The Effect of Human Mesenchymal Stem Cells Derived from Wharton’s Jelly in Spinal Cord Injury Treatment Is Dose-Dependent and Can Be Facilitated by Repeated Application
Abstract
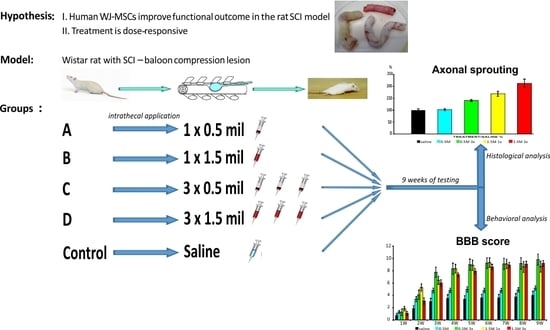
:1. Introduction
2. Results
2.1. Cell Culture
2.2. Behavioral Analysis
2.2.1. BBB Test
2.2.2. Rotarod Test
2.2.3. Beam Walk Test
2.3. Histology and Immunohistochemistry
2.3.1. Gray and White Matter Sparing
2.3.2. Astrogliosis and Distribution of Protoplasmic Astrocytes
2.3.3. Axonal Sprouting
2.4. qRT-PCR
Expression of Intrinsic Genes after Stem Cell Transplantation, after SCI
2.5. Cell Survival
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Animals
4.3. Spinal Cord Injury Model
4.4. Transplantation
4.5. Behavioral Analysis
4.5.1. BBB Test
4.5.2. Rotarod Test
4.5.3. Beam Walk Test
4.6. Histological and Immunohistochemical Analyses
4.6.1. Cresyl Violet-Luxol Staining
4.6.2. GFAP Staining
4.6.3. GAP43 Staining
4.7. qRT-PCR
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fawcett, J.W.; Asher, R.A. The glial scar and central nervous system repair. Brain Res. Bull. 1999, 49, 377–391. [Google Scholar] [CrossRef]
- Zai, L.J.; Wrathall, J.R. Cell proliferation and replacement following contusive spinal cord injury. Glia 2005, 50, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Mbalaviele, G.; Jaiswal, N.; Meng, A.; Cheng, L.; Van Den Bos, C.; Thiede, M. Human mesenchymal stem cells promote human osteoclast differentiation from CD34+ bone marrow hematopoietic progenitors. Endocrinology 1999, 140, 3736–3743. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, D.; Schwarz, E.J.; Prockop, D.J.; Black, I.B. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 2000, 61, 364–370. [Google Scholar] [CrossRef]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002, 105, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Lennon, D.P.; Eaton, V.; Maier, K.; Caplan, A.I.; Miller, S.D.; Miller, R.H. Human bone marrow-derived mesenchymal stem cells induce th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia 2009, 57, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Kim, K.S.; Bae, S.; Son, H.K.; Myung, P.K.; Hong, H.J.; Kim, H. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int. J. Stem Cells 2009, 2, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Torres-Espin, A.; Corona-Quintanilla, D.L.; Fores, J.; Allodi, I.; Gonzalez, F.; Udina, E.; Navarro, X. Neuroprotection and axonal regeneration after lumbar ventral root avulsion by re-implantation and mesenchymal stem cells transplant combined therapy. Neurotherapeutics 2013, 10, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal stem cells: Time to change the name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Amable, P.R.; Teixeira, M.V.; Carias, R.B.; Granjeiro, J.M.; Borojevic, R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and wharton’s jelly. Stem Cell Res. Ther. 2014, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yang, B.; Tian, Y.; Jiao, H.; Zheng, W.; Wang, J.; Guan, F. Immunomodulatory effect of human umbilical cord wharton’s jelly-derived mesenchymal stem cells on lymphocytes. Cell. Immunol. 2011, 272, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Staples, M.; Shinozuka, K.; Pantcheva, P.; Kang, S.D.; Borlongan, C.V. Wharton’s jelly-derived mesenchymal stem cells: Phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int. J. Mol. Sci. 2013, 14, 11692–11712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Chen, J.; Yang, M.; Katakowski, M.; Lu, M.; Chopp, M. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res. 2004, 1030, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Wei, J.; Feng, M.; Lu, S.; Li, G.; Dou, W.; Ma, W.; Ma, S.; An, Y.; Qin, C.; et al. Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res. 2011, 1367, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, R.S.; Bello, S.; Biering-Sorensen, F. Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: Systematic review with meta-analyses of rat models. Neurobiol. Dis. 2014, 62, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Saito, F.; Nakatani, T.; Iwase, M.; Maeda, Y.; Murao, Y.; Suzuki, Y.; Fukushima, M.; Ide, C. Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: A pilot study. Restor. Neurol. Neurosci. 2012, 30, 127–136. [Google Scholar] [PubMed]
- Forostyak, S.; Jendelova, P.; Sykova, E. The role of mesenchymal stromal cells in spinal cord injury, regenerative medicine and possible clinical applications. Biochimie 2013, 95, 2257–2270. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, D.; Novotna, I.; Slovinska, L.; Vanicky, I.; Jergova, S.; Rosocha, J.; Radonak, J. Repetitive intrathecal catheter delivery of bone marrow mesenchymal stromal cells improves functional recovery in a rat model of contusive spinal cord injury. J. Neurotrauma 2011, 28, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.; Mayle, R.E.; Cox, C.A.; Park, D.Y.; Smith, R.L.; Corcoran-Schwartz, I.; Ponnusamy, K.E.; Oshtory, R.; Smuck, M.W.; Mitra, R.; et al. Functional assessment of the acute local and distal transplantation of human neural stem cells after spinal cord injury. Spine J. 2012, 12, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Urdzikova, L.M.; Ruzicka, J.; LaBagnara, M.; Karova, K.; Kubinova, S.; Jirakova, K.; Murali, R.; Sykova, E.; Jhanwar-Uniyal, M.; Jendelova, P. Human mesenchymal stem cells modulate inflammatory cytokines after spinal cord injury in rat. Int. J. Mol. Sci. 2014, 15, 11275–11293. [Google Scholar] [CrossRef] [PubMed]
- Vanicky, I.; Urdzikova, L.; Saganova, K.; Cizkova, D.; Galik, J. A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J. Neurotrauma 2001, 18, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Antonic, A.; Sena, E.S.; Lees, J.S.; Wills, T.E.; Skeers, P.; Batchelor, P.E.; Macleod, M.R.; Howells, D.W. Stem cell transplantation in traumatic spinal cord injury: A systematic review and meta-analysis of animal studies. PLoS Biol. 2013, 11, e1001738. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, J.; Machova-Urdzikova, L.; Gillick, J.; Amemori, T.; Romanyuk, N.; Karova, K.; Zaviskova, K.; Dubisova, J.; Kubinova, S.; Murali, R.; et al. A comparative study of three different types of stem cells for treatment of rat spinal cord injury. Cell Transplant 2017, 26, 585–603. [Google Scholar] [CrossRef] [PubMed]
- Amemori, T.; Ruzicka, J.; Romanyuk, N.; Jhanwar-Uniyal, M.; Sykova, E.; Jendelova, P. Comparison of intraspinal and intrathecal implantation of induced pluripotent stem cell-derived neural precursors for the treatment of spinal cord injury in rats. Stem Cell Res. Ther. 2015, 6, 257. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Samdani, A.F.; Betz, R.R.; Fischer, I.; Neuhuber, B. Grafting of human bone marrow stromal cells into spinal cord injury: A comparison of delivery methods. Spine 2009, 34, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Sareen, D.; Gowing, G.; Sahabian, A.; Staggenborg, K.; Paradis, R.; Avalos, P.; Latter, J.; Ornelas, L.; Garcia, L.; Svendsen, C.N. Human induced pluripotent stem cells are a novel source of neural progenitor cells (iNPCs) that migrate and integrate in the rodent spinal cord. J. Comp. Neurol. 2014, 522, 2707–2728. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Gopinath, C.; Rao, N.M.; Banerjee, P.; Krishnamoorthy, V.; Venkataramana, N.K.; Totey, S. Functional recovery after transplantation of bone marrow-derived human mesenchymal stromal cells in a rat model of spinal cord injury. Cytotherapy 2010, 12, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Iwatsuki, K.; Ohnishi, Y.; Ohkawa, T.; Yoshimine, T. Intranasal delivery of bone marrow stromal cells to spinal cord lesions. J. Neurosurg. Spine 2015, 23, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Ishii, K.; Yamane, J.; Iwanami, A.; Ikegami, T.; Katoh, H.; Iwamoto, Y.; Nakamura, M.; Miyoshi, H.; Okano, H.J.; et al. In vivo imaging of engrafted neural stem cells: Its application in evaluating the optimal timing of transplantation for spinal cord injury. FASEB J. 2005, 19, 1839–1841. [Google Scholar] [CrossRef] [PubMed]
- Parr, A.M.; Kulbatski, I.; Tator, C.H. Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury. J. Neurotrauma 2007, 24, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, F.; Yao, C.; Shu, S.; Feng, J.; Hu, X.; Hai, Q.; Yao, S.; Chen, X. Intrathecal injection of human umbilical cord-derived mesenchymal stem cells ameliorates neuropathic pain in rats. Neurochem. Res. 2016, 41, 3250–3260. [Google Scholar] [CrossRef] [PubMed]
- Himes, B.T.; Neuhuber, B.; Coleman, C.; Kushner, R.; Swanger, S.A.; Kopen, G.C.; Wagner, J.; Shumsky, J.S.; Fischer, I. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabilit. Neural Repair 2006, 20, 278–296. [Google Scholar] [CrossRef] [PubMed]
- Amemori, T.; Jendelova, P.; Ruzickova, K.; Arboleda, D.; Sykova, E. Co-transplantation of olfactory ensheathing glia and mesenchymal stromal cells does not have synergistic effects after spinal cord injury in the rat. Cytotherapy 2010, 12, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.R.; Kim, Y.R.; Kang, H.S.; Yim, S.H.; Park, C.I.; Min, Y.H.; Lee, B.H.; Shin, J.C.; Lim, J.B. Functional recovery after the transplantation of neurally differentiated mesenchymal stem cells derived from bone marrow in a rat model of spinal cord injury. Cell Transplant. 2009, 18, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.L.; Luo, H.S.; Li, J.T.; Xia, Y.Z.; Li, L.; Zhang, L.J.; Meng, H.; Cui, G.Y.; Chen, Z.; Wu, N.; et al. Functional recovery in acute traumatic spinal cord injury after transplantation of human umbilical cord mesenchymal stem cells. Crit. Care Med. 2010, 38, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wen, Y.; Luo, Y.; Lan, X.; Wang, D.; Sun, Z.; Hu, L. Transplantation of bone marrow mesenchymal stem cells into spinal cord injury: A comparison of delivery different times. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2010, 24, 180–184. [Google Scholar] [PubMed]
- Bollini, S.; Gentili, C.; Tasso, R.; Cancedda, R. The regenerative role of the fetal and adult stem cell secretome. J. Clin. Med. 2013, 2, 302–327. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Thej, C.; Venugopal, P.; Priya, N.; Zakaria, Z.; Sundarraj, S.; Majumdar, A.S. Higher propensity of wharton’s jelly derived mesenchymal stromal cells towards neuronal lineage in comparison to those derived from adipose and bone marrow. Cell Biol. Int. 2013, 37, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.Y.; Wang, H.W.; Chang, S.J.; Liao, K.H.; Lee, I.H.; Lin, W.S.; Wu, C.H.; Lin, W.Y.; Cheng, S.M. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS ONE 2013, 8, e72604. [Google Scholar] [CrossRef] [PubMed]
- Drela, K.; Lech, W.; Figiel-Dabrowska, A.; Zychowicz, M.; Mikula, M.; Sarnowska, A.; Domanska-Janik, K. Enhanced neuro-therapeutic potential of wharton’s jelly-derived mesenchymal stem cells in comparison with bone marrow mesenchymal stem cells culture. Cytotherapy 2016, 18, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Shi, C. Recent progress toward understanding the physiological function of bone marrow mesenchymal stem cells. Immunology 2012, 136, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, J.; Ji, X.; Li, R.; Xuan, Y.; Wang, Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int. J. Mol. Med. 2014, 34, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, X.; Qiao, S.; Liu, X.; Liu, C.; Zhu, D.; Su, J.; Wang, Z. Effects of wharton’s jelly cells of the human umbilical cord on acute spinal cord injury in rats, and expression of interleukin-1beta and nerve growth factor in spinal cord tissues. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Suh-Kim, H.; Choi, J.S.; Jeun, S.S.; Kim, E.J.; Kim, S.S.; Yoon, D.H.; Lee, B.H. Human mesenchymal stem cell transplantation promotes functional recovery following acute spinal cord injury in rats. Acta Neurobiol. Exp. 2007, 67, 13–22. [Google Scholar]
- Nakajima, H.; Uchida, K.; Guerrero, A.R.; Watanabe, S.; Sugita, D.; Takeura, N.; Yoshida, A.; Long, G.; Wright, K.T.; Johnson, W.E.; et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J. Neurotrauma 2012, 29, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.S.; Park, I.S.; Kim, K.N.; Yoon, D.H.; Kim, S.H.; Ha, Y. Transplantation of an adipose stem cell cluster in a spinal cord injury. Neuroreport 2012, 23, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Lim, J.Y.; Jeong, C.H.; Kim, S.M.; Jun, J.A.; Jeun, S.S.; Oh, W.I. Human umbilical cord blood-derived mesenchymal stem cell therapy promotes functional recovery of contused rat spinal cord through enhancement of endogenous cell proliferation and oligogenesis. J. Biomed. Biotechnol. 2012, 2012, 362473. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.J.; Hong, S.Q.; Xu, Q.; Wang, H.Y.; Yang, Y.; Wang, Z.F.; Xu, B.N.; Jiang, X.D.; Xu, R.X. Nt-3-secreting human umbilical cord mesenchymal stromal cell transplantation for the treatment of acute spinal cord injury in rats. Brain Res. 2011, 1391, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Matyas, J.J.; Stewart, A.N.; Goldsmith, A.; Nan, Z.; Skeel, R.L.; Rossignol, J.; Dunbar, G.L. Effects of bone-marrow-derived msc transplantation on functional recovery in a rat model of spinal cord injury: Comparisons of transplant locations and cell concentrations. Cell Transplant. 2017, 26, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Gabr, H.; El-Kheir, W.A.; Farghali, H.A.; Ismail, Z.M.; Zickri, M.B.; El Maadawi, Z.M.; Kishk, N.A.; Sabaawy, H.E. Intrathecal transplantation of autologous adherent bone marrow cells induces functional neurological recovery in a canine model of spinal cord injury. Cell Transplant. 2015, 24, 1813–1827. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.H.; Kang, B.J.; Park, S.S.; Kim, Y.; Sung, G.J.; Woo, H.M.; Kim, W.H.; Kweon, O.K. Comparison of mesenchymal stem cells derived from fat, bone marrow, wharton’s jelly, and umbilical cord blood for treating spinal cord injuries in dogs. J. Vet. Med. Sci. 2012, 74, 1617–1630. [Google Scholar] [CrossRef] [PubMed]
- Satti, H.S.; Waheed, A.; Ahmed, P.; Ahmed, K.; Akram, Z.; Aziz, T.; Satti, T.M.; Shahbaz, N.; Khan, M.A.; Malik, S.A. Autologous mesenchymal stromal cell transplantation for spinal cord injury: A phase I pilot study. Cytotherapy 2016, 18, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, M.V.; Larocca, T.F.; de Freitas Souza, B.S.; Villarreal, C.F.; Silva, L.F.; Matos, A.C.; Novaes, M.A.; Bahia, C.M.; de Oliveira Melo Martinez, A.C.; Kaneto, C.M.; et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 2014, 5, 126. [Google Scholar] [CrossRef] [PubMed]
- Koci, Z.; Vyborny, K.; Dubisova, J.; Vackova, I.; Jager, A.; Lunov, O.; Jirakova, K.; Kubinova, S. Extracellular matrix hydrogel derived from human umbilical cord as a scaffold for neural tissue repair and its comparison with extracellular matrix from porcine tissues. Tissue Eng. Part C Methods 2017, 23, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Parr, A.M.; Kulbatski, I.; Zahir, T.; Wang, X.; Yue, C.; Keating, A.; Tator, C.H. Transplanted adult spinal cord-derived neural stem/progenitor cells promote early functional recovery after rat spinal cord injury. Neuroscience 2008, 155, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.R.; Liao, C.H.; Pang, C.Y.; Huang, L.L.; Chen, Y.L.; Shiue, Y.L.; Chen, L.R. Transplantation of porcine embryonic stem cells and their derived neuronal progenitors in a spinal cord injury rat model. Cytotherapy 2013, 15, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Dunham, N.W.; Miya, T.S. A note on a simple apparatus for detecting neurological deficit in rats and mice. J. Am. Pharm. Assoc. 1957, 46, 208–209. [Google Scholar] [CrossRef]
- Metz, G.A.; Whishaw, I.Q. The ladder rung walking task: A scoring system and its practical application. J. Vis. Exp. 2009. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupa, P.; Vackova, I.; Ruzicka, J.; Zaviskova, K.; Dubisova, J.; Koci, Z.; Turnovcova, K.; Urdzikova, L.M.; Kubinova, S.; Rehak, S.; et al. The Effect of Human Mesenchymal Stem Cells Derived from Wharton’s Jelly in Spinal Cord Injury Treatment Is Dose-Dependent and Can Be Facilitated by Repeated Application. Int. J. Mol. Sci. 2018, 19, 1503. https://doi.org/10.3390/ijms19051503
Krupa P, Vackova I, Ruzicka J, Zaviskova K, Dubisova J, Koci Z, Turnovcova K, Urdzikova LM, Kubinova S, Rehak S, et al. The Effect of Human Mesenchymal Stem Cells Derived from Wharton’s Jelly in Spinal Cord Injury Treatment Is Dose-Dependent and Can Be Facilitated by Repeated Application. International Journal of Molecular Sciences. 2018; 19(5):1503. https://doi.org/10.3390/ijms19051503
Chicago/Turabian StyleKrupa, Petr, Irena Vackova, Jiri Ruzicka, Kristyna Zaviskova, Jana Dubisova, Zuzana Koci, Karolina Turnovcova, Lucia Machova Urdzikova, Sarka Kubinova, Svatopluk Rehak, and et al. 2018. "The Effect of Human Mesenchymal Stem Cells Derived from Wharton’s Jelly in Spinal Cord Injury Treatment Is Dose-Dependent and Can Be Facilitated by Repeated Application" International Journal of Molecular Sciences 19, no. 5: 1503. https://doi.org/10.3390/ijms19051503
APA StyleKrupa, P., Vackova, I., Ruzicka, J., Zaviskova, K., Dubisova, J., Koci, Z., Turnovcova, K., Urdzikova, L. M., Kubinova, S., Rehak, S., & Jendelova, P. (2018). The Effect of Human Mesenchymal Stem Cells Derived from Wharton’s Jelly in Spinal Cord Injury Treatment Is Dose-Dependent and Can Be Facilitated by Repeated Application. International Journal of Molecular Sciences, 19(5), 1503. https://doi.org/10.3390/ijms19051503