradation during leaf senescence. Plant Cell 2007, 19, 1362–1375. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Morita, R.; Katsuma, S.; Nishimura, M.; Tanaka, A.; Kusaba, M. Two short-chain dehydrogenase/reductases, NON-YELLOW COLORING 1 and NYC1-LIKE, are required for chlorophyll b and light-harvesting complex II degradation during senescence in rice. Plant J. 2009, 57, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Pruzinska, A.; Tanner, G.; Aubry, S.; Anders, I.; Moser, S.; Muller, T.; Ongania, K.H.; Krautler, B.; Youn, J.Y.; Liljegren, S.J.; et al. Chlorophyll breakdown in senescent Arabidopsis leaves. Characterization of chlorophyll catabolites and of chlorophyll catabolic enzymes involved in the degreening reaction. Plant Physiol. 2005, 139, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Hirashima, M.; Satoh, S.; Tanaka, A. The Arabidopsis-accelerated cell death gene ACD1 is involved in oxygenation of pheophorbide a: Inhibition of the pheophorbide a oxygenase activity does not lead to the “Stay-Green” phenotype in Arabidopsis. Plant Cell Physiol. 2003, 44, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H. Sid—A Mendelian Locus Controlling Thylakoid Membrane Disassembly in Senescing Leaves of Festuca-Pratensis. Theor. Appl. Genet. 1987, 73, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Armstead, I.; Donnison, I.; Aubry, S.; Harper, J.; Hoertensteiner, S.; James, C.; Mani, J.; Moffet, M.; Ougham, H.; Roberts, L.; et al. From crop to model to crop: Identifying the genetic basis of the staygreen mutation in the Lolium/Festuca forage and amenity grasses. New Phytol. 2006, 172, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Armstead, I.; Donnison, I.; Aubry, S.; Harper, J.; Hortensteiner, S.; James, C.; Mani, J.; Moffet, M.; Ougham, H.; Roberts, L.; et al. Cross-species identification of Mendel’s/locus. Science 2007, 315, 73. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; An, K.; Liao, Y.; Zhou, X.; Cao, Y.; Zhao, H.; Ge, X.; Kuai, B. Identification of a novel chloroplast protein AtNYE1 regulating chlorophyll degradation during leaf senescence in Arabidopsis. Plant Physiol. 2007, 144, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, M.; Liang, N.; Yan, H.; Wei, Y.; Xu, X.; Liu, J.; Xu, Z.; Chen, F.; Wu, G. Molecular cloning and function analysis of the stay green gene in rice. Plant J. 2007, 52, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Yu, J.-W.; Park, J.-S.; Li, J.; Yoo, S.-C.; Lee, N.-Y.; Lee, S.-K.; Jeong, S.-W.; Seo, H.S.; Koh, H.-J.; et al. The senescence-induced staygreen protein regulates chlorophyll degradation. Plant Cell 2007, 19, 1649–1664. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Morita, R.; Nishimura, M.; Yamaguchi, H.; Kusaba, M. Mendel’s green cotyledon gene encodes a positive regulator of the chlorophyll-degrading pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 14169–14174. [Google Scholar] [CrossRef] [PubMed]
- Aubry, S.; Mani, J.; Hortensteiner, S. Stay-green protein, defective in Mendel’s green cotyledon mutant, acts independent and upstream of pheophorbide a oxygenase in the chlorophyll catabolic pathway. Plant Mol. Biol. 2008, 67, 243–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogson, B.J.; Woo, N.S.; Foerster, B.; Small, I.D. Plastid signalling to the nucleus and beyond. Trends Plant Sci. 2008, 13, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Borovsky, Y.; Paran, I. Chlorophyll breakdown during pepper fruit ripening in the chlorophyll retainer mutation is impaired at the homolog of the senescence-inducible stay-green gene. Theor. Appl. Genet. 2008, 117, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Barry, C.S. The stay-green revolution: Recent progress in deciphering the mechanisms of chlorophyll degradation in higher plants. Plant Sci. 2009, 176, 325–333. [Google Scholar] [CrossRef]
- Hoertensteiner, S. Update on the biochemistry of chlorophyll breakdown. Plant Mol. Biol. 2013, 82, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Burgess, P.; Jespersen, D.; Huang, B. Heat-Induced Leaf Senescence Associated with Chlorophyll Metabolism in Bentgrass Lines Differing in Heat Tolerance. Crop Sci. 2017, 57, S169–S178. [Google Scholar] [CrossRef]
- Todorov, D.T.; Karanov, E.N.; Smith, A.R.; Hall, M.A. Chlorophyllase activity and chlorophyll content in wild type and eti 5 mutant of Arabidopsis thaliana subjected to low and high temperatures. Biol. Plant. 2003, 46, 633–636. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.V.; Boyle, D.L.; Schapaugh, W.T. High-Temperature Stress and Soybean Leaves: Leaf Anatomy and Photosynthesis. Crop Sci. 2011, 51, 2125–2131. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.V.; Murugan, M.; Perumal, R.; Reddy, U.K. Physiological differences among sorghum (Sorghum bicolor L. Moench) genotypes under high temperature stress. Environ. Exp. Bot. 2014, 100, 43–54. [Google Scholar] [CrossRef]
- Ristic, Z.; Momcilovic, I.; Fu, J.M.; Callegaric, E.; DeRidder, B.P. Chloroplast protein synthesis elongation factor, EF-Tu, reduces thermal aggregation of Rubisco activase. J. Plant Physiol. 2007, 164, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Akter, N.; Islam, M.R. Heat stress effects and management in wheat. A review. Agron. Sustain. Dev. 2017, 37, 37. [Google Scholar] [CrossRef]
- Liu, X.Z.; Huang, B.R. Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci. 2000, 40, 503–510. [Google Scholar] [CrossRef]
- Jespersen, D.; Zhang, J.; Huang, B. Chlorophyll loss associated with heat-induced senescence in bentgrass. Plant Sci. 2016, 249, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-P.; Wu, M.-C.; Charng, Y.-Y. Identification of a Chlorophyll Dephytylase Involved in Chlorophyll Turnover in Arabidopsis. Plant Cell 2016, 28, 2974–2990. [Google Scholar] [CrossRef] [PubMed]
- Veerasamy, M.; He, Y.; Huang, B. Leaf senescence and protein metabolism in creeping bentgrass exposed to heat stress and treated with cytokinins. J. Am. Soc. Hortic. Sci. 2007, 132, 467–472. [Google Scholar]
- Chen, Y.; Cothren, J.T.; Chen, D.-H.; Ibrahim, A.M.H.; Lombardini, L. Ethylene-inhibiting compound 1-MCP delays leaf senescence in cotton plants under abiotic stress conditions. J. Integr. Agric. 2015, 14, 1321–1331. [Google Scholar] [CrossRef]
- Huberman, M.; Riov, J.; Goldschmidt, E.E.; Apelbaum, A.; Goren, R. The novel ethylene antagonist, 3-cyclopropyl-1-enyl-propanoic acid sodium salt (CPAS), increases grain yield in wheat by delaying leaf senescence. Plant Growth Regul. 2014, 73, 249–255. [Google Scholar] [CrossRef]
- Abdelrahman, M.; El-Sayed, M.; Jogaiah, S.; Burritt, D.J.; Lam-Son Phan, T. The “STAY-GREEN” trait and phytohormone signaling networks in plants under heat stress. Plant Cell Rep. 2017, 36, 1009–1025. [Google Scholar] [CrossRef] [PubMed]
- Suzuky Pinto, R.; Lopes, M.S.; Collins, N.C.; Reynolds, M.P. Modelling and genetic dissection of staygreen under heat stress. Theor. Appl. Genet. 2016, 129, 2055–2074. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.N.; Ligterink, W.; Franca-Neto, J.D.B.; Hilhorst, H.W.M.; da Silva, E.A.A. Gene expression profiling of the green seed problem in Soybean. BMC Plant Biol. 2016, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, K.; Fritz, A.K.; Paulsen, G.M.; Bai, G.; Pandravada, S.; Gill, B.S. Modeling and mapping QTL for senescence-related traits in winter wheat under high temperature. Mol. Breed. 2010, 26, 163–175. [Google Scholar] [CrossRef]
- Chen, Y.-E.; Zhang, C.-M.; Su, Y.-Q.; Ma, J.; Zhang, Z.-W.; Yuan, M.; Zhang, H.-Y.; Yuan, S. Responses of photosystem II and antioxidative systems to high light and high temperature co-stress in wheat. Environ. Exp. Bot. 2017, 135, 45–55. [Google Scholar] [CrossRef]
- Shirdelmoghanloo, H.; Cozzolino, D.; Lohraseb, I.; Collins, N.C. Truncation of grain filling in wheat (Triticum aestivum) triggered by brief heat stress during early grain filling: Association with senescence responses and reductions in stem reserves. Funct. Plant Biol. 2016, 43, 919–930. [Google Scholar] [CrossRef]
- Shirdelmoghanloo, H.; Lohraseb, I.; Rabie, H.S.; Brien, C.; Parent, B.; Collins, N.C. Heat susceptibility of grain filling in wheat (Triticum aestivum L.) linked with rapid chlorophyll loss during a 3-day heat treatment. Acta Physiol. Plant. 2016, 38, 208. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Pospisil, P.; Prasad, A. Formation of singlet oxygen and protection against its oxidative damage in Photosystem II under abiotic stress. J. Photochem. Photobiol. B Biol. 2014, 137, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; LopezDelgado, H.; Dat, J.F.; Scott, I.M. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol. Plant. 1997, 100, 241–254. [Google Scholar] [CrossRef]
- Dat, J.F.; Lopez-Delgado, H.; Foyer, C.H.; Scott, I.M. Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol. 1998, 116, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Vallelian-Bindschedler, L.; Schweizer, P.; Mosinger, E.; Metraux, J.P. Heat-induced resistance in barley to powdery mildew (Blumeria graminis f.sp. hordei) is associated with a burst of active oxygen species. Physiol. Mol. Plant Pathol. 1998, 52, 185–199. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Signaling in Plants. Antioxid. Redox Signal. 2013, 18, 2087–2090. [Google Scholar] [CrossRef] [PubMed]
- Vacca, R.A.; Valenti, D.; Bobba, A.; Merafina, R.S.; Passarella, S.; Marra, E. Cytochrome c is released in a reactive oxygen species-dependent manner and is degraded via caspase-like proteases in tobacco bright-yellow 2 cells en route to heat shock-induced cell death. Plant Physiol. 2006, 141, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Duan, W.; Takabayashi, A.; Endo, T.; Shikanai, T.; Ye, J.Y.; Mi, H.L. Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress. Plant Physiol. 2006, 141, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Nijo, N.; Pospisil, P.; Morita, N.; Takenaka, D.; Aminaka, R.; Yamamoto, Y.; Yamamoto, Y. Quality control of photosystem II—Reactive oxygen species are responsible for the damage to photosystem II under moderate heat stress. J. Biol. Chem. 2008, 283, 28380–28391. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-P.; Lee, T.-Y.; Tanaka, A.; Charng, Y.-Y. Analysis of an Arabidopsis heat-sensitive mutant reveals that chlorophyll synthase is involved in reutilization of chlorophyllide during chlorophyll turnover. Plant J. 2014, 80, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Edreva, A. Generation and scavenging of reactive oxygen species in chloroplasts: A submolecular approach. Agric. Ecosyst. Environ. 2005, 106, 119–133. [Google Scholar] [CrossRef]
- Foyer, C.H.; Neukermans, J.; Queval, G.; Noctor, G.; Harbinson, J. Photosynthetic control of electron transport and the regulation of gene expression. J. Exp. Bot. 2012, 63, 1637–1661. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox Regulation in Photosynthetic Organisms: Signaling, Acclimation, and Practical Implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Ascorbate and Glutathione: The Heart of the Redox Hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Managing the cellular redox hub in photosynthetic organisms. Plant Cell Environ. 2012, 35, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Sainz, M.; Diaz, P.; Monza, J.; Borsani, O. Heat stress results in loss of chloroplast Cu/Zn superoxide dismutase and increased damage to Photosystem II in combined drought-heat stressed Lotus japonicus. Physiol. Plant. 2010, 140, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.Q.; Rodrigues, W.P.; Fortunato, A.S.; Leitao, A.E.; Rodrigues, A.P.; Pais, I.P.; Martins, L.D.; Silva, M.J.; Reboredo, F.H.; Partelli, F.L.; et al. Protective Response Mechanisms to Heat Stress in Interaction with High [CO2] Conditions in Coffea spp. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Yoshida, N.; Fujita, M. Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul. 2014, 73, 31–44. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Wollenweber, B.; Jiang, D. Multiple heat priming enhances thermo-tolerance to a later high temperature stress via improving subcellular antioxidant activities in wheat seedlings. Plant Physiol. Biochem. 2014, 74, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhou, P.; Huang, B. Antioxidant enzymatic activities and gene expression associated with heat tolerance in a cool-season perennial grass species. Environ. Exp. Bot. 2013, 87, 159–166. [Google Scholar] [CrossRef]
- Kong, F.; Deng, Y.; Wang, G.; Wang, J.; Liang, X.; Meng, Q. LeCDJ1, a chloroplast DnaJ protein, facilitates heat tolerance in transgenic tomatoes. J. Integr. Plant Biol. 2014, 56, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Yuan, L.; Zhu, S.; Liu, S.; Ge, J.; Wang, C. Effects of heat stress on photosynthetic characteristics and chloroplast ultrastructure of a heat-sensitive and heat-tolerant cultivar of wucai (Brassica campestris L.). Acta Physiol. Plant. 2017, 39. [Google Scholar] [CrossRef]
- Ara, N.; Nakkanong, K.; Lv, W.; Yang, J.; Hu, Z.; Zhang, M. Antioxidant Enzymatic Activities and Gene Expression Associated with Heat Tolerance in the Stems and Roots of Two Cucurbit Species (“Cucurbita maxima” and “Cucurbita moschata”) and Their Interspecific Inbred Line “Maxchata”. Int. J. Mol. Sci. 2013, 14, 24008–24028. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, J.; Dehesh, K. Review of stress specific organelles-to-nucleus metabolic signal molecules in plants. Plant Sci. 2013, 212, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Komayama, K.; Khatoon, M.; Takenaka, D.; Horie, J.; Yamashita, A.; Yoshioka, M.; Nakayama, Y.; Yoshida, M.; Ohira, S.; Morita, N.; et al. Quality control of photosystem II: Cleavage and aggregation of heat-damaged D1 protein in spinach thylakoids. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; Uchida, S.; Mori, H.; Komayama, K.; Ohira, S.; Morita, N.; Nakanishi, T.; Yamamoto, Y. Quality control of photosystem II—Cleavage of reaction center D1 protein in spinach thylakoids by FtsH protease under moderate heat stress. J. Biol. Chem. 2006, 281, 21660–21669. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, M.; Inagawa, K.; Pospisil, P.; Yamashita, A.; Yoshioka, M.; Lundin, B.; Horie, J.; Morita, N.; Jajoo, A.; Yamamoto, Y.; et al. Quality Control of Photosystem II thylakoid unstacking is necessary to avoid further damage to the D1 protein and to facilitate D1 degradation under light stress in spinach thylakoids. J. Biol. Chem. 2009, 284, 25343–25352. [Google Scholar] [CrossRef] [PubMed]
- Jarvi, S.; Suorsa, M.; Aro, E.M. Photosystem II repair in plant chloroplasts—Regulation, assisting proteins and shared components with photosystem II biogenesis. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Vass, I.; Cser, K. Janus-faced charge recombinations in photosystem II photoinhibition. Trends Plant Sci. 2009, 14, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Telfer, A.; Dhami, S.; Bishop, S.M.; Phillips, D.; Barber, J. Beta-Carotene Quenches Singlet Oxygen Formed by Isolated Photosystem-II Reaction Centers. Biochemistry 1994, 33, 14469–14474. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Trebst, A. Plastoquinol as a singlet oxygen scavenger in photosystem II. Biochim. Biophys. Acta Bioenerg. 2008, 1777, 154–162. [Google Scholar]
- Weisz, D.A.; Gross, M.L.; Pakrasi, H.B. Reactive oxygen species leave a damage trail that reveals water channels in Photosystem II. Sci. Adv. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Pospisil, P.; Yamamoto, Y. Damage to photosystem II by lipid peroxidation products. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.I.; Chow, W.S.; Anderson, J.M. Light Inactivation of Functional Photosystem-Ii in Leaves of Peas Grown in Moderate Light Depends on Photon Exposure. Planta 1995, 196, 401–411. [Google Scholar] [CrossRef]
- Tyystjarvi, E.; Aro, E.M. The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc. Natl. Acad. Sci. USA 1996, 93, 2213–2218. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.J.; Alexova, R.; Jacoby, R.P.; Millar, A.H. Proteins with High Turnover Rate in Barley Leaves Estimated by Proteome Analysis Combined with in Planta Isotope Labeling. Plant Physiol. 2014, 166, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Bergantino, E.; Brunetta, A.; Touloupakis, E.; Segalla, A.; Szabo, I.; Giacometti, G.M. Role of the PSII-H subunit in photoprotection—Novel aspects of D1 turnover in Synechocystis 6803. J. Biol. Chem. 2003, 278, 41820–41829. [Google Scholar] [CrossRef] [PubMed]
- Rokka, A.; Suorsa, M.; Saleem, A.; Battchikova, N.; Aro, E.M. Synthesis and assembly of thylakoid protein complexes: Multiple assembly steps of photosystem II. Biochem. J. 2005, 388, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Sakamoto, W. Protein Quality Control in Chloroplasts: A Current Model of D1 Protein Degradation in the Photosystem II Repair Cycle. J. Biochem. 2009, 146, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Barber, J.; Andersson, B. Too Much of a Good Thing—Light Can Be Bad for Photosynthesis. Trends Biochem. Sci. 1992, 17, 61–66. [Google Scholar] [CrossRef]
- Aro, E.M.; Virgin, I.; Andersson, B. Photoinhibition of Photosystem-2—Inactivation, Protein Damage and Turnover. Biochim. Biophys. Acta 1993, 1143, 113–134. [Google Scholar] [CrossRef]
- Adir, N.; Zer, H.; Shochat, S.; Ohad, I. Photoinhibition—A historical perspective. Photosynth. Res. 2003, 76, 343–370. [Google Scholar] [CrossRef] [PubMed]
- Aro, E.M.; Suorsa, M.; Rokka, A.; Allahverdiyeva, Y.; Paakkarinen, V.; Saleem, A.; Battchikova, N.; Rintamaki, E. Dynamics of photosystem II: A proteomic approach to thylakoid protein complexes. J. Exp. Bot. 2005, 56, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Baena-Gonzalez, E.; Aro, E.M. Biogenesis, assembly and turnover of photosystem II units. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2002, 357, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Cheregi, O.; Wagner, R.; Funk, C. Insights into the Cyanobacterial Deg/HtrA Proteases. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Huesgen, P.F.; Schuhmann, H.; Adamska, I. Deg/HtrA proteases as components of a network for photosystem II quality control in chloroplasts and cyanobacteria. Res. Microbiol. 2009, 160, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, H.; Huesgen, P.F.; Adamska, I. The family of Deg/HtrA proteases in plants. BMC Plant Biol. 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, H.; Adamska, I. Deg proteases and their role in protein quality control and processing in different subcellular compartments of the plant cell. Physiol. Plant. 2012, 145, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, L.; Zhang, L. Involvement of DEG5 and DEG8 proteases in the turnover of the photosystem II reaction center D1 protein under heat stress in Arabidopsis thaliana. Chin. Sci. Bull. 2007, 52, 1742–1745. [Google Scholar] [CrossRef]
- Kato, Y.; Sun, X.; Zhang, L.; Sakamoto, W. Cooperative D1 Degradation in the Photosystem II Repair Mediated by Chloroplastic Proteases in Arabidopsis. Plant Physiol. 2012, 159, 1428–1439. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Burke, J.J.; Velten, J.; Xin, Z.U. FtsH11 protease plays a critical role in Arabidopsis thermotolerance. Plant J. 2006, 48, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; Yamamoto, Y. Quality control of Photosystem II: Where and how does the degradation of the D1 protein by FtsH proteases start under light stress?—Facts and hypotheses. J. Photochem. Photobiol. B Biol. 2011, 104, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Peltier, J.B.; Emanuelsson, O.; Kalume, D.E.; Ytterberg, J.; Friso, G.; Rudella, A.; Liberles, D.A.; Soderberg, L.; Roepstorff, P.; von Heijne, G.; et al. Central functions of the lumenal and peripheral thylakoid proteome of Arabidopsis determined by experimentation and genome-wide prediction. Plant Cell 2002, 14, 211–236. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Petersson, U.A.; Haas, B.J.; Funk, C.; Schroder, W.P.; Kieselbach, T. Proteome map of the chloroplast lumen of Arabidopsis thaliana. J. Biol. Chem. 2002, 277, 8354–8365. [Google Scholar] [CrossRef] [PubMed]
- Kapri-Pardes, E.; Naveh, L.; Adam, Z. The thylakoid lumen protease Deg1 is involved in the repair of photosystem II from photoinhibition in Arabidopsis. Plant Cell 2007, 19, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Peng, L.; Guo, J.; Chi, W.; Ma, J.; Lu, C.; Zhang, L. Formation of DEG5 and DEG8 complexes and their involvement in the degradation of photodamaged photosystem II reaction center D1 protein in Arabidopsis. Plant Cell 2007, 19, 1347–1361. [Google Scholar] [CrossRef] [PubMed]
- Kley, J.; Schmidt, B.; Boyanov, B.; Stolt-Bergner, P.C.; Kirk, R.; Ehrmann, M.; Knopf, R.R.; Naveh, L.; Adam, Z.; Clausen, T. Structural adaptation of the plant protease Deg1 to repair photosystem II during light exposure. Nat. Struct. Mol. Biol. 2011, 18, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ouyang, M.; Guo, J.; Ma, J.; Lu, C.; Adam, Z.; Zhang, L. The thylakoid protease Deg1 is involved in photosystem-II assembly in Arabidopsis thaliana. Plant J. 2010, 62, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Chassin, Y.; Kapri-Pardes, E.; Sinvany, G.; Arad, T.; Adam, Z. Expression and characterization of the thylakoid lumen protease DegP1 from Arabidopsis. Plant Physiol. 2002, 130, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Aro, E.M.; McCaffery, S.; Anderson, J.M. Photoinhibition and D1 Protein-Degradation in Peas Acclimated to Different Growth Irradiances. Plant Physiol. 1993, 103, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Tyedmers, J.; Mogk, A.; Bukau, B. Cellular strategies for controlling protein aggregation. Nat. Rev. Mol. Cell Biol. 2010, 11, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Vierling, E. The Roles of Heat-Shock Proteins in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 579–620. [Google Scholar] [CrossRef]
- Chen, Q.; Vierling, E. Analysis of Conserved Domains Identifies a Unique Structural Feature of a Chloroplast Heat-Shock Protein. Mol. Gen. Genet. 1991, 226, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lauzon, L.M.; Derocher, A.E.; Vierling, E. Accumulation, Stability, and Localization of a Major Chloroplast Heat-Shock Protein. J. Cell Biol. 1990, 110, 1873–1883. [Google Scholar] [CrossRef] [PubMed]
- Vierling, E.; Harris, L.M.; Chen, Q. The Major Low-Molecular-Weight Heat-Shock Protein in Chloroplasts Shows Antigenic Conservation among Diverse Higher-Plant Species. Mol. Cell. Biol. 1989, 9, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Heckathorn, S.A.; Downs, C.A.; Sharkey, T.D.; Coleman, J.S. The small, methionine-rich chloroplast heat-shock protein protects photosystem II electron transport during heat stress. Plant Physiol. 1998, 116, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Heckathorn, S.A.; Ryan, S.L.; Baylis, J.A.; Wang, D.F.; Hamilton, E.W.; Cundiff, L.; Luthe, D.S. In vivo evidence from an Agrostis stolonifera selection genotype that chloroplast small heat-shock proteins can protect photosystem II during heat stress. Funct. Plant Biol. 2002, 29, 933–944. [Google Scholar] [CrossRef]
- Kim, K.-H.; Alam, I.; Kim, Y.-G.; Sharmin, S.A.; Lee, K.-W.; Lee, S.-H.; Lee, B.-H. Overexpression of a chloroplast-localized small heat shock protein OsHSP26 confers enhanced tolerance against oxidative and heat stresses in tall fescue. Biotechnol. Lett. 2012, 34, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, S.; Ul Haq, N.; Heckathorn, S.A.; Hamilton, E.W.; Luthe, D.S. Ecotypic variation in chloroplast small heat-shock proteins and related thermotolerance in Chenopodium album. Plant Physiol. Biochem. 2011, 49, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.F.; Luthe, D.S. Heat sensitivity in a bentgrass variant. Failure to accumulate a chloroplast heat shock protein isoform implicated in heat tolerance. Plant Physiol. 2003, 133, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Harndahl, U.; Hall, R.B.; Osteryoung, K.W.; Vierling, E.; Bornman, J.F.; Sundby, C. The chloroplast small heat shock protein undergoes oxidation-dependent conformational changes and may protect plants from oxidative stress. Cell Stress Chaperones 1999, 4, 129–138. [Google Scholar] [CrossRef]
- Neta-Sharir, I.; Isaacson, T.; Lurie, S.; Weiss, D. Dual role for tomato heat shock protein 21: Protecting photosystem II from oxidative stress and promoting color changes during fruit maturation. Plant Cell 2005, 17, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Downs, C.A.; Coleman, J.S.; Heckathorn, S.A. The chloroplast 22-Ku heat-shock protein: A lumenal protein that associates with the oxygen evolving complex and protects photosystem II during heat stress. J. Plant Physiol. 1999, 155, 477–487. [Google Scholar] [CrossRef]
- Bernfur, K.; Rutsdottir, G.; Emanuelsson, C. The chloroplast-localized small heat shock protein Hsp21 associates with the thylakoid membranes in heat-stressed plants. Protein Sci. Publ. Protein Soc. 2017, 26, 1773–1784. [Google Scholar] [CrossRef] [PubMed]
- Spreitzer, R.J.; Salvucci, M.E. Rubisco: Structure, regulatory interactions, and possibilities for a better enzyme. Annu. Rev. Plant Biol. 2002, 53, 449–475. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, J.A.; Capo-Bauca, S.; Carmo-Silva, E.; Galmes, J. Rubisco and Rubisco Activase Play an Important Role in the Biochemical Limitations of Photosynthesis in Rice, Wheat, and Maize under High Temperature and Water Deficit. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F.; Way, D.A.; Kubien, D.S. Rubisco, Rubisco activase, and global climate change. J. Exp. Bot. 2008, 59, 1581–1595. [Google Scholar] [CrossRef] [PubMed]
- Portis, A.R. Rubisco activase—Rubisco’s catalytic chaperone. Photosynth. Res. 2003, 75, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Schrader, S.M.; Wise, R.R.; Wacholtz, W.F.; Ort, D.R.; Sharkey, T.D. Thylakoid membrane responses to moderately high leaf temperature in Pima cotton. Plant Cell Environ. 2004, 27, 725–735. [Google Scholar] [CrossRef]
- Yamori, W.; Noguchi, K.; Kashino, Y.; Terashima, I. The role of electron transport in determining the temperature dependence of the photosynthetic rate in spinach leaves grown at contrasting temperatures. Plant Cell Physiol. 2008, 49, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Carmo-Silva, A.E.; Salvucci, M.E. The activity of Rubisco’s molecular chaperone, Rubisco activase, in leaf extracts. Photosynth. Res. 2011, 108, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Salvucci, M.E.; Crafts-Brandner, S.J. Mechanism for deactivation of Rubisco under moderate heat stress. Physiol. Plant. 2004, 122, 513–519. [Google Scholar] [CrossRef]
- Robinson, S.P.; Streusand, V.J.; Chatfield, J.M.; Portis, A.R. Purification and Assay of Rubisco activase from Leaves. Plant Physiol. 1988, 88, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, G.P.; Galasinski, S.C.; Salvucci, M.E. Regulation of 2-Carboxyarabinitol 1-Phosphatase. Plant Physiol. 1991, 97, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Feller, U.; Crafts-Brandner, S.J.; Salvucci, M.E. Moderately high temperatures inhibit ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase-mediated activation of Rubisco. Plant Physiol. 1998, 116, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Salvucci, M.E.; Osteryoung, K.W.; Crafts-Brandner, S.J.; Vierling, E. Exceptional sensitivity of Rubisco activase to thermal denaturation in vitro and in vivo. Plant Physiol. 2001, 127, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Schrader, S.M.; Kleinbeck, K.R.; Sharkey, T.D. Rapid heating of intact leaves reveals initial effects of stromal oxidation on photosynthesis. Plant Cell Environ. 2007, 30, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Sharkey, T.D. Photosynthetic electron transport and proton flux under moderate heat stress. Photosynth. Res. 2009, 100, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Way, D.A.; Yamori, W. Thermal acclimation of photosynthesis: On the importance of adjusting our definitions and accounting for thermal acclimation of respiration. Photosynth. Res. 2014, 119, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C-3, C-4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Salvucci, M.E.; Crafts-Brandner, S.J. Inhibition of photosynthesis by heat stress: The activation state of Rubisco as a limiting factor in photosynthesis. Physiol. Plant. 2004, 120, 179–186. [Google Scholar] [CrossRef] [PubMed]
- DeRidder, B.P.; Salvucci, M.E. Modulation of Rubisco activase gene expression during heat stress in cotton (Gossypium hirsutum L.) involves post-transcriptional mechanisms. Plant Sci. 2007, 172, 246–254. [Google Scholar] [CrossRef]
- Kumar, R.R.; Goswami, S.; Singh, K.; Dubey, K.; Singh, S.; Sharma, R.; Verma, N.; Kala, Y.K.; Rai, G.K.; Grover, M.; et al. Identification of Putative RuBisCo Activase (TaRca1)-The Catalytic Chaperone Regulating Carbon Assimilatory Pathway in Wheat (Triticum aestivum) under the Heat Stress. Front. Plant Sci. 2016, 7, 986. [Google Scholar] [CrossRef] [PubMed]
- Kurek, I.; Chang, T.K.; Bertain, S.M.; Madrigal, A.; Liu, L.; Lassner, M.W.; Zhu, G. Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell 2007, 19, 3230–3241. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Li, C.; Portis, A.R., Jr. Arabidopsis thaliana expressing a thermostable chimeric Rubisco activase exhibits enhanced growth and higher rates of photosynthesis at moderately high temperatures. Photosynth. Res. 2009, 100, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Ristic, Z.; Momcilovic, I.; Bukovnik, U.; Prasad, P.V.V.; Fu, J.; DeRidder, B.P.; Elthon, T.E.; Mladenov, N. Rubisco activase and wheat productivity under heat-stress conditions. J. Exp. Bot. 2009, 60, 4003–4014. [Google Scholar] [CrossRef] [PubMed]
- Shivhare, D.; Mueller-Cajar, O. In Vitro Characterization of Thermostable CAM Rubisco Activase Reveals a Rubisco Interacting Surface Loop. Plant Physiol. 2017, 174, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Salvucci, M.E. Association of Rubisco activase with chaperonin-60 beta: A possible mechanism for protecting photosynthesis during heat stress. J. Exp. Bot. 2008, 59, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Scales, J.C.; Parry, M.A.J.; Salvucci, M.E. A non-radioactive method for measuring Rubisco activase activity in the presence of variable ATP: ADP ratios, including modifications for measuring the activity and activation state of Rubisco. Photosynth. Res. 2014, 119, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Carmo-Silva, A.E.; Salvucci, M.E. The Regulatory Properties of Rubisco activase Differ among Species and Affect Photosynthetic Induction during Light Transitions. Plant Physiol. 2013, 161, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, X.-F.; Zhou, Z.-J.; Feng, X.-P.; Yang, W.-J.; Jiang, D.-A. Two Rubisco activase isoforms may play different roles in photosynthetic heat acclimation in the rice plant. Physiol. Plant. 2010, 139, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Hozain, M.D.I.; Salvucci, M.E.; Fokar, M.; Holaday, A.S. The differential response of photosynthesis to high temperature for a boreal and temperate Populus species relates to differences in Rubisco activation and Rubisco activase properties. Tree Physiol. 2010, 30, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Feller, U. Drought stress and carbon assimilation in a warming climate: Reversible and irreversible impacts. J. Plant Physiol. 2016, 203, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.-L.; Chen, J.-H.; He, N.-Y.; Guo, F.-Q. Metabolic Reprogramming in Chloroplasts under Heat Stress in Plants. Int. J. Mol. Sci. 2018, 19, 849. https://doi.org/10.3390/ijms19030849
Wang Q-L, Chen J-H, He N-Y, Guo F-Q. Metabolic Reprogramming in Chloroplasts under Heat Stress in Plants. International Journal of Molecular Sciences. 2018; 19(3):849. https://doi.org/10.3390/ijms19030849
Chicago/Turabian StyleWang, Qing-Long, Juan-Hua Chen, Ning-Yu He, and Fang-Qing Guo. 2018. "Metabolic Reprogramming in Chloroplasts under Heat Stress in Plants" International Journal of Molecular Sciences 19, no. 3: 849. https://doi.org/10.3390/ijms19030849
APA StyleWang, Q.-L., Chen, J.-H., He, N.-Y., & Guo, F.-Q. (2018). Metabolic Reprogramming in Chloroplasts under Heat Stress in Plants. International Journal of Molecular Sciences, 19(3), 849. https://doi.org/10.3390/ijms19030849




