Treatment, Therapy and Management of Metabolic Epilepsy: A Systematic Review
Abstract
1. Introduction
2. Results and Discussion
2.1. Biotinidase Deficiency
2.2. Cerebral Folate Deficiency
2.3. Creatine Disorders
2.4. Disorders of Urea Cycle
2.5. Folinic Acid-Responsive Seizures
2.6. Glucose Transporter Type 1 Deficiency Syndrome
2.7. Glutaric Aciduria
2.8. Mitochondrial Disorders
2.9. Molybdenum Cofactor Deficiency
2.10. Non-Ketotic Hyperglycaemia
2.11. Non-Ketotic Hyperglycinemia
2.12. Peroxisomal Disorders
2.13. Pyridoxine-Dependent Epilepsy/ Pyridox(am)ine-5′-phosphate oxidase Deficiency
2.14. Succinic Semialdehyde Dehydrogenase (SSADH) Deficiency
3. Materials and Methods
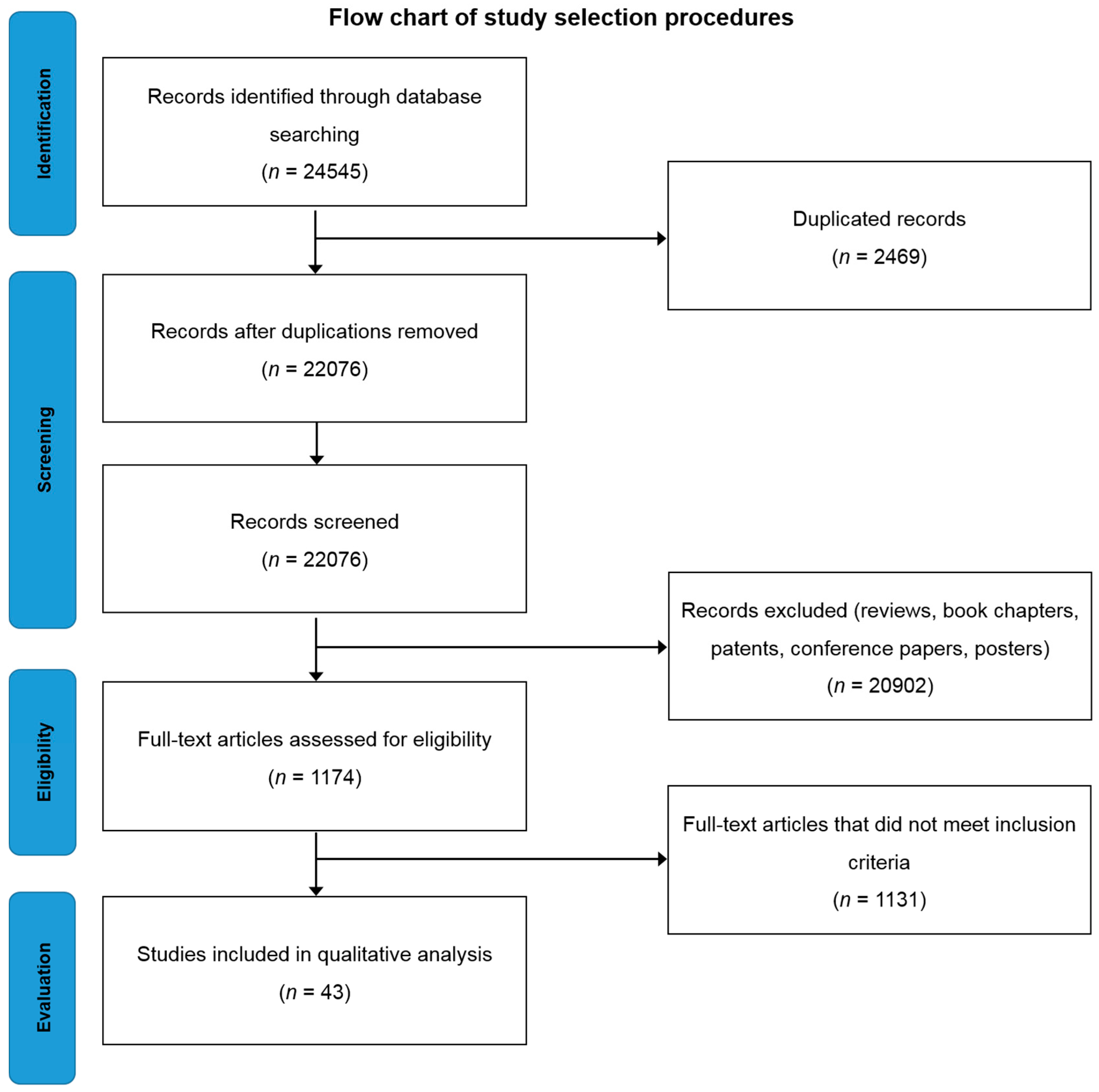
3.1. Data Sourced and Search Strategy
3.2. Study Selection and Exclusion/Inclusion Criteria
3.3. Data Extraction and Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 5-MTHF | 5-methyltetrahydrofolate |
| AASA | α-aminodipic semialdehyde |
| AEDs | Anti-epileptic drugs |
| ALDH5A1 | Aldehyde Dehydrogenase 5 Family Member A1 |
| ALDH7A1 | Aldehyde Dehydrogenase 7 Family Member A1 |
| ATQ | Antiquitin |
| Cr | Creatine |
| CFD | Cerebral folate deficiency |
| CNS | Central nervous system |
| C: PGR | CSF: Plasma glycine ratio |
| CSF | Cerebrospinal fluid |
| EEG | Electroencephalogram |
| FOLR1 | Folate receptor 1 |
| GA | Glutaric acid |
| GAA | Guanidoacetic acid |
| GAMT | Guanidinoacetate methyltransferase |
| GLUT-1 | Glucose transporter type-1 |
| HGA | Hydroxyglutaric aciduria |
| IEM | Inborn errors of metabolism |
| ILAE | International League Against Epilepsy |
| KD | Ketogenic diet |
| l-DOPA | l-3,4-dihydroxyphenylalanine |
| MAD | Modified Atkins diet |
| MAE | Myoclonic-astatic epilepsy |
| MELAS | Mitochondrial myopathy, encephalopathy, lactic acidosis, stroke-like episodes |
| MERRF | Myoclonic epilepsy with ragged red fibers |
| MIDs | Mitochondrial disorders |
| MRI | Magnetic resonance imaging |
| NKH | Non-ketotic hyperglycinemia |
| OCD | Obsessive compulsive disorder |
| PCr | Phosphocreatine |
| PDE | Pyridoxine-dependent epilepsy |
| PED | Paroxysmal exertional dyskinesia |
| Phe | Phenylalanine |
| PLP | Pyridoxal phosphate |
| PNPO | Pridox(am)ine-5′-phosphate oxidase |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SLC2A1 | Solute carrier family 2 member 1 |
| UCD | Urea cycle disorders |
| VPA | Valproic acid |
References
- Berg, A.T.; Berkovic, S.F.; Brodie, M.J.; Buchhalter, J.; Cross, J.H.; van Emde Boas, W.; Engel, J.; French, J.; Glauser, T.A.; Mathern, G.W.; et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ilae commission on classification and terminology, 2005–2009. Epilepsia 2010, 51, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Mahjoub, S.Z. Presentation of adult mitochondrial epilepsy. Seizure 2013, 22, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Epilepsy, Who, Fact Sheet. Available online: http://www.who.int/mediacentre/factsheets/fs999/en/ (accessed on 20 February 2017).
- Messing, R.; Simon, R. Seizures as a manifestation of systemic disease. Neurol. Clin. 1986, 4, 563–584. [Google Scholar] [PubMed]
- Gupta, N.; Kabra, M.; Haberle, J. Mutation analysis of Indian patients with urea cycle defects. Indian Pediatr. 2012, 49, 585–586. [Google Scholar] [CrossRef] [PubMed]
- Eeg-Olofsson, O.; Zhang, W.W.; Olsson, Y.; Jagell, S.; Hagenfeldt, L. D-2-hydroxyglutaric aciduria with cerebral, vascular, and muscular abnormalities in a 14-year-old boy. J. Child Neurol. 2000, 15, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Teksam, O.; Yurdakok, M.; Coskun, T. Molybdenum cofactor deficiency presenting with severe metabolic acidosis and intracranial hemorrhage. J. Child Neurol. 2005, 20, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Pro, S.; Randi, F.; Pulitano, P.; Vicenzini, E.; Mecarelli, O. Non-convulsive status epilepticus characterised exclusively by a language disorder induced by non-ketotic hyperglycaemia. Epileptic Disord. Int. Epilepsy J. Videotape 2011, 13, 193–196. [Google Scholar]
- Korman, S.H.; Gutman, A. Pitfalls in the diagnosis of glycine encephalopathy (non-ketotic hyperglycinemia). Dev. Med. Child Neurol. 2002, 44, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Pearl, P.L.; Shukla, L.; Theodore, W.H.; Jakobs, C.; Michael Gibson, K. Epilepsy in succinic semialdehyde dehydrogenase deficiency, a disorder of GABA metabolism. Brain Dev. 2011, 33, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Shorvon, S.D. The etiologic classification of epilepsy. Epilepsia 2011, 52, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Kozyraki, R.; Cases, O. Vitamin b12 absorption: Mammalian physiology and acquired and inherited disorders. Biochimie 2013, 95, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, G.; Krummel, T.; Sabourdy, C.; Ryvlin, P.; Hirsch, E. Optimizing therapy of seizures in patients with renal or hepatic dysfunction. Neurology 2006, 67, S28–S33. [Google Scholar] [CrossRef] [PubMed]
- Papetti, L.; Parisi, P.; Leuzzi, V.; Nardecchia, F.; Nicita, F.; Ursitti, F.; Marra, F.; Paolino, M.C.; Spalice, A. Metabolic epilepsy: An update. Brain Dev. 2013, 35, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Wolf, N.I.; Bast, T.; Surtees, R. Epilepsy in inborn errors of metabolism. Epileptic Disord. 2005, 7, 67–81. [Google Scholar] [PubMed]
- Pascual, J.M.; Campistol, J.; Antonio Gil-Nagel, M. Epilepsy in inherited metabolic disorders. Neurologist 2008, 14, S2–S14. [Google Scholar] [CrossRef] [PubMed]
- Singhi, P.; Ray, M. Ohtahara syndrome with biotinidase deficiency. J. Child Neurol. 2011, 26, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Masri, A.; Al Wahsh, S. Manifestations and treatment of epilepsy in children with neurometabolic disorders: A series from Jordan. Seizure 2014, 23, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Al-Baradie, R.S.; Chaudhary, M.W. Diagnosis and management of cerebral folate deficiency: A form of folinic acid-responsive seizures. Neurosciences 2014, 19, 312–316. [Google Scholar] [PubMed]
- Bianchi, M.C.; Tosetti, M.; Fornai, F.; Alessandri, M.G.; Cipriani, P.; De Vito, G.; Canapicchi, R. Reversible brain creatine deficiency in two sisters with normal blood creatine level. Ann. Neurol. 2000, 47, 511–513. [Google Scholar] [CrossRef]
- Leuzzi, V.; Bianchi, M.C.; Tosetti, M.; Carducci, C.; Cerquiglini, C.A.; Cioni, G.; Antonozzi, I. Brain creatine depletion: Guanidinoacetate methyltransferase deficiency (improving with creatine supplementation). Neurology 2000, 55, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Lubana, S.S.; Alfishawy, M.; Singh, N.; Atkinson, S. Vitamin b12 deficiency and elevated folate levels: An unusual cause of generalized tonic-clonic seizure. Am. J. Case Rep. 2015, 16, 386. [Google Scholar] [PubMed]
- McClelland, V.M.; Bakalinova, D.B.; Hendriksz, C.; Singh, R.P. Glutaric aciduria type 1 presenting with epilepsy. Dev. Med. Child Neurol. 2009, 51, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Jovic, N.J.; Kosac, A.; Koprivsek, K. L-2-hydroxyglutaric aciduria: A case report. Srpski Arhiv za Celokupno Lekarstvo 2014, 142, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, V.; Rudwan, M.; Yadav, G.; Khan, R.A. Epilepsy in a young adult caused by L-2-hydroxyglutaric aciduria: A case report. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2008, 17, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Mete, A.; Isikay, S.; Sirikci, A.; Ozkur, A.; Bayram, M. Eyelid myoclonia with absence seizures in a child with l-2 hydroxyglutaric aciduria: Findings of magnetic resonance imaging. Pediatr. Neurol. 2012, 46, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Seijo-Martinez, M.; Navarro, C.; Castro del Rio, M.; Vila, O.; Puig, M.; Ribes, A.; Butron, M. L-2-hydroxyglutaric aciduria: Clinical, neuroimaging, and neuropathological findings. Arch. Neurol. 2005, 62, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Calik, M.; Tuncer, F.N.; Sarikaya, S.; Karakas, O.; Cece, H.; Iscan, A. A case of l-2 hydroxyglutaric aciduria presenting as febrile seizure. Genet. Couns. 2014, 25, 363–367. [Google Scholar] [PubMed]
- Larnaout, A.; Amouri, R.; Kefi, M.; Hentati, F. L-2-hydroxyglutaric aciduria: Clinical and molecular study in three Tunisian families. Identification of a new mutation and inter-familial phenotype variability. J. Inherit. Metab. Dis. 2008, 31 (Suppl. S2), S375–S379. [Google Scholar] [CrossRef] [PubMed]
- Kamate, M.; Prashanth, G.P.; Hattiholi, V. L-2-hydroxyglutaric aciduria: Report of two Indian families. Indian J. Pediatr. 2014, 81, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Goffette, S.M.; Duprez, T.P.; Nassogne, M.C.; Vincent, M.F.; Jakobs, C.; Sindic, C.J. L-2-hydroxyglutaric aciduria: Clinical, genetic, and brain mri characteristics in two adult sisters. Eur. J. Neurol. 2006, 13, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Pong, A.W.; Geary, B.R.; Engelstad, K.M.; Natarajan, A.; Yang, H.; De Vivo, D.C. Glucose transporter type i deficiency syndrome: Epilepsy phenotypes and outcomes. Epilepsia 2012, 53, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Oguni, H.; Ito, S.; Oguni, M.; Osawa, M. A modified Atkins diet is promising as a treatment for glucose transporter type 1 deficiency syndrome. Dev. Med. Child Neurol. 2011, 53, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Tzadok, M.; Nissenkorn, A.; Porper, K.; Matot, I.; Marcu, S.; Anikster, Y.; Menascu, S.; Bercovich, D.; Zeev, B.B. The many faces of GLUT-1 deficiency syndrome. J. Child Neurol. 2014, 29, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Szczepanik, E.; Terczyńska, I.; Kruk, M.; Lipiec, A.; Dudko, E.; Tryfon, J.; Jurek, M.; Hoffman-Zacharska, D. Glucose transporter type 1 deficiency due to slc2a1 gene mutations a rare but treatable cause of metabolic epilepsy and extrapyramidal movement disorder; own experience and literature review. Dev. Period Med. 2015, 4, 454–463. [Google Scholar]
- Mullen, S.A.; Marini, C.; Suls, A.; Mei, D.; Della Giustina, E.; Buti, D.; Arsov, T.; Damiano, J.; Lawrence, K.; De Jonghe, P.; et al. Glucose transporter 1 deficiency as a treatable cause of myoclonic astatic epilepsy. Arch. Neurol. 2011, 68, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, G.; Castellotti, B.; Zibordi, F.; Gellera, C.; Nardocci, N. Paroxysmal movement disorders in GLUT-1 deficiency syndrome. Neurology 2008, 71, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Zsurka, G.; Becker, F.; Heinen, M.; Gdynia, H.-J.; Lerche, H.; Kunz, W.S.; Weber, Y.G. Mutation in the mitochondrial trna ile gene causes progressive myoclonus epilepsy. Seizure 2013, 22, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Veeravigrom, M.; Damrongphol, P.; Ittiwut, R.; Ittiwut, C.; Suphapeetiporn, K.; Shotelersuk, V. Pyridoxal 5ꞌ-phosphate-responsive epilepsy with novel mutations in the pnpo gene: A case report. Genet. Mol. Res. 2015, 14, 14130–14135. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.; Pereira, C.; Rodrigues, F.; Alfaite, C.; Garcia, P.; Robalo, C.; Fineza, I.; Gonçalves, O.; Struys, E.; Salomons, G. Pyridoxine-dependent epilepsy due to antiquitin deficiency: Achieving a favourable outcome. Epileptic Disord. 2013, 15, 400–406. [Google Scholar] [PubMed]
- Ville, D.; Ginguene, C.; Marignier, S.; Des Portes, V.; De Bellescize, J. Early diagnosis of pyridoxine-dependent epilepsy: Video-eeg monitoring and biochemical and genetic investigation. Eur. J. Paediatr. Neurol. 2013, 17, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Coulter-Mackie, M.B.; Li, A.; Lian, Q.; Struys, E.; Stockler, S.; Waters, P.J. Overexpression of human antiquitin in E. coli: Enzymatic characterization of twelve aldh7a1 missense mutations associated with pyridoxine-dependent epilepsy. Mol. Genet. Metab. 2012, 106, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Van Karnebeek, C.D.; Hartmann, H.; Jaggumantri, S.; Bok, L.A.; Cheng, B.; Connolly, M.; Coughlin, C.R.; Das, A.M.; Gospe, S.M.; Jakobs, C. Lysine restricted diet for pyridoxine-dependent epilepsy: First evidence and future trials. Mol. Genet. Metab. 2012, 107, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Millet, A.; Salomons, G.; Cneude, F.; Corne, C.; Debillon, T.; Jakobs, C.; Struys, E.; Hamelin, S. Novel mutations in pyridoxine-dependent epilepsy. Eur. J. Paediatr. Neurol. 2011, 15, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Khayat, M.; Korman, S.H.; Frankel, P.; Weintraub, Z.; Hershckowitz, S.; Sheffer, V.F.; Elisha, M.B.; Wevers, R.A.; Falik-Zaccai, T.C. Pnpo deficiency: An under diagnosed inborn error of pyridoxine metabolism. Mol. Genet. Metab. 2008, 94, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Alkan, A.; Kutlu, R.; Aslan, M.; Sigirci, A.; Orkan, I.; Yakinci, C. Pyridoxine-dependent seizures: Magnetic resonance spectroscopy findings. J. Child Neurol. 2004, 19, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Garcia-Villoria, J.; Ormazabal, A.; Zschocke, J.; Fiol, M.; Navarro-Sastre, A.; Artuch, R.; Vilaseca, M.A.; Ribes, A. A new fatal case of pyridox(am)ine 5′-phosphate oxidase (pnpo) deficiency. Mol. Genet. Metab. 2008, 93, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Leuzzi, V.; Di Sabato, M.L.; Deodato, F.; Rizzo, C.; Boenzi, S.; Carducci, C.; Malaspina, P.; Liberanome, C.; Dionisi-Vici, C. Vigabatrin improves paroxysmal dystonia in succinic semialdehyde dehydrogenase deficiency. Neurology 2007, 68, 1320–1321. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Weng, W.C.; Lee, W.T. A novel mutation of aldh5a1 gene associated with succinic semialdehyde dehydrogenase deficiency. J. Child Neurol. 2015, 30, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; El Melegy, E.M.; Talaat, I.; Hosny, A.; Abu-Amero, K.K. Neurometabolic disorders-related early childhood epilepsy: A single-center experience in saudi arabia. Pediatr. Neonatol. 2015, 56, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A. Creatine deficiency syndromes. Handb. Clin. Neurol. 2013, 113, 1837–1843. [Google Scholar] [PubMed]
- Kossoff, E.H.; Dorward, J.L. The modified atkins diet. Epilepsia 2008, 49, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Gokcen, C.; Isikay, S.; Yilmaz, K. L-2 hydroxyglutaric aciduria presenting with anxiety symptoms. BMJ Case Rep. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Lee, J.H.; Lee, S.A.; No, Y.J.; Im, J.H.; Lee, M.C. Co-occurrence of seizure and chorea in a patient with nonketotic hyperglycemia. Eur. Neurol. 2005, 54, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandusse, S.; Ebberink, M.S.; Vaz, F.M.; Waterham, H.R.; Wanders, R.J. The important role of biochemical and functional studies in the diagnostics of peroxisomal disorders. J. Inherit. Metab. Dis. 2016, 39, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Waterham, H.R.; Ebberink, M.S. Genetics and molecular basis of human peroxisome biogenesis disorders. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 1430–1441. [Google Scholar] [CrossRef] [PubMed]
- Van Veldhoven, P.P. Biochemistry and genetics of inherited disorders of peroxisomal fatty acid metabolism. J. Lipid Res. 2010, 51, 2863–2895. [Google Scholar] [CrossRef] [PubMed]
| Type of Metabolic Epilepsy | Study Design | Intervention | Major Outcome | Reference |
|---|---|---|---|---|
| Biotinidase and holocarboxylase synthase deficiency | Case study (n = 1) | Biotin treatment (10–40 mg/day) | ◦ Biotin treatment normalized metabolic acidosis within hours. ◦ Encephalopathy and seizures became passive within 48 h. | [17] |
| Retrospective study (n = 1) | Biotin treatment | ◦ Biotin treatment yielded complete control of seizures. | [18] | |
| Cerebral folate deficiency | Case study (n = 2) | Pyridoxine (6 mg/kg/day) & folinic acid (1.7–2 mg/kg/day) treatment | ◦ Pyridoxine and folinic acid were effective in treating intractable seizures in young children. ◦ EEG and neurological improvement was observed. ◦ Diagnosis: DNA mutation analysis- homozygous mutation in FOLR1 gene. | [19] |
| Creatine disorders | Case study (n = 2) | l-arginine (300 mg/kg/day) & Cr monohydrate (400 mg/kg/day) | ◦ Blood concentrations of Cr and GAA turned out to be within normal values, thus excluding a systemic Cr synthesis deficit. ◦ Oral Cr monohydrate was given when MRI showed no increase in Cr after 2 months ◦ Brain Cr level reached 40% of the normal after 3 months and 80% at 9 months. After 16 months, brain Cr was restored to normal in gray matter and the cerebellum but was still slightly less than normal in the hemispheric white matter. ◦ Acceleration of the rate of cognitive development. ◦ Seizures stopped one month after oral Cr monohydrate treatment alone. | [20] |
| Case study (n = 1) | Creatine monohydrate (350 mg/kg/day) | ◦ AEDs were ineffective against seizures. ◦ Seizures stopped and the patient appeared less irritable and more interested in their environment after an oral dose of creatine monohydrate of 350 mg/kg/day. | [21] | |
| Disorders of urea cycle | Case report (n = 4) | Sodium benzoate | ◦ Patient 1 was on sodium benzoate therapy and had hyperammonemia intermittently. Patient developed intractable seizures and chronic encephalopathy which led to coma and death. ◦ Patient 2 did not have seizures although hyperammonia was present. ◦ Patient 3 presented with seizures and hyperammonemia and unfortunately died at the age of 1 week. ◦ Patient 4 had recurrent episodes of seizures and encephalopathy. Sodium benzoate was given for hyperammonemia and peritoneal dialysis was done but the encephalopathy did not improve and the patient died on day 5 of life. | [5] |
| Folinic acid-responsive seizures | Case study (n = 1) | Levetiracetam and Vitamin B12 injection | ◦ Six months after beginning vitamin B12 injections, the levels of Vitamin B12, methylmalonic acid, folate and homocysteine normalized. ◦ Patient was on levetiracetam for 5 months and was off for another 5 months but remained seizure free during the period he was off Levetiracetam. | [22] |
| Glutaric aciduria | Case study (n = 1) | AEDs | ◦ Valproate therapy decreased the urinary excretion of d-2-hydroxyglutarate but the excretion level was still very high. ◦ Patient died at the age of 14.5 with a history of pyloric stenosis in the neonatal period leading to surgery, epilepsy and developmental delay from the third month of life. | [6] |
| Case study (n = 1) | AEDs Carnitine | ◦ Patient was initially given carbamazepine, but was changed to valproate acid, with an apparent initial improvement. ◦ 2 months later, her seizures worsened and lamotrigine was added. ◦ She commenced regular carnitine therapy, but her seizures continued to be difficult to control and she was then commenced on levetiracetam therapy, with good clinical and electrophysiological response. ◦ She remained seizure-free for 12 months on levetiracetam and low-dose lamotrigine. ◦ A low-protein diet (restricted to 1.5 g/kg/day) was introduced. Neurological examination results remained normal, with no dystonia. | [23] | |
| Case study (n = 1) | AEDs Co-enzyme Q10 (400 mg/day) Riboflavin (200 mg/day) | ◦ Partial seizure control with valproate (92µg/mL) therapy. ◦ Comedication with lamotrigine (300 mg/day) improved seizure control. ◦ Patient became seizure-free with a combination therapy of valproate, levetiracetam, and lamotrigine. ◦ Therapeutic trials with oral coenzyme Q10 (400 mg/day) for 6 months and oral riboflavin (200 mg/day) for 4 months did not produce any effects. | [24] | |
| Case study (n = 1) | AEDs | ◦ Determination of the level of l-2-hydroxyglutaric acid in urine, plasma, and cerebrospinal fluid as well as a brain MRI were needed to confirm the diagnosis of l-2-hydroxylutaric aciduria. ◦ Complete seizure control was achieved following phenytoin and oxycarbamazepine therapy. | [25] | |
| Case study (n = 1) | AEDs Riboflavin l-carnitine | ◦ Complete seizure control achieved from lamotrigine (5 mg/kg/day) and clonazepam (0.1 mg/kg/day) therapy but the seizures returned after a month. ◦ Complete seizure control achieved from levetiracetam (40 mg/kg/day), riboflavin (200 mg/day), and l-carnitine (100 mg/kg/day) therapy. ◦ Patient remained seizure-free for 12 months after that on lamotrigine, clonazepam and levetiracetam combination therapy. | [26] | |
| Case study (n = 1) | AEDs Folic acid | ◦ Seizures stopped after treatment with phenobarbital. ◦ Folic acid supplementation (10 mg/day) started 6 months after diagnosis. ◦ However, he died 10 months later after an episode of spontaneous abdominal pain and lower gastrointestinal tract bleeding and multiorgan failure. | [27] | |
| Case study (n = 1) | AEDs Carnitine Riboflavin | ◦ After diagnosis, the patient was given carnitine (100 mg/kg/day) and riboflavin (200 mg/day) which improved patient’s reflexes. ◦ When partial seizures occurred and EEG recordings showed focal epileptic discharges, oxcarbazepine (20 mg/kg/day) treatment was started and it significantly improved her seizures. ◦ She continued the oxcarbazepine treatment and remained seizure-free till date. | [28] | |
| Case study (n = 3) | AEDs | ◦ One patient had complete relief from epileptic seizures with phenobarbital (50 mg/day) treatment. ◦ Another patient had partial relief from myoclonic jerks with clonazepam (0.5 mg, 3 times a day) treatment. | [29] | |
| Case study (n = 3) | Carnitine (100 mg/kg) Riboflavin (50–100 mg/day) AEDs | ◦ All patients were advised moderate protein restriction and were prescribed oral carnitine (100 mg/kg), riboflavin (50–100 mg/day) supplementation, and symptomatic treatment for epilepsy. ◦ No further worsening was documented with the use of carnitine and riboflavin. | [30] | |
| Case study (n = 2) | AEDs | ◦ Patient 1 suffered from recurrent seizures despite initial treatment with phenobarbital and VPA. ◦ Seizure control was achieved with a combination of VPA and primidone. ◦ Patient 2 suffered from seizures during childhood until the age of 5. ◦ Her parents stopped all treatments without any recurrence of seizures. | [31] | |
| GLUT-1 deficiency | Retrospective study (n = 87) | KD AEDs l-carnitine | ◦ KD together with carbonic anhydrase inhibitors increased the risk of acidosis and urolithiasis and therefore they should not be used together. ◦ Valproate worsens hypocarnitinemia, inhibits fatty acid oxidation and cannot be combined with KD. ◦ l-carnitine supplementation is given if hypocarnitinemia is observed. | [32] |
| Case study (n = 6) | Modified Atkins diet (MAD) | ◦ Reduced frequency of epileptic seizures. ◦ Improvement of cognitive activity. ◦ Urinary ketosis less than that achieved with the ketogenic diet. | [33] | |
| Case study (n = 8) | MAD AEDs | ◦ MAD was found to be as effective as the ketogenic diet. ◦ AEDs with a carbonic anhydrase inhibitor mechanism (acetazolamide, zonisamide) was able to control seizures and improve EEG readings. ◦ Recommended diagnosis methods: lumbar puncture and SLC2A1 gene analysis/sequencing. | [34] | |
| Case study (n = 3) | KD AEDs | ◦ KD can effectively treat epilepsy due to GLUT-1 deficiency. ◦Basic diagnostic hallmark: CSF hypoglycorrhachia and glucose ratio below 0.6 | [35] | |
| Case study (n = 3) | KD | ◦ GLUT-1 deficiency should be suspected in all patients with MAE though clinical or EEG features cannot be used to exclude the diagnosis. ◦ On the other hand, the presence of PED should heighten suspicion of GLUT-1 deficiency. ◦ KD is likely to improve cognitive outcome as GLUT-1 deficiency is often associated with intellectual impairment which arises from this metabolic defect. | [36] | |
| Case study (n = 3) | AEDs | ◦ Seizures persisted over time, but were satisfactorily controlled by antiepileptic drugs (valproate, carbamazepine). ◦ All patients were reported to have paroxysmal movement disorders that did not go away after AED treatment. | [37] | |
| Mitochondrial disorders | Retrospective study (n = 7) | AEDs | ◦ Complete seizure control with clonazepam and phenobarbital monotherapy ◦ Partial seizure control with phenobarbital + clonazepam polytherapy ◦Topiramate, phenobarbital, and levetiracetam polytherapy is ineffective. | [18] |
| Retrospective study (n = 441) | AEDs | ◦ VPA, carbamazepine, and oxcarbazepine cause mitochondrial toxicity. ◦ Levetiracetam, lamotrigine, and lacosamide are effective treatments ◦ Antioxidants are proposed as adjunctive agents to reduce the increased oxidative stress produced by excess free radicals. | [2] | |
| Case study (n = 1) | AEDs | ◦ VPA deteriorated the patient’s condition because it influenced the mitochondrial metabolism of fatty acids. ◦ Levetiracetam at a dose of 2000 mg/day yielded seizure freedom. ◦ Diagnosis: histology and genetic analyses of skeletal muscle biopsy. | [38] | |
| Molybdenum Cofactor Deficiency | Case study | AEDs Folinic acid Pyridoxine Corticosteroid | ◦ Phenytoin was initiated, and then phenobarbital, pyridoxine, folinic acid and corticosteroid to control the seizure. ◦ Patient responded well to intravenous infusion therapy of midazolam. ◦ Unfortunately, patient suffered from feeding difficulties, motor, and mental retardation and died of pneumonia at 4 months of age. | [7] |
| Non-ketotic hyperglycaemia | Case study (n = 1) | AEDs | ◦ Patient was placed on carbamazepine monotherapy, titrated up to (600 mg/day). ◦ His blood glucose levels returned to normal and hypoglycaemic agents combined with strict dietary control were prescribed. ◦ Ten months later, the patient was seizure-free, without any language disorders, and had a normal EEG reading. ◦ The patient was compliant to therapy and carbamazepine was reduced to the therapeutic range (6.8 ng/mL). | [8] |
| Non-ketotic hyperglycinemia | Case study (n = 5) | AEDs Benzoate Ketamine Parenteral nutrition Adrenocorticotropic hormone therapy | ◦ Patient 1 had convulsions that were controlled with phenobarbitone. Treatment with oral sodium benzoate and ketamine improved attentiveness. Convulsions recurred and significant development delay with microcephaly, hypotonia and hyperreflexia were observed. ◦ Patient 2 was a false positive. ◦ Patient 3 was initially treated with phenobarbitone, hydantoin, and then clonazepam but without any significant response and subsequently with VPA and vigabatrin. Vigabatrin treatment was reintroduced together with adrenocorticotropic hormone therapy which led to an improvement in seizure frequency and duration as well as EEG findings. ◦ Patient 4 and 5 did not have epileptic seizures. | [9] |
| Pyridoxine dependent epilepsy (PDE)/PNPO deficiency | Case study (n = 1) | AEDs Pyridoxal 5′-phosphate (PLP, 30 mg/kg/day) Cefotaxime Acyclovir | ◦ AEDs such as phenytoin, phenobarbital, levetiracetam, and VPA as well as treatment with cefotaxime and acyclovir did not stop the clinical seizure ◦ PLP successfully controlled seizures. ◦ Early and accurate diagnosis (genetic diagnosis/mutation analysis) is a crucial management step. | [39] |
| Case study (n = 4) | Pyridoxine | ◦ Pharmacological doses of pyridoxine are able to control seizures | [40] | |
| Retrospective study (n = 4) | Pyridoxine and AEDs | ◦ Complete seizure control from pyridoxine monotherapy and pyridoxine and levetiracetam and phenobarbital polytherapy. ◦ Partial control from pyridoxine and valproate acid polytherapy. | [18] | |
| Case study (n = 1) | - | ◦ EEG monitoring could potentially be an effective diagnostic tool. ◦ In addition to the stereotypical ictal pattern which is considered as an intermediate between myoclonia and spasms, short interictal interval was highlighted as a helpful marker for the disease. | [41] | |
| Preclinical study | - | ◦ ATQ missense mutation screening system using a recombinant lat gene from S. clavuligerus can contribute to the diagnostic work-up of patients suspected of PDE. | [42] | |
| Observational study (n = 7) | Lysine-restricted diet Pyridoxine | ◦ Effectively reduces the chemical biomarkers: CSF AASA and pipecolic acid. ◦ Seizure were controlled, leading to a pyridoxine dosage reduction. | [43] | |
| Case study | AEDs Pyridoxine Pyridoxine phosphate | ◦ Biomarkers: elevated urinary excretion of AASA, elevated concentration of pipecolic acid in plasma, and mutation of the ALDH7A1 gene. ◦ Diagnosis: Thorough metabolic examination, early performance of pyridoxine trial and a pyridoxine phosphate trial. | [44] | |
| Case study (n = 1) | PLP | ◦ Seizures decreased significantly in patients administered PLP, following ineffective treatment with pyridoxine. ◦ PNPO deficiency is a potentially treatable disease that may be under or misdiagnosed. | [45] | |
| Case study (n = 1) | - | ◦ Magnetic resonance spectroscopy demonstrated a decreased N-acetylaspartate-to-creatine ratio in the frontal and parieto-occipital cortices ◦ Could potentially be an effective diagnostic tool to represent neuronal loss for the diagnosis of PDE | [46] | |
| Case study (n = 1) | PLP (50 mg/kg/day) | ◦ Patient presented with neonatal epileptic encephalopathy with severe seizures which do not respond to anticonvulsant drugs or pyridoxine. ◦ Patient showed a dramatic response to PLP treatment (50 mg/kg/day). ◦ 48 h after treatment, an EEG showed the disappearance of burst-suppression patterns which were present before treatment; despite persistence of multi-focal alterations. ◦ Transient clinical improvement was observed, but convulsions re-appeared after 72 h. ◦ A fungal infection could not be eliminated and multi-organ failure occurred, leading to death at 48 days of life. | [47] | |
| Succinic semialdehyde dehydrogenase deficiency | Case study (n = 1) | AEDs | ◦ Patient presented with severe hyperactivity and new onset generalized convulsion. ◦ VPA therapy was given but was associated with lethargy. ◦ Then, the patient was treated with lamotrigine which resulted in fair seizure control but had persistent problems with expressive aphasia, OCD and anxiety. | [10] |
| Case study (n = 2) | AEDs | ◦ In patient 1, vigabatrin (30 mg/kg/day) partially improved PED but did not influence epilepsy and language disorder. ◦ Patient 2 had sporadic generalized seizures and under vigabatrin (20 mg/kg/day), gait clumsiness and exercise-induced dystonia disappeared. ◦ Patient 2 remained seizure free for 1 year. Sertraline (1 mg/kg/day) improved OCD and after 16 months of vigabatrin treatment, his clinical condition remained stationary. | [48] | |
| Case report (n = 1) | AEDs | ◦ Succinic semialdehyde dehydrogenase deficiency was diagnosed after mutation analysis of the ALDH5A1 gene showed heterozygous mutations. ◦ Myoclonic and tonic-clonic seizures were controlled with vigabatrin, levetiracetam, oxcarbazepine and clonazepam. | [49] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin Lin Lee, V.; Kar Meng Choo, B.; Chung, Y.-S.; P. Kundap, U.; Kumari, Y.; Shaikh, M.F. Treatment, Therapy and Management of Metabolic Epilepsy: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 871. https://doi.org/10.3390/ijms19030871
Lin Lin Lee V, Kar Meng Choo B, Chung Y-S, P. Kundap U, Kumari Y, Shaikh MF. Treatment, Therapy and Management of Metabolic Epilepsy: A Systematic Review. International Journal of Molecular Sciences. 2018; 19(3):871. https://doi.org/10.3390/ijms19030871
Chicago/Turabian StyleLin Lin Lee, Vanessa, Brandon Kar Meng Choo, Yin-Sir Chung, Uday P. Kundap, Yatinesh Kumari, and Mohd. Farooq Shaikh. 2018. "Treatment, Therapy and Management of Metabolic Epilepsy: A Systematic Review" International Journal of Molecular Sciences 19, no. 3: 871. https://doi.org/10.3390/ijms19030871
APA StyleLin Lin Lee, V., Kar Meng Choo, B., Chung, Y.-S., P. Kundap, U., Kumari, Y., & Shaikh, M. F. (2018). Treatment, Therapy and Management of Metabolic Epilepsy: A Systematic Review. International Journal of Molecular Sciences, 19(3), 871. https://doi.org/10.3390/ijms19030871







