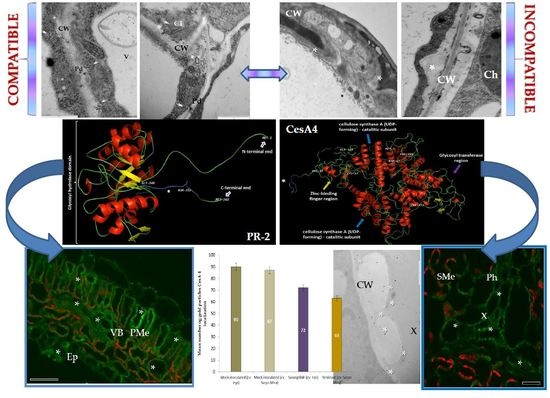
Plant Cell Wall Dynamics in Compatible and Incompatible Potato Response to Infection Caused by Potato Virus Y (PVYNTN)
Abstract
:1. Introduction
2. Results
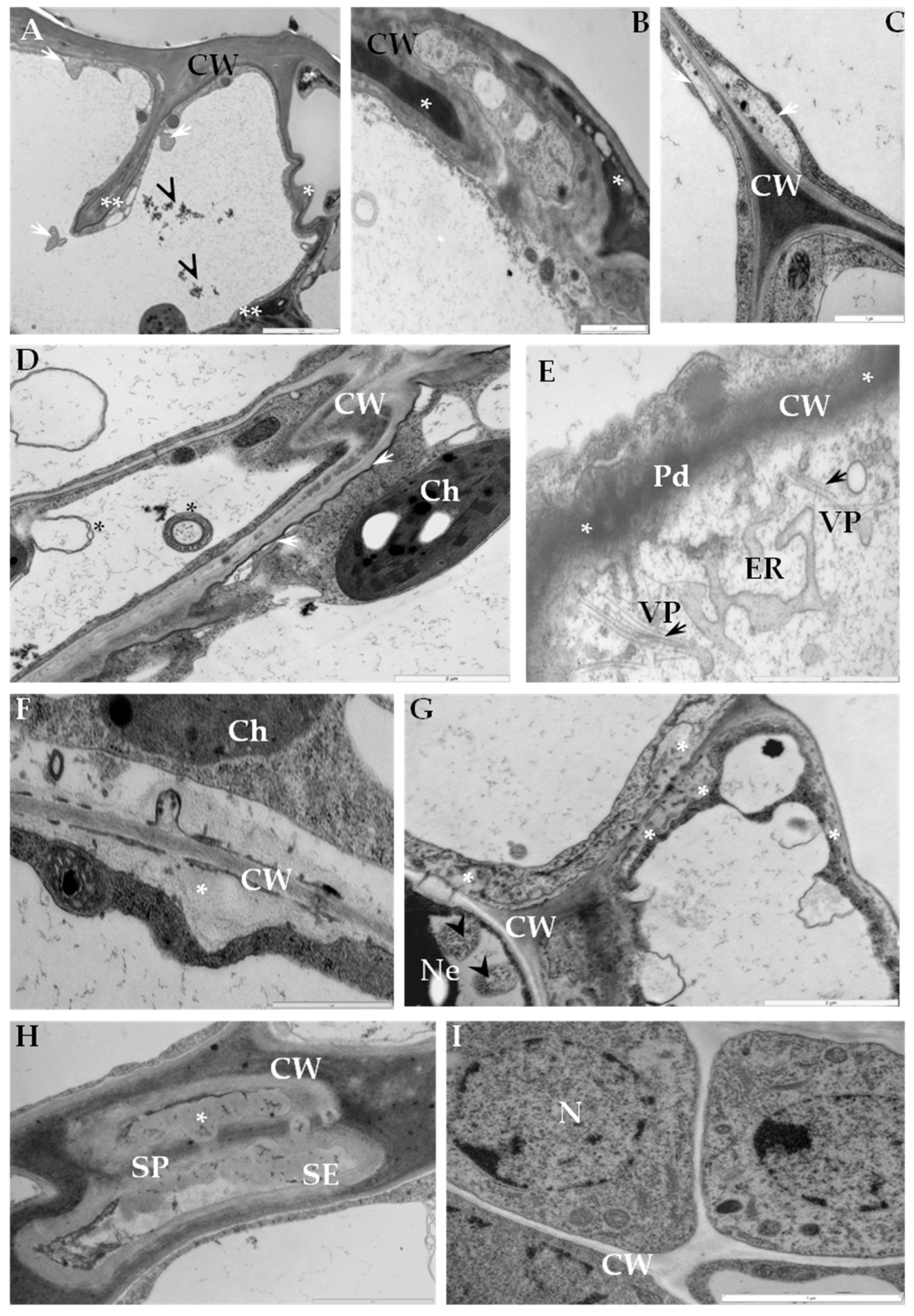
2.1. Ultrastructural Analyses of Adjoining Cell Wall Connections in Compatible and Incompatible PVYNTN-Potato Interactions
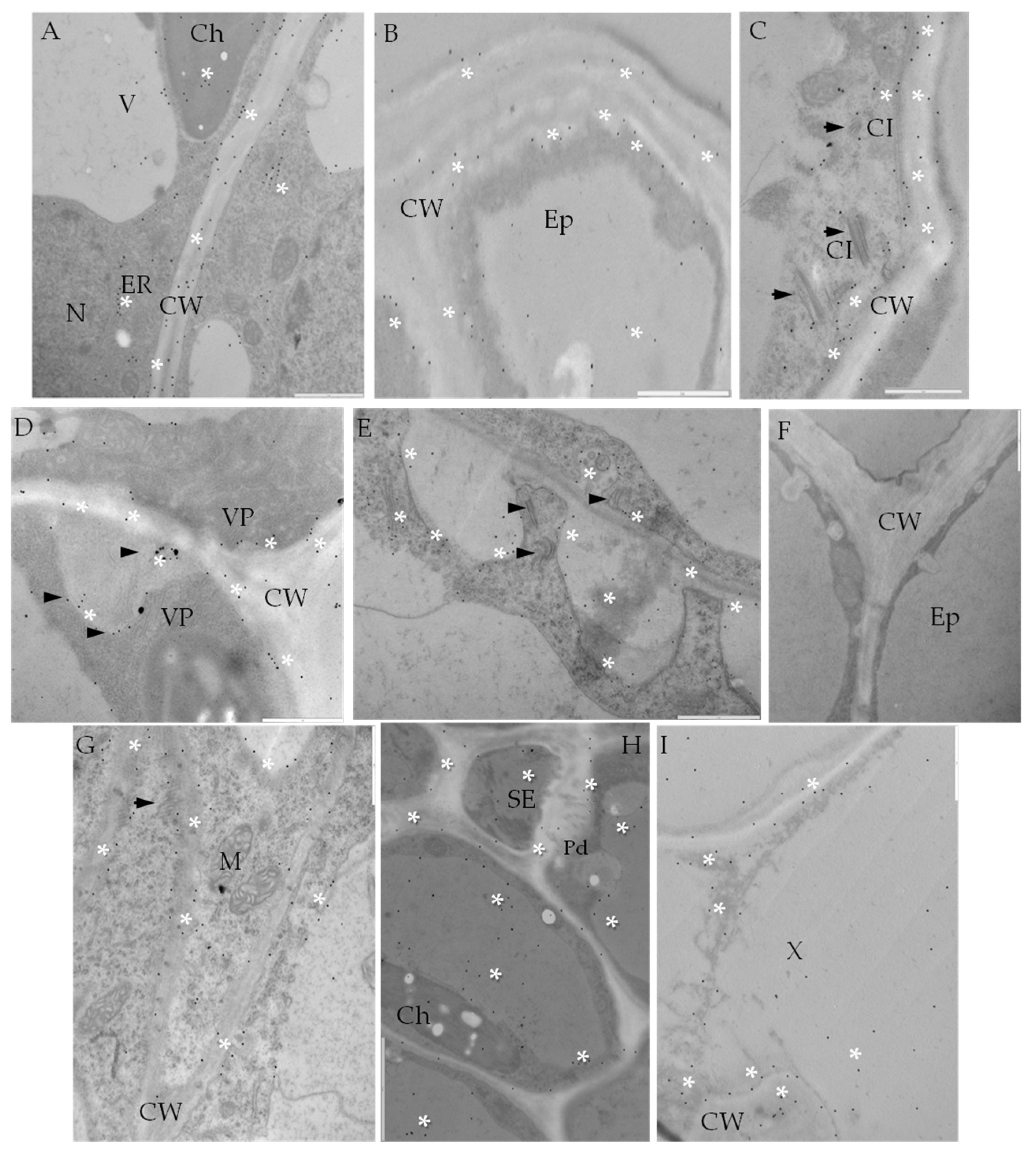
2.2. Localization of Pathogenesis Related Protein 2 (PR-2, β-1,3 Glucanase, EC 3.2.1.39)
2.3. Localization of Cellulose Synthase 4 (CesA4, EC 2.4.1.12)
2.4. Localization of Extensin (HRGPs) in Susceptible and Resistant Potato-PVYNTN Interactions
3. Discussion
4. Materials and Methods
4.1. Plant Material and Virus Inoculation
4.2. Ultrastructural Examinations
4.3. Immunofluorescence Localization
4.4. Immunogold Labeling
4.5. Statistical Quantification of Tissue and Cell Distribution of Immunogold-Labeled Cell Wall-Associated Proteins PR-2, CesA4 and HRGP (Extensin)
5. Conclusions
Acknowledgments
Author Contributions
Conflict of Interest
References
- Carpita, N.C. Progress in the biological synthesis of the plant cell wall: New ideas for improving biomass for bioenergy. Curr. Opin. Biotechnol. 2012, 23, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Showalter, A.M. Structure and function of plant cell wall proteins. Plant Cell 1993, 5, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Malinovsky, F.G.; Fangel, J.U.; Willats, W.G.T. The role of the cell wall in plant immunity. Front. Plant Sci. 2014, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rybicki, E.P. A top ten list for economically important plant viruses. Arch. Virol. 2015, 160, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Chikh Ali, M.; Karasev, A.V.; Furutani, N.; Taniguchi, M.; Kano, Y.; Sato, M.; Natsuaki, T.; Maoka, T. Occurrence of Potato virus Y strain PVY NTN in foundation seed potatoes in Japan, and screening for symptoms in Japanese potato cultivars. Plant Pathol. 2013, 62, 1157–1165. [Google Scholar] [CrossRef]
- Dangl, J.L.; Dietrich, R.A.; Richberg, M.H. Death don’t have no mercy: cell death programs in plant-microbe interactions. Plant Cell 1996, 8, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.M.; Cilia, M. A molecular tug-of-war: Global plant proteome changes during viral infection. Curr. Plant Biol. 2016, 5, 13–24. [Google Scholar] [CrossRef]
- Stavolone, L.; Lionetti, V. Extracellular matrix in plants and animals: Hooks and locks for viruses. Front. Microbiol. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, V.; Cervone, F.; Bellincampi, D. Methyl esterification of pectin plays a role during plant-pathogen interactions and affects plant resistance to diseases. J. Plant Physiol. 2012, 169, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Hull, R. Plant Virology, 5th ed.; Elsevier Academic Press: London, UK, 2013; pp. 669–754. ISBN 9780123848710. [Google Scholar]
- Otulak, K.; Kozieł, E.; Garbaczewska, G. “Seeing is believing”. The use of light, fluorescent and transmission electron microscopy in the observation of pathological changes during different plant—Virus interactions. In Microscopy: Advances in Scientific Research and Education, 6th ed.; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2014; Volume 1, pp. 367–376. ISBN 978-84-942134-3-4. [Google Scholar]
- Nguema-Ona, E.; Vicré-Gibouin, M.; Cannesan, M.A.; Driouich, A. Arabinogalactan proteins in root–microbe interactions. Trends Plant Sci. 2013, 18, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Bethke, G.; Thao, A.; Xiong, G.; Li, B.; Soltis, N.E.; Hatsugai, N.; Hillmer, R.A.; Katagiri, F.; Kliebenstein, D.J.; Pauly, M.; et al. Pectin biosynthesis is critical for cell wall integrity and immunity in Arabidopsis thaliana. Plant Cell 2016, 28, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lilley, C.J.; Imren, M.; Knox, J.P.; Urwin, P.E. The complex cell wall composition of syncytia induced by plant parasitic cyst nematodes reflects both function and host plant. Front. Plant Sci. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, D.; Voigt, C.A. Callose biosynthesis in Arabidopsis with a focus on pathogen response: What we have learned within the last decade. Ann. Bot. 2014, 114, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.E.; Funnell-Harris, D.L. Modifying lignin to improve bioenergy feedstocks: Strengthening the barrier against pathogens. Front. Plant Sci. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Di Carli, M.; Benvenuto, E.; Donini, M. Recent insights into plant–virus interactions through proteomic analysis. J. Proteome Res. 2012, 11, 4765–4780. [Google Scholar] [CrossRef] [PubMed]
- Bruknard, J.O.; Zambryski, P.C. Plasmodesmata enable multicellularity: New insights into their evolution, biogenesis, and functions in development and immunity. Curr. Opin. Plant Biol. 2017, 35, 76–83. [Google Scholar] [CrossRef]
- Harries, P.A.; Schoelz, J.E.; Nelson, R.S. Intracellular transport of viruses and their components: Utilizing the cytoskeleton and membrane highways. Mol. Plant Microbe Interact. 2010, 23, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Underwood, W. The plant cell wall: A dynamic barrier against pathogen invasion. Front. Plant Sci. 2012, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Büttner, D.; He, S.Y. Type III protein secretion in plant pathogenic bacteria. Plant Physiol. 2009, 150, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- The European Cultivated Potato Database. Available online: https://www.europotato.org/quick_search.php (accessed on 24 December 2017).
- Tomczyńska, I.; Jupe, F.; Hein, I.; Marczewski, W.; Śliwka, J. Hypersensitive response to Potato virus Y in potato cultivar Sárpo Mira is conferred by the Ny-Smira gene located on the long arm of chromosome IX. Mol. Breed. 2014, 34, 471–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Schwessinger, B.; Ronald, P.C. Plant innate immunity: Perception of conserved microbial signatures. Annu. Rev. Plant Biol. 2012, 63, 451–482. [Google Scholar] [CrossRef] [PubMed]
- Hammond-Kosack, K.E.; Jones, J.D. Resistance gene-dependent plant defense responses. Plant Cell 1996, 8, 1773–1791. [Google Scholar] [CrossRef] [PubMed]
- De León, P. The moss Physcomitrella patens as a model system to study interactions between plants and phytopathogenic fungi and Oomycetesinés. J. Pathog. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jayamohan, N.S.; Kumudini, B.S. Host pathogen interaction at the plant cell wall. Int. Res. J. Pharm. Pharmacol. 2011, 1, 242–249. [Google Scholar]
- Otulak, K.; Garbaczewska, G. Cellular localisation of calcium ions during potato hypersensitive response to Potato virus Y. Micron 2011, 42, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Otulak, K.; Garbaczewska, G. The participation of plant cell organelles in compatible and incompatible Potato virus Y-tobacco and -potato plant interaction. Acta Physiol. Plant. 2013, 36, 85–99. [Google Scholar] [CrossRef]
- Allie, F.; Pierce, E.J.; Okoniewski, M.J.; Rey, C. Transcriptional analysis of South African cassava mosaic virus-infected susceptible and tolerant landraces of cassava highlights differences in resistance, basal defense and cell wall associated genes during infection. BMC Genom. 2014, 14, 1–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Movahed, N.; Patarroyo, C.; Sun, J.; Vali, H.; Laliberté, J.F.; Zheng, H. Cylindrical inclusion protein of Turnip mosaic virus serves as a docking point for the intercellular movement of viral replication vesicles. Plant Physiol. 2017, 175, 1732–1744. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Ehlers, K.; Kogel, K.H.; van Bel, A.J.E.; Hückelhoven, R. Multivesicular compartments proliferate in susceptible and resistant MLA12-barley leaves in response to infection by the biotrophic powdery mildew fungus. New Phytol. 2006, 172, 563–576. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Hückelhoven, R.; Kogel, K.H.; van Bel, A.J.E. Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell. Microbiol. 2006, 8, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.D.; Hiruki, C. Electron microscopy of cell wall thickening in local lesions of potato virus M-infected red kidney bean. Phytopathology 1971, 6, 862–868. [Google Scholar] [CrossRef]
- Chappell, J.; Levine, A.; Tenhaken, R.; Lusso, M.; Lamb, C. Characterization of a diffusible signal capable of inducing defense gene expression in tobacco. Plant Physiol. 1997, 113, 621–629. [Google Scholar] [CrossRef] [PubMed]
- McLusky, S.R.; Bennett, M.H.; Beale, M.H.; Lewis, M.J.; Gaskin, P.; Mansfield, J.W. Cell wall alterations and localized accumulation of feruloyl-3′-methoxytyramine in onion epidermis at sites of attempted penetration by Botrytis allii are associated with actin polarisation, peroxidase activity and suppression of flavonoid biosynthesis. Plant J. 1999, 17, 523–534. [Google Scholar] [CrossRef]
- Bolwell, P.P.; Page, A.; Piślewska, M.; Wojtaszek, P. Pathogenic infection and the oxidative defences in plant apoplast. Protoplasma 2001, 217, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Benhamou, N.; Kloepper, J.W.; Quadt-Hallman, A.; Tuzun, S. Induction of defence-related ultrastructural modifications in pea root tissues inoculated with endophytic bacteria. Plant Physiol. 1996, 112, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Otulak, K.; Garbaczewsk, G. Localisation of hydrogen peroxide accumulation during Solanum tuberosum cv. Rywal hypersensitive response to Potato virus Y. Micron 2010, 41, 327–335. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.A.; Daudi, A.; Butt, V.S.; Bolwell, G.P. Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 2012, 236, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Tse, Y.C.; Mo, B.; Hillmer, S.; Zhao, M.; Lo, S.W.; Robinson, D.G.; Jiang, L. Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 2004, 16, 672–693. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Ma, L.; Zhao, J.; Li, Z.; Sun, F.; Lu, X. Comparative Transcriptome Analysis of Two Rice Varieties in Response to Rice Stripe Virus and Small Brown Planthoppers during Early Interaction. PLoS ONE 2013, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Satoh, K.; Kikuchi, S.; Omura, T. The repression of cell wall and plastid-related genes and the induction of defense-related genes in rice plants infected with Rice dwarf virus. Mol. Plant-Microbe Interact. 2007, 20, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Ogamino, T.; Hiraguri, A.; Nakazono-Nagaoka, E.; Uehara-Ichiki, T.; Nakajima, M.; Akutsu, K.; Omura, T.; Sasaya, T. Strong resistance against Rice grassy stunt virus is induced in transgenic rice plants expressing double-stranded RNA of the viral genes for nucleocapsid or movement proteins as targets for RNA interference. Phytopathology 2013, 103, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Bucher, G.L.; Tarina, C.; Heinlein, M.; Di Serio, F.; Meins, F., Jr.; Iglesias, V.A. Local expression of enzymatically active class I beta-1,3-glucanase enhances symptoms of TMV infection in tobacco. Plant J. 2001, 28, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Kauss, H. Callose synthesis. In Membranes: Specialized Function in Plants, 2nd ed.; Smallwood, M., Knox, J.P., Bowles, D.J., Eds.; BIOS Scientific: Oxford, UK, 1996; Volume 1, pp. 77–92. ISBN 978-1859962008. [Google Scholar]
- Beffa, R.; Meins, F., Jr. Pathogenesis-related functions of plant beta-1,3-glucanases investigated by antisense transformation—A review. Gene 1996, 179, 97–103. [Google Scholar] [CrossRef]
- Iglesias, V.A.; Meins, F., Jr. Movement of plant viruses is delayed in a beta-1,3-glucanase-deficient mutant showing a reduced plasmodesmatal size exclusion limit and enhanced callose deposition. Plant J. 2000, 21, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Dobnik, D.; Baebler, S.; Kogovšek, P.; Pompe-Novak, M.; Štebih, D.; Panter, G.; Janež, N.; Morisset, D.; Žel, J.; Gruden, K. β-1,3-glucanase class III promotes spread of PVYNTN and improves in planta protein production. Plant Biotechnol. Rep. 2013, 7, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Keefe, D.; Hinz, U.; Meins, F., Jr. The effect of ethylene on the cell-type-specific and intracellular localization of β-1,3-glucanase and chitinase in tobacco leaves. Planta 1990, 182, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Epel, B.L. Plant viruses spread by diffusion on ER-associated movement-protein-rafts through plasmodesmata gated by viral induced host beta-1,3-glucanases. Semin. Cell Dev. Biol. 2009, 20, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, F.; Liu, D.; Jiang, C.; Cui, L.; Shen, L.; Liu, G.; Yang, A. Dynamic expression analysis of early response genes induced by potato virus Y in PVY-resistant Nicotiana tabacum. Plant Cell Rep. 2016, 36, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Baebler, S.; Witek, K.; Petek, M.; Stare, K.; Tušek-Žnidarič, M.; Pompe-Novak, M.; Renaut, J.; Szajko, K.; Strzelczyk-Żyta, D.; Marczewski, W.; et al. Salicylic acid is an indispensable component of the Ny-1 resistance-gene-mediated response against Potato virus Y infection in potato. J. Exp. Bot. 2014, 65, 1095–1109. [Google Scholar] [CrossRef] [PubMed]
- Delmer, D.P. Cellulose biosynthesis: exciting times for a difficult field of study. Annu. Rev. Plant Physiol. Plant Mol Biol. 1999, 50, 245–276. [Google Scholar] [CrossRef] [PubMed]
- Bashline, L.; Li, S.; Gu, Y. The trafficking of the cellulose synthase complex in higher plants. Ann. Bot. 2014, 114, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Atanassov, I.I.; Pittman, J.K.; Turner, S.R. Elucidating the mechanisms of assembly and subunit interaction of the cellulose synthase complex of Arabidopsis secondary cell walls. J. Biol. Chem. 2009, 284, 3833–3841. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.G.; Howells, R.M.; Huttly, A.K.; Vickers, K.; Turner, S.R. Interactions among three distinct CesA proteins essential for cellulose synthesis. Proc. Natl. Acad. Sci. USA 2003, 100, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Blanco, C.; Feng, D.X.; Hu, J.; Sánchez-Vallet, A.; Deslandes, L.; Llorente, F.; Berrocal-Lobo, M.; Keller, H.; Barlet, X.; Sánchez-Rodríguez, C.; et al. Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell 2007, 19, 890–903. [Google Scholar] [CrossRef]
- Humphrey, T.V.; Bonetta, D.T.; Goring, D.R. Sentinels at the wall: cell wall receptors and sensors. New Phytol. 2007, 176, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.A.; Gibeaut, D.M.; Bacic, A.; Findlay, K.; Roberts, K.; Hamilton, A.; Baulcombe, D.C.; Fincher, G.B. Virus-induced silencing of a plant cellulose synthase gene. Plant Cell 2000, 12, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Hanak, T.; Persson, S.; Voigt, C.A. Cellulose and callose synthesis and organization in focus, what’s new? Curr. Opin. Plant Biol. 2016, 34, 9–16. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, H.E.; Watanabe, Y.; Yang, W.; Huang, Y.; Ohlrogge, J.; Lacey Samuels, A. Golgi- and trans-golgi network-mediated vesicle trafficking is required for wax secretion from epidermal cells. Plant Physiol. 2014, 164, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.J.; Brown, I.R.; Mansfield, J.W.; Bailey, J.A.; Mazau, D.; Rumeau, D.; Esquerré-Tugayé, M.T. Immunocytochemical localization of hydroxyproline-rich glycoproteins accumulating in melon and bean at sites of resistance to bacteria and fungi. Mol. Plant Microbe Interact. 1990, 3, 33–40. [Google Scholar] [CrossRef]
- Benhamou, N.; Mazau, D.; Esquerré-Tugayé, M.T. Immunocytochemical localization of hydroxyproline-rich glycoproteins in tomato root cells infected by Fusarium oxysporum f. sp. radicis-lycopersici: Study of a compatible interaction. Phytopathology 1990, 80, 163–173. [Google Scholar] [CrossRef]
- Wycoff, K.L.; Powell, P.A.; Gonzales, R.A.; Corbin, D.R.; Lamb, C.; Dixon, R.A. Stress activation of a bean hydroxyproline-rich glycoprotein promoter is superimposed on a pattern of tissue-specific developmental expression. Plant Physiol. 1995, 109, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Templeton, M.D.; Dixon, R.A.; Lamb, C.J.; Lawton, M.A. Hydroxyproline-rich glycoprotein transcripts exhibit different spatial pat- tems of accumulation in compatible and incompatible interactions between Phaseolus vulgaris and Colletotrichum lindemuthianum. Plant Physiol. 1990, 94, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Kieliszewski, M.J.; Lamport, D.T.A. Extensin: Repetitive motifs, functional sites, post-translational codes, and phylogeny. Plant J. 1994, 5, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Raggi, V. Hydroxyproline-rich glycoprotein accumulation in tobacco leaves protected against Erysiphe cichoracearumby potato virus Y infection. Plant Pathol. 2000, 49, 179–186. [Google Scholar] [CrossRef]
- Varner, J.E.; Lin, L.S. Plant cell wall architecture. Cell 1989, 56, 231–239. [Google Scholar] [CrossRef]
- Corbin, D.R.; Sauer, N.; Lamb, C. Differential regulation of a hydroxyproline-rich glycoprotein gene family in wounded and infected plants. Mol. Cell. Biol. 1987, 7, 4337–4344. [Google Scholar] [CrossRef] [PubMed]
- Zimmoch-Guzowska, E.; Yin, Z.; Chrzanowska, M.; Flis, B. Sources and effectiveness of potato PVY resistance in IHAR’s breeding research. Am. Potato J. 2013, 90, 21–27. [Google Scholar] [CrossRef]
- Otulak, K.; Kozieł, E.; Garbaczewska, G. Ultastructural impact of tobacco rattle virus on tobacco and pepper ovary and anther tissues. J. Phytopatol. 2016, 164, 226–241. [Google Scholar] [CrossRef]
- Chrzanowska, M.; Doroszewska, T. Comparison between PVY isolates obtained from potato and tobacco plants grown in Poland. Phytopathol. Pol. 1997, 13, 63–71. [Google Scholar]
- Otulak, K.; Garbaczewska, G. Ultrastructural events during hypersensitive response of potato cv. Rywal infected with necrotic strains of Potato virus Y. Acta Physiol. Plant. 2010, 32, 635–644. [Google Scholar] [CrossRef]
- Gubler, F. Immunofluorescence localisation of microtubules in plant root tips embedded in butyl-methyl methacrylate. Cell Biol. Int. Rep. 1989, 13, 137–145. [Google Scholar] [CrossRef]
- Luschin-Ebengreuth, N.; Zechmann, B. Compartment-specific investigations of antioxidants and hydrogen peroxide in leaves of Arabidopsis thaliana during dark-induced senescence. Acta Physiol. Plant. 2016, 38, 1–15. [Google Scholar] [CrossRef] [PubMed]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otulak-Kozieł, K.; Kozieł, E.; Lockhart, B.E.L. Plant Cell Wall Dynamics in Compatible and Incompatible Potato Response to Infection Caused by Potato Virus Y (PVYNTN). Int. J. Mol. Sci. 2018, 19, 862. https://doi.org/10.3390/ijms19030862
Otulak-Kozieł K, Kozieł E, Lockhart BEL. Plant Cell Wall Dynamics in Compatible and Incompatible Potato Response to Infection Caused by Potato Virus Y (PVYNTN). International Journal of Molecular Sciences. 2018; 19(3):862. https://doi.org/10.3390/ijms19030862
Chicago/Turabian StyleOtulak-Kozieł, Katarzyna, Edmund Kozieł, and Benham E. L. Lockhart. 2018. "Plant Cell Wall Dynamics in Compatible and Incompatible Potato Response to Infection Caused by Potato Virus Y (PVYNTN)" International Journal of Molecular Sciences 19, no. 3: 862. https://doi.org/10.3390/ijms19030862
APA StyleOtulak-Kozieł, K., Kozieł, E., & Lockhart, B. E. L. (2018). Plant Cell Wall Dynamics in Compatible and Incompatible Potato Response to Infection Caused by Potato Virus Y (PVYNTN). International Journal of Molecular Sciences, 19(3), 862. https://doi.org/10.3390/ijms19030862