An Anion Conductance, the Essential Component of the Hydroxyl-Radical-Induced Ion Current in Plant Roots
Abstract
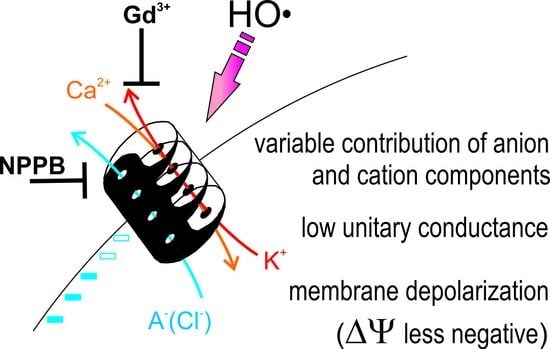
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Membrane Potential Measurements
4.3. Non-Invasive Ion Flux (MIFE) Measurements
4.4. Patch-Clamp Measurements on Root Protoplasts
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Kurusu, T.; Kuchitsu, K.; Tada, Y. Plant signaling networks involving Ca2+ and Rboh/Nox-mediated ROS production under salinity stress. Front. Plant Sci. 2015, 6, 427. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Pottosin, I.; Velarde-Buendía, A.M.; Bose, J.; Zepeda-Jazo, I.; Shabala, S.; Dobrovinskaya, O. Cross-talk between reactive oxygen species and polyamines in regulation of ion transport across the plasma membrane: Implications for plant adaptive responses. J. Exp. Bot. 2014, 65, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Shabala, S. Mechanisms of cytosolic calcium elevation in plants: The role of ion channels, calcium extrusion systems and NADPH oxidase-mediated ‘ROS-Ca2+ Hub’. Funct. Plant Biol. 2017, 45, 9–27. [Google Scholar] [CrossRef]
- Kaur, G.; Sharma, A.; Guruprasad, K.; Pati, P.K. Versatile roles of plant NADPH oxidases and emerging concepts. Biotechnol. Adv. 2014, 32, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, S.; Białasek, M.; Suzuki, N.; Górecka, M.; Devireddy, A.R.; Karpiński, S.; Mittler, R. ROS, calcium, and electric signals: Key mediators of rapid systemic signaling in plants. Plant Physiol. 2016, 171, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Shabala, L.; Zhang, J.; Pottosin, I.; Bose, J.; Zhu, M.; Fuglsang, A.T.; Velarde-Buendia, A.; Massart, A.; Hill, C.B.; Roessner, U.; et al. Cell-Type-Specific H+-ATPase activity in root tissues enables K+ retention and mediates acclimation of barley (Hordeum vulgare) to salinity stress. Plant Physiol. 2016, 172, 2445–2458. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V. Mechanisms and physiological roles of K+ efflux from root cells. J. Plant Physiol. 2014, 171, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S. Signalling by potassium: Another second messenger to add to the list? J. Exp. Bot. 2017, 68, 4003–4007. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Shabala, L.; Volkenburgh, E.V.; Newman, I. Effect of divalent cations on ion fluxes and leaf photochemistry in salinized barley leaves. J. Exp. Bot. 2005, 56, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Shabala, L.; Bose, J.; Cuin, T.; Newman, I. Ion flux measurements using the MIFE technique. In Plant Mineral Nutrients: Methods and Protocols (Methods in Molecular Biology); Maathuis, F.J.M., Ed.; Humana Press: Totowa, NJ, USA, 2013; Volume 953, pp. 171–183. ISBN 978-1-62703-151-6. [Google Scholar]
- Demidchik, V.; Shabala, S.N.; Davies, J.M. Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. Plant J. 2007, 49, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Cuin, T.A.; Svistunenko, D.; Smith, S.J.; Miller, A.J.; Shabala, S.; Sokolik, A.; Yurin, V. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: Single-channel properties, genetic basis and involvement in stress-induced cell death. J. Cell Sci. 2010, 123, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Shabala, S.N.; Coutts, K.B.; Tester, M.A.; Davies, J.M. Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J. Cell Sci. 2003, 116, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Laohavisit, A.; Shang, Z.; Rubio, L.; Cuin, T.A.; Véry, A.A.; Wang, A.; Mortimer, J.C.; Macpherson, N.; Coxon, K.M.; Battey, N.H.; et al. Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca²+- and K+-permeable conductance in root cells. Plant Cell 2012, 24, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Jazo, I.; Velarde-Buendía, A.M.; Enríquez-Figueroa, R.; Bose, J.; Shabala, S.; Muñiz-Murguía, J.; Pottosin, I.I. Polyamines interact with hydroxyl radicals in activating Ca2+ and K+ transport across the root epidermal plasma membranes. Plant Physiol. 2011, 157, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Barbier-Brygoo, H.; Vinauger, M.; Colcombet, J.; Ephritikhine, G.; Frachisse, J.-M.; Maurel, C. Anion channels in higher plants: Functional characterization, molecular structure and physiological role. Biochim. Biophys. Acta Biomembr. 2000, 1465, 199–218. [Google Scholar] [CrossRef]
- Demidchik, V.; Maathuis, F.J. Physiological roles of nonselective cation channels in plants: From salt stress to signalling and development. New Phytol. 2007, 175, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Velarde-Buendía, A.M.; Shabala, S.; Cvikrova, M.; Dobrovinskaya, O.; Pottosin, I. Salt-sensitive and salt-tolerant barley varieties differ in the extent of potentiation of the ROS-induced K+ efflux by polyamines. Plant Physiol. Biochem. 2012, 61, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.H.; Conti, F. Nonstationary noise analysis and application to patch clamp recordings. Methods Enzymol. 1992, 207, 131–148. [Google Scholar] [PubMed]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Makavitskaya, M.; Svistunenko, D.; Navaselsky, I.; Hryvusevich, P.; Mackievic, V.; Rabadanova, C.; Tyutereva, E.; Samokhina, V.; Straltsova, D.; Sokolik, A.; et al. l-Ascorbic acid induces elevation of cytosolic free calcium and leaks from plant cells through anion channels under stress conditions. J. Exp. Bot. 2018, 69. [Google Scholar] [CrossRef]
- Parsons, H.T.; Fry, S.C. Reactive oxygen species-induced release of intracellular ascorbate in plant cell-suspension cultures and evidence for pulsing of net release rate. New Phytol. 2010, 187, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Bose, Y.; Shabala, L.; Pottosin, I.; Zeng, F.; Velarde-Buendia, A.M.; Massart, A.; Poschenrieder, C.; Hariadi, Y.; Shabala, S. Kinetics of xylem loading, membrane potential maintenance, and sensitivity of K+-permeable channels to reactive oxygen species: Physiological traits that differentiate salinity tolerance between pea and barley. Plant Cell Environ. 2014, 37, 589–600. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pottosin, I.; Zepeda-Jazo, I.; Bose, J.; Shabala, S. An Anion Conductance, the Essential Component of the Hydroxyl-Radical-Induced Ion Current in Plant Roots. Int. J. Mol. Sci. 2018, 19, 897. https://doi.org/10.3390/ijms19030897
Pottosin I, Zepeda-Jazo I, Bose J, Shabala S. An Anion Conductance, the Essential Component of the Hydroxyl-Radical-Induced Ion Current in Plant Roots. International Journal of Molecular Sciences. 2018; 19(3):897. https://doi.org/10.3390/ijms19030897
Chicago/Turabian StylePottosin, Igor, Isaac Zepeda-Jazo, Jayakumar Bose, and Sergey Shabala. 2018. "An Anion Conductance, the Essential Component of the Hydroxyl-Radical-Induced Ion Current in Plant Roots" International Journal of Molecular Sciences 19, no. 3: 897. https://doi.org/10.3390/ijms19030897
APA StylePottosin, I., Zepeda-Jazo, I., Bose, J., & Shabala, S. (2018). An Anion Conductance, the Essential Component of the Hydroxyl-Radical-Induced Ion Current in Plant Roots. International Journal of Molecular Sciences, 19(3), 897. https://doi.org/10.3390/ijms19030897