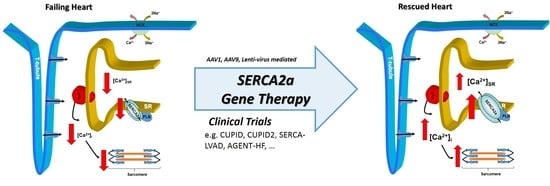
Correcting Calcium Dysregulation in Chronic Heart Failure Using SERCA2a Gene Therapy
Abstract
:1. Introduction
2. Early Techniques of SERCA2a Gene Manipulation in Animals
2.1. Plasmid and Direct DNA Injection
2.2. Adenoviral (Ad)-Based Vectors
2.3. Adeno-Associated Virus (AAV)-Based Vectors
3. Human Trials
3.1. The Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) Trial
3.2. Post-CUPID
4. The Promise of Novel Gene Delivery Techniques in Animals
5. Concluding Remarks and Future Directions
Acknowledgments
Conflicts of Interest
References
- Azad, N.; Lemay, G. Management of chronic heart failure in the older population. J. Geriatr. Cardiol. 2014, 11, 329–337. [Google Scholar] [PubMed]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States: A policy statement from the american heart association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasenfuss, G.; Pieske, B.; Holubarsch, C.; Alpert, N.R.; Just, H. Excitation-contraction coupling and contractile protein function in failing and nonfailing human myocardium. Adv. Exp. Med. Biol. 1993, 346, 91–100. [Google Scholar] [PubMed]
- Balderas-Villalobos, J.; Molina-Munoz, T.; Mailloux-Salinas, P.; Bravo, G.; Carvajal, K.; Gomez-Viquez, N.L. Oxidative stress in cardiomyocytes contributes to decreased serca2a activity in rats with metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1344–H1353. [Google Scholar] [CrossRef] [PubMed]
- Kawase, Y.; Ly, H.Q.; Prunier, F.; Lebeche, D.; Shi, Y.; Jin, H.; Hadri, L.; Yoneyama, R.; Hoshino, K.; Takewa, Y.; et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J. Am. Coll. Cardiol. 2008, 51, 1112–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, J.P. Abnormal intracellular modulation of calcium as a major cause of cardiac contractile dysfunction. N. Engl. J. Med. 1991, 325, 625–632. [Google Scholar] [CrossRef]
- Schwarzl, M.; Ojeda, F.; Zeller, T.; Seiffert, M.; Becher, P.M.; Munzel, T.; Wild, P.S.; Blettner, M.; Lackner, K.J.; Pfeiffer, N.; et al. Risk factors for heart failure are associated with alterations of the LV end-diastolic pressure-volume relationship in non-heart failure individuals: Data from a large-scale, population-based cohort. Eur. Heart J. 2016, 37, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.L.; Hashimoto, K.; Grupp, I.L.; Ji, Y.; Reed, T.; Loukianov, E.; Grupp, G.; Bhagwhat, A.; Hoit, B.; Walsh, R.; et al. Targeted overexpression of the sarcoplasmic reticulum Ca2+-atpase increases cardiac contractility in transgenic mouse hearts. Circ. Res. 1998, 83, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Del Monte, F.; Harding, S.E.; Schmidt, U.; Matsui, T.; Kang, Z.B.; Dec, G.W.; Gwathmey, J.K.; Rosenzweig, A.; Hajjar, R.J. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation 1999, 100, 2308–2311. [Google Scholar] [CrossRef]
- Studeli, R.; Jung, S.; Mohacsi, P.; Perruchoud, S.; Castiglioni, P.; Wenaweser, P.; Heimbeck, G.; Feller, M.; Hullin, R. Diastolic dysfunction in human cardiac allografts is related with reduced SERCA2a gene expression. Am. J. Transplant. 2006, 6, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Jaski, B.E.; Jessup, M.L.; Mancini, D.M.; Cappola, T.P.; Pauly, D.F.; Greenberg, B.; Borow, K.; Dittrich, H.; Zsebo, K.M.; Hajjar, R.J.; et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J. Card. Fail. 2009, 15, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Jessup, M.; Greenberg, B.; Mancini, D.; Cappola, T.; Pauly, D.F.; Jaski, B.; Yaroshinsky, A.; Zsebo, K.M.; Dittrich, H.; Hajjar, R.J.; et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID): A phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-atpase in patients with advanced heart failure. Circulation 2011, 124, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.; Butler, J.; Felker, G.M.; Ponikowski, P.; Voors, A.A.; Desai, A.S.; Barnard, D.; Bouchard, A.; Jaski, B.; Lyon, A.R.; et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): A randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet 2016, 387, 1178–1186. [Google Scholar] [CrossRef]
- Tada, M.; Yamamoto, T.; Tonomura, Y. Molecular mechanism of active calcium transport by sarcoplasmic reticulum. Physiol. Rev. 1978, 58, 1–79. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Rosing, D.R.; Bonow, R.O.; Epstein, S.E. Combination therapy with calcium-channel blockers and β-blockers for chronic stable angina-pectoris. Am. J. Cardiol. 1985, 55, B69–B80. [Google Scholar] [CrossRef]
- Cannon, R.O., 3rd; Watson, R.M.; Rosing, D.R.; Epstein, S.E. Efficacy of calcium channel blocker therapy for angina pectoris resulting from small-vessel coronary artery disease and abnormal vasodilator reserve. Am. J. Cardiol. 1985, 56, 242–246. [Google Scholar] [CrossRef]
- Antman, E.M.; Stone, P.H.; Muller, J.E.; Braunwald, E. Calcium channel blocking agents in the treatment of cardiovascular disorders. Part I: Basic and clinical electrophysiologic effects. Ann. Intern. Med. 1980, 93, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Yan, X.H.; Feng, X.; Manning, W.J.; Dillmann, W.H.; Lorell, B.H. Transgenic expression of sarcoplasmic reticulum Ca2+ atpase modifies the transition from hypertrophy to early heart failure. Circ. Res. 2001, 89, 422–429. [Google Scholar] [CrossRef] [PubMed]
- He, H.P.; Giordano, F.J.; HilalDandan, R.; Choi, D.J.; Rockman, H.A.; McDonough, P.M.; Bluhm, W.F.; Meyer, M.; Sayen, M.R.; Swanson, E.; et al. Overexpression of the rat sarcoplasmic reticulum Ca2+ atpase gene in the heart of transgenic mice accelerates calcium transients and cardiac relaxation. J. Clin. Investig. 1997, 100, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Rincon, M.Y.; VandenDriessche, T.; Chuah, M.K. Gene therapy for cardiovascular disease: Advances in vector development, targeting, and delivery for clinical translation. Cardiovasc. Res. 2015, 108, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.D.; Ranjzad, P.; Kakar, S.J.; Kingston, P.A. Development of viral vectors for use in cardiovascular gene therapy. Viruses 2010, 2, 334–371. [Google Scholar] [CrossRef] [PubMed]
- Del Monte, F.; Lebeche, D.; Guerrero, J.L.; Tsuji, T.; Doye, A.A.; Gwathmey, J.K.; Hajjar, R.J. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc. Natl. Acad. Sci. USA 2004, 101, 5622–5627. [Google Scholar] [CrossRef] [PubMed]
- Del Monte, F.; Williams, E.; Lebeche, D.; Schmidt, U.; Rosenzweig, A.; Gwathmey, J.K.; Lewandowski, E.D.; Hajjar, R.J. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca2+-atpase in a rat model of heart failure. Circulation 2001, 104, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Chaudhri, B.; del Monte, F.; Hajjar, R.J.; Harding, S.E. Contractile effects of adenovirally-mediated increases in SERCA2a activity: A comparison between adult rat and rabbit ventricular myocytes. Mol. Cell. Biochem. 2003, 251, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Nassal, M.M.; Wan, X.; Laurita, K.R.; Cutler, M.J. Atrial SERCA2a overexpression has no affect on cardiac alternans but promotes arrhythmogenic SR Ca2+ triggers. PLoS ONE 2015, 10, e0137359. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.I.; del Monte, F.; Schmidt, U.; DiSalvo, T.S.; Kang, Z.B.; Matsui, T.; Guerrero, J.L.; Gwathmey, J.K.; Rosenzweig, A.; Hajjar, R.J. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc. Natl. Acad. Sci. USA 2000, 97, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Lipskaia, L.; Hadri, L.; Le Prince, P.; Esposito, B.; Atassi, F.; Liang, L.; Glorian, M.; Limon, I.; Lompre, A.M.; Lehoux, S.; et al. SERCA2a gene transfer prevents intimal proliferation in an organ culture of human internal mammary artery. Gene Ther. 2013, 20, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Sakata, S.; Lebeche, D.; Sakata, Y.; Sakata, N.; Chemaly, E.R.; Liang, L.; Nakajima-Takenaka, C.; Tsuji, T.; Konishi, N.; del Monte, F.; et al. Transcoronary gene transfer of SERCA2a increases coronary blood flow and decreases cardiomyocyte size in a type 2 diabetic rat model. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1204–H1207. [Google Scholar] [CrossRef] [PubMed]
- Hadri, L.; Bobe, R.; Kawase, Y.; Ladage, D.; Ishikawa, K.; Atassi, F.; Lebeche, D.; Kranias, E.G.; Leopold, J.A.; Lompre, A.M.; et al. SERCA2a gene transfer enhances enos expression and activity in endothelial cells. Mol. Ther. 2010, 18, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Lu, X.; Li, X.; Niu, K.; Cai, J. Attenuation of endoplasmic reticulum stress-related myocardial apoptosis by serca2a gene delivery in ischemic heart disease. Mol. Med. 2011, 17, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Kuken, B.N.; Aikemu, A.N.; Xiang, S.Y.; Wulasihan, M.H. Effect of SERCA2a overexpression in the pericardium mediated by the AAV1 gene transfer on rapid atrial pacing in rabbits. Genet. Mol. Res. 2015, 14, 13625–13632. [Google Scholar] [CrossRef] [PubMed]
- Lipskaia, L.; Hadri, L.; Lopez, J.J.; Hajjar, R.J.; Bobe, R. Benefit of SERCA2a gene transfer to vascular endothelial and smooth muscle cells: A new aspect in therapy of cardiovascular diseases. Curr. Vasc. Pharmacol. 2013, 11, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Wobus, C.E.; Hugle-Dorr, B.; Girod, A.; Petersen, G.; Hallek, M.; Kleinschmidt, J.A. Monoclonal antibodies against the adeno-associated virus type 2 (AAV-2) capsid: Epitope mapping and identification of capsid domains involved in AAV-2-cell interaction and neutralization of AAV-2 infection. J. Virol. 2000, 74, 9281–9293. [Google Scholar] [CrossRef] [PubMed]
- Scallan, C.D.; Jiang, H.; Liu, T.; Patarroyo-White, S.; Sommer, J.M.; Zhou, S.; Couto, L.B.; Pierce, G.F. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in scid mice. Blood 2006, 107, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Moskalenko, M.; Chen, L.; van Roey, M.; Donahue, B.A.; Snyder, R.O.; McArthur, J.G.; Patel, S.D. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: Implications for gene therapy and virus structure. J. Virol. 2000, 74, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.; Butler, J.; Felker, G.M.; Ponikowski, P.; Voors, A.A.; Pogoda, J.M.; Provost, R.; Guerrero, J.; Hajjar, R.J.; Zsebo, K.M. Prevalence of AAV1 neutralizing antibodies and consequences for a clinical trial of gene transfer for advanced heart failure. Gene Ther. 2016, 23, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Hulot, J.S.; Salem, J.E.; Redheuil, A.; Collet, J.P.; Varnous, S.; Jourdain, P.; Logeart, D.; Gandjbakhch, E.; Bernard, C.; Hatem, S.N.; et al. Effect of intracoronary administration of AAV1/SERCA2a on ventricular remodelling in patients with advanced systolic heart failure: Results from the agent-hf randomized phase 2 trial. Eur. J. Heart Fail. 2017, 19, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.; Yaroshinsky, A.; Zsebo, K.M.; Butler, J.; Felker, G.M.; Voors, A.A.; Rudy, J.J.; Wagner, K.; Hajjar, R.J. Design of a phase 2b trial of intracoronary administration of AAV1/SERCA2a in patients with advanced heart failure: The CUPID 2 trial (calcium up-regulation by percutaneous administration of gene therapy in cardiac disease phase 2b). JACC Heart Fail. 2014, 2, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Zsebo, K.; Yaroshinsky, A.; Rudy, J.J.; Wagner, K.; Greenberg, B.; Jessup, M.; Hajjar, R.J. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: Analysis of recurrent cardiovascular events and mortality. Circ. Res. 2014, 114, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.D.; Rosenson, R.S.; McMurray, J.J.; Marx, N.; Remme, W.J. Clinical trials update aha congress 2010. Cardiovasc. Drugs Ther. 2011, 25, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, R.J.; Zsebo, K.; Deckelbaum, L.; Thompson, C.; Rudy, J.; Yaroshinsky, A.; Ly, H.; Kawase, Y.; Wagner, K.; Borow, K.; et al. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J. Card. Fail. 2008, 14, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Hulot, J.S.; Ishikawa, K.; Hajjar, R.J. Gene therapy for the treatment of heart failure: Promise postponed. Eur. Heart J. 2016, 37, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Yla-Herttuala, S. Gene therapy for heart failure: Back to the bench. J. Am. Soc. Gene Ther. 2015, 23, 1551–1552. [Google Scholar] [CrossRef] [PubMed]
- Mingozzi, F.; Anguela, X.M.; Pavani, G.; Chen, Y.; Davidson, R.J.; Hui, D.J.; Yazicioglu, M.; Elkouby, L.; Hinderer, C.J.; Faella, A.; et al. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci. Transl. Med. 2013, 5, 194ra192. [Google Scholar] [CrossRef] [PubMed]
- Pacak, C.A.; Mah, C.S.; Thattaliyath, B.D.; Conlon, T.J.; Lewis, M.A.; Cloutier, D.E.; Zolotukhin, I.; Tarantal, A.F.; Byrne, B.J. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ. Res. 2006, 99, e3–e9. [Google Scholar] [CrossRef] [PubMed]
- Vandendriessche, T.; Thorrez, L.; Acosta-Sanchez, A.; Petrus, I.; Wang, L.; Ma, L.; Waele, D.E.; Iwasaki, Y.; Gillijns, V.; Wilson, J.M.; et al. Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. Lentiviral vectors for hemophilia B gene therapy. J. Thromb. Haemost. 2007, 5, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inagaki, K.; Fuess, S.; Storm, T.A.; Gibson, G.A.; McTiernan, C.F.; Kay, M.A.; Nakai, H. Robust systemic transduction with AAV9 vectors in mice: Efficient global cardiac gene transfer superior to that of AAV8. Mol. Ther. 2006, 14, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Zincarelli, C.; Soltys, S.; Rengo, G.; Koch, W.J.; Rabinowitz, J.E. Comparative cardiac gene delivery of adeno-associated virus serotypes 1–9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clin. Transl. Sci. 2010, 3, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Bannister, M.L.; Collins, T.; Pearce, E.; Sepehripour, A.H.; Dubb, S.S.; Garcia, E.; O’Gara, P.; Liang, L.; Kohlbrenner, E.; et al. SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ. Arrhythm. Electrophysiol. 2011, 4, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Cutler, M.J.; Wan, X.; Plummer, B.N.; Liu, H.; Deschenes, I.; Laurita, K.R.; Hajjar, R.J.; Rosenbaum, D.S. Targeted sarcoplasmic reticulum Ca2+ ATPase 2a gene delivery to restore electrical stability in the failing heart. Circulation 2012, 126, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Bostick, B.; Yue, Y.; Hajjar, R.; Duan, D. SERCA2a gene transfer improves electrocardiographic performance in aged mdx mice. J. Transl. Med. 2011, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Zhou, X.H.; Li, Z.Q.; Fan, P.; Zhou, Q.N.; Li, Y.D.; Hou, Y.M.; Tang, B.P. Prevention of atrial fibrillation by using sarcoplasmic reticulum calcium atpase pump overexpression in a rabbit model of rapid atrial pacing. Med. Sci. Monit. 2017, 23, 3952–3960. [Google Scholar] [CrossRef] [PubMed]
- Pleger, S.T.; Shan, C.; Ksienzyk, J.; Bekeredjian, R.; Boekstegers, P.; Hinkel, R.; Schinkel, S.; Leuchs, B.; Ludwig, J.; Qiu, G.; et al. Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci. Transl. Med. 2011, 3, 92ra64. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.G.; Fargnoli, A.S.; Williams, R.D.; Steuerwald, N.M.; Isidro, A.; Ivanina, A.V.; Sokolova, I.M.; Bridges, C.R. Safety and efficacy of high-dose adeno-associated virus 9 encoding sarcoplasmic reticulum Ca2+ adenosine triphosphatase delivered by molecular cardiac surgery with recirculating delivery in ovine ischemic cardiomyopathy. J. Thorac. Cardiovasc. Surg. 2014, 148, 1065–1072, 1073e1–1073e2; discussion 1072–1073. [Google Scholar] [CrossRef] [PubMed]
- Niwano, K.; Arai, M.; Koitabashi, N.; Watanabe, A.; Ikeda, Y.; Miyoshi, H.; Kurabayashi, M. Lentiviral vector-mediated SERCA2 gene transfer protects against heart failure and left ventricular remodeling after myocardial infarction in rats. J. Am. Soc. Gene Ther. 2008, 16, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Fleury, S.; Simeoni, E.; Zuppinger, C.; Deglon, N.; Von Segesser, L.K.; Kappenberger, L.; Vassalli, G. Multiply attenuated, self-inactivating lentiviral vectors efficiently deliver and express genes for extended periods of time in adult rat cardiomyocytes in vivo. Circulation 2003, 107, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Takahashi, M.; Gage, F.H.; Verma, I.M. Stable and efficient gene transfer into the retina using an hiv-based lentiviral vector. Proc. Natl. Acad. Sci. USA 1997, 94, 10319–10323. [Google Scholar] [CrossRef] [PubMed]
- Naldini, L.; Blomer, U.; Gallay, P.; Ory, D.; Mulligan, R.; Gage, F.H.; Verma, I.M.; Trono, D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996, 272, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Merentie, M.; Lottonen-Raikaslehto, L.; Parviainen, V.; Huusko, J.; Pikkarainen, S.; Mendel, M.; Laham-Karam, N.; Karja, V.; Rissanen, R.; Hedman, M.; et al. Efficacy and safety of myocardial gene transfer of adenovirus, adeno-associated virus and lentivirus vectors in the mouse heart. Gene Ther. 2016, 23, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Renaud-Gabardos, E.; Tatin, F.; Hantelys, F.; Lebas, B.; Calise, D.; Kunduzova, O.; Masri, B.; Pujol, F.; Sicard, P.; Valet, P.; et al. Therapeutic benefit and gene network regulation by combined gene transfer of apelin, FGF2, and SERCA2a into ischemic heart. Mol. Ther. 2018, 26, 902–916. [Google Scholar] [CrossRef] [PubMed]
- Merentie, M.; Rissanen, R.; Lottonen-Raikaslehto, L.; Huusko, J.; Gurzeler, E.; Turunen, M.P.; Holappa, L.; Makinen, P.; Yla-Herttuala, S. Doxycycline modulates VEGF-A expression: Failure of doxycycline-inducible lentivirus shrna vector to knockdown VEGF-A expression in transgenic mice. PLoS ONE 2018, 13, e0190981. [Google Scholar] [CrossRef] [PubMed]
- Tilemann, L.; Lee, A.; Ishikawa, K.; Aguero, J.; Rapti, K.; Santos-Gallego, C.; Kohlbrenner, E.; Fish, K.M.; Kho, C.; Hajjar, R.J. SUMO-1 gene transfer improves cardiac function in a large-animal model of heart failure. Sci. Transl. Med. 2013, 5, 211ra159. [Google Scholar] [CrossRef] [PubMed]
- Kho, C.; Lee, A.; Jeong, D.; Oh, J.G.; Chaanine, A.H.; Kizana, E.; Park, W.J.; Hajjar, R.J. SUMO1-dependent modulation of SERCA2a in heart failure. Nature 2011, 477, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Ferrandi, M.; Barassi, P.; Tadini-Buoninsegni, F.; Bartolommei, G.; Molinari, I.; Tripodi, M.G.; Reina, C.; Moncelli, M.R.; Bianchi, G.; Ferrari, P. Istaroxime stimulates SERCA2a and accelerates calcium cycling in heart failure by relieving phospholamban inhibition. Br. J. Pharmacol. 2013, 169, 1849–1861. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.O.; Quick, A.P.; Wang, Q.; Beavers, D.L.; Philippen, L.E.; Showell, J.; Barreto-Torres, G.; Thuerauf, D.J.; Doroudgar, S.; Glembotski, C.C.; et al. Junctophilin-2 gene therapy rescues heart failure by normalizing RyR2-mediated Ca2+ release. Int. J. Cardiol. 2016, 225, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Beavers, D.L.; Landstrom, A.P.; Chiang, D.Y.; Wehrens, X.H. Emerging roles of Junctophilin-2 in the heart and implications for cardiac diseases. Cardiovasc. Res. 2014, 103, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Zhang, X.; Iyer, V.R.; Chen, B.; Zhang, C.; Kutschke, W.J.; Weiss, R.M.; Franzini-Armstrong, C.; Song, L.S. Overexpression of Junctophilin-2 does not enhance baseline function but attenuates heart failure development after cardiac stress. Proc. Natl. Acad. Sci. USA 2014, 111, 12240–12245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, B.; Guo, A.; Zhu, Y.; Miller, J.D.; Gao, S.; Yuan, C.; Kutschke, W.; Zimmerman, K.; Weiss, R.M.; et al. Microtubule-mediated defects in Junctophilin-2 trafficking contribute to myocyte transverse-tubule remodeling and Ca2+ handling dysfunction in heart failure. Circulation 2014, 129, 1742–1750. [Google Scholar] [CrossRef] [PubMed]


| National Clinical Trial Code | Status | Study Title | Conditions | Interventions | Study Results | References |
|---|---|---|---|---|---|---|
| NCT02772068 | Recruiting | Hemodynamic Response to Exercise in HFpEF Patients After Upregulation of SERCA2a | Congestive Heart Failure | Drug: Istaroxime Other: Exercise | No Published Results | - |
| NCT01966887 | Terminated | AAV1-CMV-Serca2a GENe Therapy Trial in Heart Failure (AGENT-HF) | Congestive Heart Failure Ischemic and non-ischemic Cardiomyopathies | Genetic: AAV1/SERCA2a (MYDICAR)-single intracoronary infusion Genetic: Placebo; single intracoronary infusion | No Positive or Negative Effects | [38] |
| NCT00534703 | Terminated | Investigation of the Safety and Feasibility of AAV1/SERCA2a Gene Transfer in Patients with Chronic Heart Failure (SERCA-LVAD) | Chronic Heart Failure Left Ventricular Assist Device | Genetic: AAV1/SERCA2a Drug: Placebo | Terminated Early—No Results | - |
| NCT01643330 | Completed | A Study of Genetically Targeted Enzyme Replacement Therapy for Advanced Heart Failure (CUPID-2b) | Ischemic and non-ischemic Cardiomyopathies Heart Failure | Genetic: AAV1/SERCA2a (MYDICAR) Genetic: Placebo | No Positive or Negative Effects | [12,13,14,37,39,40,41] |
| NCT00454818 | Completed | Efficacy and Safety Study of Genetically Targeted Enzyme Replacement Therapy for Advanced Heart Failure (CUPID) | Heart Failure, Congestive Dilated Cardiomyopathy | Genetic: MYDICAR Phase 1 (Open-label, Serial Dose-Escalation Study) Procedure: Placebo Infusion Genetic: MYDICAR Phase 2 (Placebo-controlled, Randomized Study) | Positive Results | [12,13,40,41,42] |
| NCT02346422 | Terminated | A Phase 1/2 Study of High-Dose Genetically Targeted Enzyme Replacement Therapy for Advanced Heart Failure | Heart Failure Cardiomyopathy | Genetic: MYICAR Phase 1/2 (Dose 2.5 × 1013 DRP) | Terminated Early—No Results | [12,13,40] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samuel, T.J.; Rosenberry, R.P.; Lee, S.; Pan, Z. Correcting Calcium Dysregulation in Chronic Heart Failure Using SERCA2a Gene Therapy. Int. J. Mol. Sci. 2018, 19, 1086. https://doi.org/10.3390/ijms19041086
Samuel TJ, Rosenberry RP, Lee S, Pan Z. Correcting Calcium Dysregulation in Chronic Heart Failure Using SERCA2a Gene Therapy. International Journal of Molecular Sciences. 2018; 19(4):1086. https://doi.org/10.3390/ijms19041086
Chicago/Turabian StyleSamuel, T. Jake, Ryan P. Rosenberry, Seungyong Lee, and Zui Pan. 2018. "Correcting Calcium Dysregulation in Chronic Heart Failure Using SERCA2a Gene Therapy" International Journal of Molecular Sciences 19, no. 4: 1086. https://doi.org/10.3390/ijms19041086
APA StyleSamuel, T. J., Rosenberry, R. P., Lee, S., & Pan, Z. (2018). Correcting Calcium Dysregulation in Chronic Heart Failure Using SERCA2a Gene Therapy. International Journal of Molecular Sciences, 19(4), 1086. https://doi.org/10.3390/ijms19041086