Calcium-Activated Cl− Channel: Insights on the Molecular Identity in Epithelial Tissues
Abstract
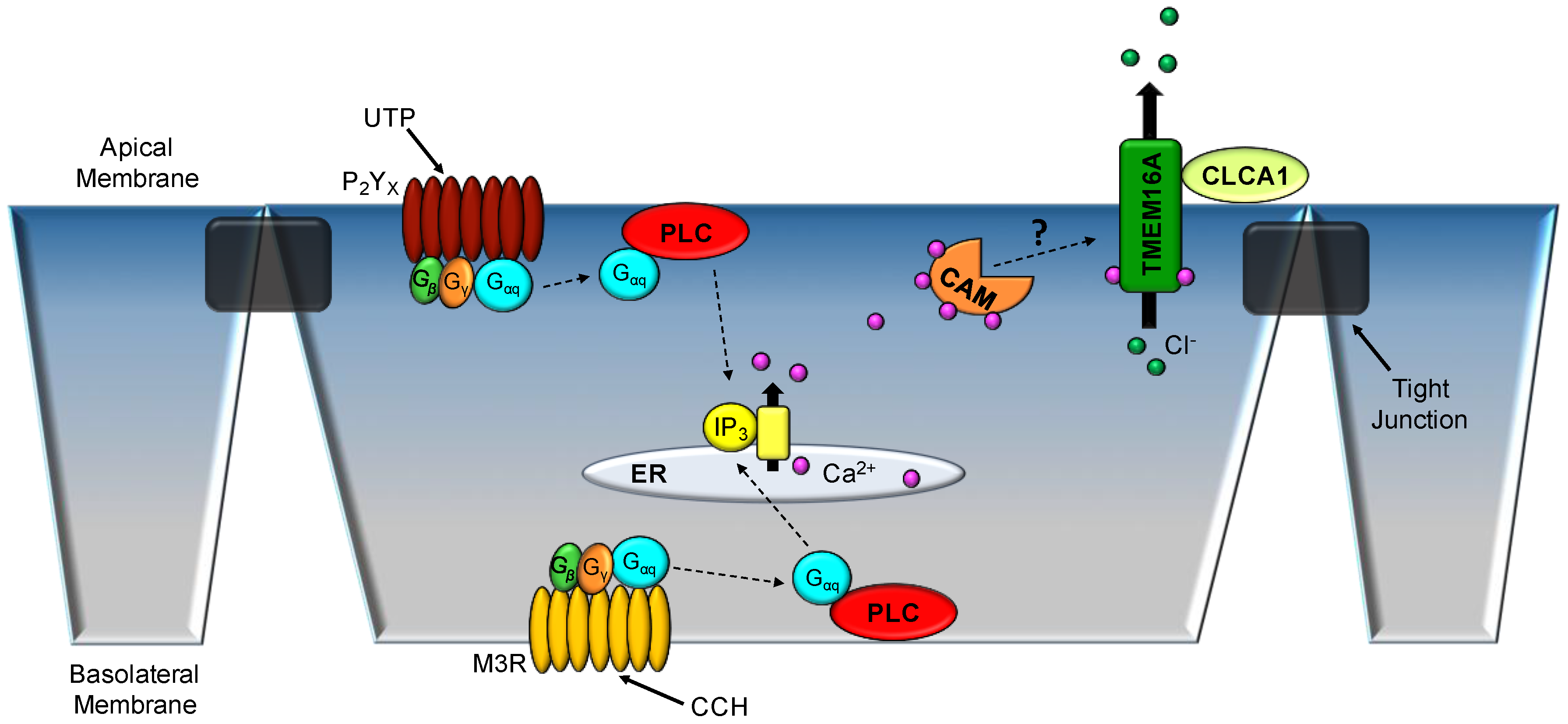
:1. Introduction
2. TMEM16A Characterization
3. TMEM16A in Epithelial Tissues
3.1. Respiratory Epithelium
3.2. Colonic Epithelium
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| CaCC | calcium-activated chloride channel |
| CaM | calmodulin |
| cAMP | cyclic adenosine monophosphate |
| CCH | carbachol |
| CF | Cystic Fibrosis |
| CFTR | Cystic Fibrosis Transmembrane Conductance Regulator |
| CLCA | Cl− channel accessory |
| cRNA | complementary ribonucleic acid |
| DTT | dithiothreitol |
| EC50 | half-maximal effective concentration |
| FRET | fluorescence resonance energy transfer |
| GFP | green fluorescent protein |
| HEK 293 | human embryonic kidney 293 cells |
| IL-4 | interleukin 4 |
| ISC | short-circuit current |
| kDa | kilodalton |
| mM | millimolar |
| mRNA | messenger ribonucleic acid |
| mV | millivolts |
| µM | micromolar |
| siRNA | small interfering ribonucleic acid |
| TMEM16A | transmembrane member 16A |
| UTP | uridine triphosphate |
| VTE | trans-epithelial voltage |
| WT | wild type |
References
- Browner, M.; Ferkany, J.W.; Enna, S.J. Biochemical identification of pharmacologically and functionally distinct gaba receptors in rat brain. J. Neurosci. 1981, 1, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Cozens, A.L.; Yezzi, M.J.; Kunzelmann, K.; Ohrui, T.; Chin, L.; Eng, K.; Finkbeiner, W.E.; Widdicombe, J.H.; Gruenert, D.C. Cftr expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 1994, 10, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Devor, D.C.; Singh, A.K.; Lambert, L.C.; DeLuca, A.; Frizzell, R.A.; Bridges, R.J. Bicarbonate and chloride secretion in calu-3 human airway epithelial cells. J. Gen. Physiol. 1999, 113, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Gallos, G.; Remy, K.E.; Danielsson, J.; Funayama, H.; Fu, X.W.; Chang, H.Y.; Yim, P.; Xu, D.; Emala, C.W., Sr. Functional expression of the TMEM16 family of calcium-activated chloride channels in airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L625–L634. [Google Scholar] [CrossRef] [PubMed]
- Manoury, B.; Tamuleviciute, A.; Tammaro, P. TMEM16A/anoctamin 1 protein mediates calcium-activated chloride currents in pulmonary arterial smooth muscle cells. J. Physiol. 2010, 588, 2305–2314. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.A.; Winpenny, J.P.; Porteous, D.J.; Dorin, J.R.; Argent, B.E. CFTR and calcium-activated chloride currents in pancreatic duct cells of a transgenic cf mouse. Am. J. Physiol. 1994, 266, C213–C221. [Google Scholar] [CrossRef] [PubMed]
- Willumsen, N.J.; Boucher, R.C. Activation of an apical Cl- conductance by Ca2+ ionophores in cystic fibrosis airway epithelia. Am. J. Physiol. 1989, 256, C226–C233. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.R.; Clarke, L.L.; Boucher, R.C. Activation by extracellular nucleotides of chloride secretion in the airway epithelia of patients with cystic fibrosis. N. Engl. J. Med. 1991, 325, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.A.; Cozens, A.L.; Schulman, H.; Gruenert, D.C.; Stryer, L.; Gardner, P. Activation of chloride channels in normal and cystic fibrosis airway epithelial cells by multifunctional calcium/calmodulin-dependent protein kinase. Nature 1991, 349, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.L.; Grubb, B.R.; Yankaskas, J.R.; Cotton, C.U.; McKenzie, A.; Boucher, R.C. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in CFTR(-/-) mice. Proc. Natl. Acad. Sci. USA 1994, 91, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Kartner, N.; Hanrahan, J.W.; Jensen, T.J.; Naismith, A.L.; Sun, S.Z.; Ackerley, C.A.; Reyes, E.F.; Tsui, L.C.; Rommens, J.M.; Bear, C.E.; et al. Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. Cell 1991, 64, 681–691. [Google Scholar] [CrossRef]
- Cunningham, S.A.; Awayda, M.S.; Bubien, J.K.; Ismailov, I.I.; Pia Arrate, M.P.; Berdiev, B.K.; Benos, D.J.; Fuller, C.M. Cloning of an epithelial chloride channel from bovine trachea. J. Biol. Chem. 1995, 270, 31016–31026. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.D.; Pauli, B.U. Molecular cloning and biochemical characterization of a truncated, secreted member of the human family of Ca2+-activated Cl- channels. Biochim. Biophys. Acta 1999, 1444, 418–423. [Google Scholar] [CrossRef]
- Leverkoehne, I.; Gruber, A.D. The murine MCLCA3 (alias Gob-5) protein is located in the mucin granule membranes of intestinal, respiratory, and uterine goblet cells. J. Histochem. Cytochem. 2002, 50, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Elble, R.C.; Gruber, A.D.; Schreur, K.D.; Ji, H.L.; Fuller, C.M.; Pauli, B.U. Molecular and functional characterization of a calcium-sensitive chloride channel from mouse lung. J. Biol. Chem. 1998, 273, 32096–32101. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.D.; Elble, R.C.; Ji, H.L.; Schreur, K.D.; Fuller, C.M.; Pauli, B.U. Genomic cloning, molecular characterization, and functional analysis of human clca1, the first human member of the family of Ca2+-activated Cl- channel proteins. Genomics 1998, 54, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Neher, E.; Sakmann, B. Rat brain serotonin receptors in xenopus oocytes are coupled by intracellular calcium to endogenous channels. Proc. Natl. Acad. Sci. USA 1987, 84, 5063–5067. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.D.; Schreur, K.D.; Ji, H.L.; Fuller, C.M.; Pauli, B.U. Molecular cloning and transmembrane structure of HClCa2 from human lung, trachea, and mammary gland. Am. J. Physiol. 1999, 276, C1261–C1270. [Google Scholar] [CrossRef] [PubMed]
- Caputo, A.; Caci, E.; Ferrera, L.; Pedemonte, N.; Barsanti, C.; Sondo, E.; Pfeffer, U.; Ravazzolo, R.; Zegarra-Moran, O.; Galietta, L.J. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 2008, 322, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.C.; Cheng, T.; Jan, Y.N.; Jan, L.Y. Expression cloning of tmem16a as a calcium-activated chloride channel subunit. Cell 2008, 134, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.D.; Cho, H.; Koo, J.Y.; Tak, M.H.; Cho, Y.; Shim, W.S.; Park, S.P.; Lee, J.; Lee, B.; Kim, B.M.; et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 2008, 455, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Jeng, G.; Aggarwal, M.; Yu, W.P.; Chen, T.Y. Independent activation of distinct pores in dimeric tmem16a channels. J. Gen. Physiol. 2016, 148, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Lim, N.K.; Lam, A.K.; Dutzler, R. Independent activation of ion conduction pores in the double-barreled calcium-activated chloride channel TMEM16A. J. Gen. Physiol. 2016, 148, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Paulino, C.; Neldner, Y.; Lam, A.K.; Kalienkova, V.; Brunner, J.D.; Schenck, S.; Dutzler, R. Structural basis for anion conduction in the calcium-activated chloride channel TMEM16A. eLife 2017, 6, e26232. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, J.T.; Worthington, E.N.; Yu, K.; Gabriel, S.E.; Hartzell, H.C.; Tarran, R. Characterization of the oligomeric structure of the Ca(2+)-activated Cl- channel ANO1/TMEM16A. J. Biol. Chem. 2011, 286, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Tien, J.; Lee, H.Y.; Minor, D.L., Jr.; Jan, Y.N.; Jan, L.Y. Identification of a dimerization domain in the TMEM16A calcium-activated chloride channel (CACC). Proc. Natl. Acad. Sci. USA 2013, 110, 6352–6357. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Yu, K.; Perez-Cornejo, P.; Cui, Y.; Arreola, J.; Hartzell, H.C. Voltage- and calcium-dependent gating of TMEM16A/ANO1 chloride channels are physically coupled by the first intracellular loop. Proc. Natl. Acad. Sci. USA 2011, 108, 8891–8896. [Google Scholar] [CrossRef] [PubMed]
- Tien, J.; Peters, C.J.; Wong, X.M.; Cheng, T.; Jan, Y.N.; Jan, L.Y.; Yang, H.H. A comprehensive search for calcium binding sites critical for TMEM16A calcium-activated chloride channel activity. eLife 2014, 3, e02772. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, S.; Ren, S.; Chen, Y.; Yuan, H.; Chai, R.; Yu, H.; Zhang, H.; Zhan, Y.; An, H. Two Ca(2+)-binding sites cooperatively couple together in TMEM16A channel. J. Membr. Biol. 2016, 249, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Scudieri, P.; Musante, I.; Gianotti, A.; Moran, O.; Galietta, L.J. Intermolecular interactions in the TMEM16A dimer controlling channel activity. Sci. Rep. 2016, 6, 38788. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.J.; Yu, H.B.; Tien, J.; Jan, Y.N.; Li, M.; Jan, L.Y. Four basic residues critical for the ion selectivity and pore blocker sensitivity of TMEM16A calcium-activated chloride channels. Proc. Natl. Acad. Sci. USA 2015, 112, 3547–3552. [Google Scholar] [CrossRef] [PubMed]
- Brunner, J.D.; Lim, N.K.; Schenck, S.; Duerst, A.; Dutzler, R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature 2014, 516, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Feng, S.; Tien, J.; Peters, C.J.; Bulkley, D.; Lolicato, M.; Zhao, J.; Zuberbuhler, K.; Ye, W.; Qi, L.; et al. Cryo-em structures of the TMEM16A calcium-activated chloride channel. Nature 2017, 552, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Paulino, C.; Kalienkova, V.; Lam, A.K.M.; Neldner, Y.; Dutzler, R. Activation mechanism of the calcium-activated chloride channel tmem16a revealed by cryo-em. Nature 2017, 552, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Barro-Soria, R.; Rebolledo, S.; Liin, S.I.; Perez, M.E.; Sampson, K.J.; Kass, R.S.; Larsson, H.P. Kcne1 divides the voltage sensor movement in KCNQ1/KCNE1 channels into two steps. Nat. Commun. 2014, 5, 3750. [Google Scholar] [CrossRef] [PubMed]
- Hullin, R.; Khan, I.F.; Wirtz, S.; Mohacsi, P.; Varadi, G.; Schwartz, A.; Herzig, S. Cardiac l-type calcium channel beta-subunits expressed in human heart have differential effects on single channel characteristics. J. Biol. Chem. 2003, 278, 21623–21630. [Google Scholar] [CrossRef] [PubMed]
- Orio, P.; Latorre, R. Differential effects of beta 1 and beta 2 subunits on BK channel activity. J. Gen. Physiol. 2005, 125, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Kongsuphol, P.; Hug, M.; Ousingsawat, J.; Witzgall, R.; Schreiber, R.; Kunzelmann, K. Calmodulin-dependent activation of the epithelial calcium-dependent chloride channel TMEM16A. FASEB J. 2011, 25, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, P.S.; Warner, B.B.; Zhou, Y.; Weinstock, G.M.; Sodergren, E.; Hall-Moore, C.M.; Stevens, H.J.; Bennett, W.E., Jr.; Shaikh, N.; Linneman, L.A.; et al. Patterned progression of bacterial populations in the premature infant gut. Proc. Natl. Acad. Sci. USA 2014, 111, 12522–12527. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Zhu, J.; Qu, Z.; Cui, Y.Y.; Hartzell, H.C. Activation of the ano1 (TMEM16A) chloride channel by calcium is not mediated by calmodulin. J. Gen. Physiol. 2014, 143, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Kuan, A.S.; Chen, T.Y. Calcium-calmodulin does not alter the anion permeability of the mouse tmem16a calcium-activated chloride channel. J. Gen. Physiol. 2014, 144, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Terashima, H.; Picollo, A.; Accardi, A. Purified TMEM16A is sufficient to form Ca2+-activated Cl-channels. Proc. Natl. Acad. Sci. USA 2013, 110, 19354–19359. [Google Scholar] [CrossRef] [PubMed]
- Mura, C.V.; Delgado, R.; Delgado, M.G.; Restrepo, D.; Bacigalupo, J. A clca regulatory protein present in the chemosensory cilia of olfactory sensory neurons induces a Ca(2+)-activated Cl(−) current when transfected into HEK293. BMC Neurosci. 2017, 18, 61. [Google Scholar] [CrossRef] [PubMed]
- Sala-Rabanal, M.; Yurtsever, Z.; Berry, K.N.; Nichols, C.G.; Brett, T.J. Modulation of TMEM16A channel activity by the von willebrand factor type a (VWA) domain of the calcium-activated chloride channel regulator 1 (CLCA1). J. Biol. Chem. 2017, 292, 9164–9174. [Google Scholar] [CrossRef] [PubMed]
- Sala-Rabanal, M.; Yurtsever, Z.; Nichols, C.G.; Brett, T.J. Secreted clca1 modulates tmem16a to activate ca(2+)-dependent chloride currents in human cells. eLife 2015, 4, e05875. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K.; Khimji, A.K.; Kresge, C.; Bugde, A.; Dougherty, M.; Esser, V.; Ueno, Y.; Glaser, S.S.; Alpini, G.; Rockey, D.C.; et al. Identification and functional characterization of TMEM16A, a Ca2+-activated Cl- channel activated by extracellular nucleotides, in biliary epithelium. J. Biol. Chem. 2011, 286, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Rock, J.R.; Harfe, B.D.; Cheng, T.; Huang, X.; Jan, Y.N.; Jan, L.Y. Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc. Natl. Acad. Sci. USA 2009, 106, 21413–21418. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.R.; Futtner, C.R.; Harfe, B.D. The transmembrane protein tmem16a is required for normal development of the murine trachea. Dev. Biol. 2008, 321, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Ousingsawat, J.; Martins, J.R.; Schreiber, R.; Rock, J.R.; Harfe, B.D.; Kunzelmann, K. Loss of TMEM16A causes a defect in epithelial Ca2+-dependent chloride transport. J. Biol. Chem. 2009, 284, 28698–28703. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, R.; Ousingsawat, J.; Wanitchakool, P.; Zhang, Y.; Holtzman, M.J.; Amaral, M.; Rock, J.R.; Schreiber, R.; Kunzelmann, K. Epithelial chloride transport by CFTR requires TMEM16A. Sci. Rep. 2017, 7, 12397. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Liu, W.; Guan, L.; Lu, M.; Wang, K. Inhibition of calcium-activated chloride channel ANO1/TMEM16A suppresses tumor growth and invasion in human lung cancer. PLoS ONE 2015, 10, e0136584. [Google Scholar] [CrossRef] [PubMed]
- Lerias, J.; Pinto, M.; Benedetto, R.; Schreiber, R.; Amaral, M.; Aureli, M.; Kunzelmann, K. Compartmentalized crosstalk of CFTR and TMEM16A (ANO1) through EPAC1 and ADCY1. Cell. Signal. 2018, 44, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Galietta, L.J.; Pagesy, P.; Folli, C.; Caci, E.; Romio, L.; Costes, B.; Nicolis, E.; Cabrini, G.; Goossens, M.; Ravazzolo, R.; et al. IL-4 is a potent modulator of ion transport in the human bronchial epithelium in vitro. J. Immunol. 2002, 168, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.X.; Lu, L.W.; Liu, W.J.; Huang, M. Plasma inflammatory cytokine IL-4, IL-8, IL-10, and tnf-alpha levels correlate with pulmonary function in patients with asthma-chronic obstructive pulmonary disease (copd) overlap syndrome. Med. Sci. Monit. 2016, 22, 2800–2808. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Qiu, Y.; Valobra, M.; Qiu, S.; Majumdar, S.; Matin, D.; De Rose, V.; Jeffery, P.K. Plasma cells and IL-4 in chronic bronchitis and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007, 175, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, H.; Wu, M.; Yang, H.; Kudo, M.; Peters, C.J.; Woodruff, P.G.; Solberg, O.D.; Donne, M.L.; Huang, X.; et al. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc. Natl. Acad. Sci. USA 2012, 109, 16354–16359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Wang, H.; Jiao, J.; Li, Y.; Fan, E.; Zhang, L.; Bachert, C. TMEM16A-mediated mucin secretion in IL-13-induced nasal epithelial cells from chronic rhinosinusitis patients. Allergy Asthma Immunol. Res. 2015, 7, 367–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonneville, F.; Ruffin, M.; Coraux, C.; Rousselet, N.; Le Rouzic, P.; Blouquit-Laye, S.; Corvol, H.; Tabary, O. Microrna-9 downregulates the ano1 chloride channel and contributes to cystic fibrosis lung pathology. Nat. Commun. 2017, 8, 710. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.R.; O’Neal, W.K.; Gabriel, S.E.; Randell, S.H.; Harfe, B.D.; Boucher, R.C.; Grubb, B.R. Transmembrane protein 16a (TMEM16A) is a Ca2+-regulated Cl-secretory channel in mouse airways. J. Biol. Chem. 2009, 284, 14875–14880. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Harris, W.T.; Kortyka, S.; Kotha, K.; Ostmann, A.J.; Rezayat, A.; Sridharan, A.; Sanders, Y.; Naren, A.P.; Clancy, J.P. Tgf-beta downregulation of distinct chloride channels in cystic fibrosis-affected epithelia. PLoS ONE 2014, 9, e106842. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Schreiber, R.; Wanitchakool, P.; Kongsuphol, P.; Sousa, M.; Uliyakina, I.; Palma, M.; Faria, D.; Traynor-Kaplan, A.E.; Fragata, J.I.; et al. Control of tmem16a by ino-4995 and other inositolphosphates. Br. J. Pharmacol. 2013, 168, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Namkung, W.; Phuan, P.W.; Verkman, A.S. Tmem16a inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J. Biol. Chem. 2011, 286, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Stutts, M.J.; Lazarowski, E.R.; Paradiso, A.M.; Boucher, R.C. Activation of CFTR Cl- conductance in polarized T84 cells by luminal extracellular atp. Am. J. Physiol. 1995, 268, C425–C433. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.M.; Tian, P.; Zeng, C.Q.; Morris, A.P.; Estes, M.K. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 1996, 272, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.P.; Scott, J.K.; Ball, J.M.; Zeng, C.Q.; O’Neal, W.K.; Estes, M.K. Nsp4 elicits age-dependent diarrhea and Ca(2+)mediated i(-) influx into intestinal crypts of cf mice. Am. J. Physiol. 1999, 277, G431–G444. [Google Scholar] [PubMed]
- Ousingsawat, J.; Mirza, M.; Tian, Y.; Roussa, E.; Schreiber, R.; Cook, D.I.; Kunzelmann, K. Rotavirus toxin NSP4 induces diarrhea by activation of tmem16a and inhibition of na+ absorption. Pflugers Arch. 2011, 461, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Sun, M.; Wu, F.; Yang, L.; Di, W.; Zhang, G.; Zhong, L.; Ma, Z.; Zheng, J.; Fang, X.; et al. Inhibition of TMEM16A expression suppresses growth and invasion in human colorectal cancer cells. PLoS ONE 2014, 9, e115443. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Wu, F.; Lv, J.; Li, H.; Li, X.; Du, Z.; Sun, M.; Zheng, Y.; Yang, L.; Zhong, L.; et al. Identification of the novel TMEM16A inhibitor dehydroandrographolide and its anticancer activity on SW620 cells. PLoS ONE 2015, 10, e0144715. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Wee, J.; Jung, J.; Jang, Y.; Lee, B.; Hong, G.S.; Chang, B.C.; Choi, Y.L.; Shin, Y.K.; Min, H.Y.; et al. Anoctamin 1 (TMEM16A) is essential for testosterone-induced prostate hyperplasia. Proc. Natl. Acad. Sci. USA 2015, 112, 9722–9727. [Google Scholar] [CrossRef] [PubMed]
- Godse, N.R.; Khan, N.; Yochum, Z.A.; Gomez-Casal, R.; Kemp, C.; Shiwarski, D.J.; Seethala, R.S.; Kulich, S.; Seshadri, M.; Burns, T.F.; et al. TMEM16A/ANO1 inhibits apoptosis via downregulation of bim expression. Clin. Cancer Res. 2017, 23, 7324–7332. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lu, M.; Liu, B.; Huang, Y.; Wang, K. Inhibition of Ca(2+)-activated Cl(−) channel ANO1/TMEM16A expression suppresses tumor growth and invasiveness in human prostate carcinoma. Cancer Lett. 2012, 326, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Rottgen, T.S.; Nickerson, A.J.; Minor, E.A.; Stewart, A.B.; Harold, A.D.; Rajendran, V.M. Dextran sulfate sodium (DSS)-induced chronic colitis attenuates Ca(2+)-activated Cl(−) secretion in murine colon by down-regulating TMEM16A. Am. J. Physiol. Cell Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Almaca, J.; Tian, Y.; Aldehni, F.; Ousingsawat, J.; Kongsuphol, P.; Rock, J.R.; Harfe, B.D.; Schreiber, R.; Kunzelmann, K. TMEM16 proteins produce volume-regulated chloride currents that are reduced in mice lacking TMEM16A. J. Biol. Chem. 2009, 284, 28571–28578. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dutta, A.; Kresge, C.; Bugde, A.; Feranchak, A.P. Bile acids stimulate cholangiocyte fluid secretion by activation of membraneTMEM16A Cl(-) channels. Hepatology 2018. [Google Scholar] [CrossRef]
- Sauter, D.R.P.; Novak, I.; Pedersen, S.F.; Larsen, E.H.; Hoffmann, E.K. Ano1 (TMEM16A) in pancreatic ductal adenocarcinoma (PDAC). Pflugers Arch. 2015, 467, 1495–1508. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rottgen, T.S.; Nickerson, A.J.; Rajendran, V.M. Calcium-Activated Cl− Channel: Insights on the Molecular Identity in Epithelial Tissues. Int. J. Mol. Sci. 2018, 19, 1432. https://doi.org/10.3390/ijms19051432
Rottgen TS, Nickerson AJ, Rajendran VM. Calcium-Activated Cl− Channel: Insights on the Molecular Identity in Epithelial Tissues. International Journal of Molecular Sciences. 2018; 19(5):1432. https://doi.org/10.3390/ijms19051432
Chicago/Turabian StyleRottgen, Trey S., Andrew J. Nickerson, and Vazhaikkurichi M. Rajendran. 2018. "Calcium-Activated Cl− Channel: Insights on the Molecular Identity in Epithelial Tissues" International Journal of Molecular Sciences 19, no. 5: 1432. https://doi.org/10.3390/ijms19051432
APA StyleRottgen, T. S., Nickerson, A. J., & Rajendran, V. M. (2018). Calcium-Activated Cl− Channel: Insights on the Molecular Identity in Epithelial Tissues. International Journal of Molecular Sciences, 19(5), 1432. https://doi.org/10.3390/ijms19051432



