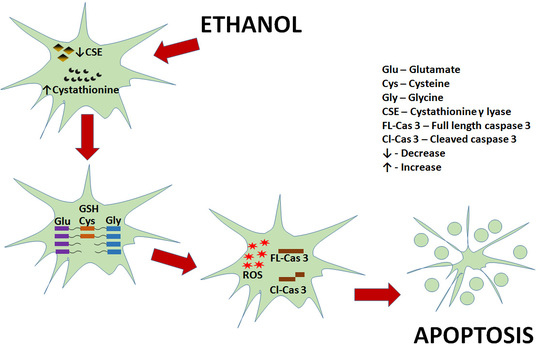
Role for Cystathionine γ Lyase (CSE) in an Ethanol (E)-Induced Lesion in Fetal Brain GSH Homeostasis
Abstract
:1. Introduction
2. Results
2.1. Ethanol Increases Cystathionine Levels in Primary Cerebral Cortical Neurons (PCNs) and Fetal Brains
2.2. Ethanol Decreases Cystathionine-γ Lyase (CSE) Protein in PCNs and Fetal Cortices of In Utero Binge Alcohol Exposed Rats
2.3. Ethanol-Induced Reduction of CSE Protein Is Associated with a Decrease in Its Transcript Levels
2.4. Differentiation of Rat Cortical Neuroblasts to Neurons and Inhibition of CSE Expression by Small Interfering RNA (siRNA)
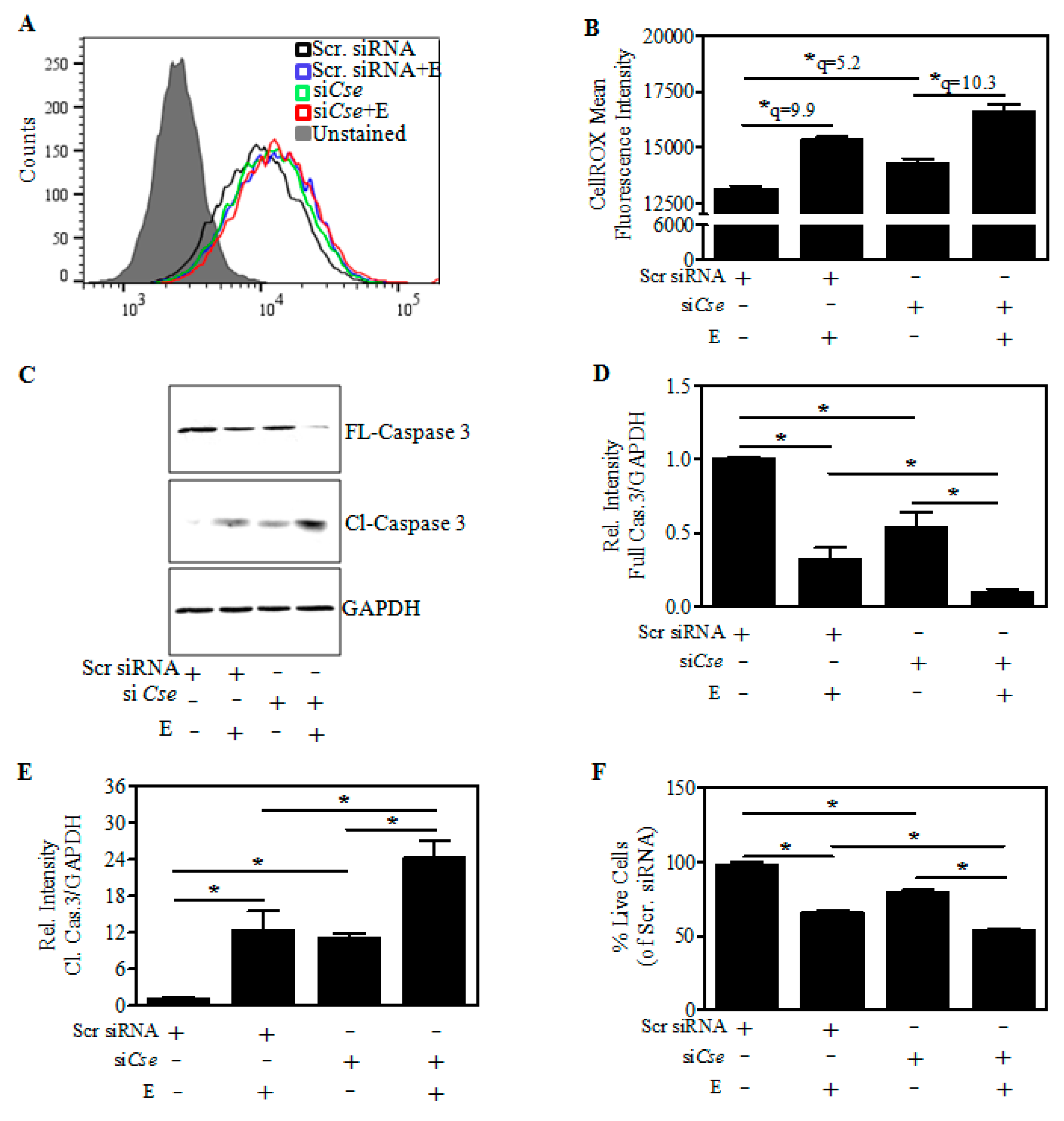
2.5. Cse Knockdown Reduces Neuron GSH and Potentiates Ethanol-Mediated GSH Depletion
2.6. Effect of Cse Silencing on Ethanol-Mediated Reactive Oxygen Species (ROS) induction and Cell Death
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Primary Cortical Neuron (PCN) Cultures and Ethanol Treatment
4.3. Rat Brain Cortical Neuroblasts
4.4. Small Interfering RNA (siRNA) Transfection
4.5. In Vivo Binge Model
4.6. In Vivo Postnatal (PN7) Model
4.7. HPLC Based Determination of Cystathionine
4.8. RNA Extraction and Real-Time qRT-PCR Analysis
4.9. Immunoblotting
4.10. Immunofluorescence
4.11. Reactive Oxygen Species (ROS) Detection With CellROX-Green Reagent Using Flow Cytometry
4.12. MTT Assay
4.13. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| GSH | Reduced Glutathione |
| ROS | Reactive Oxygen Species |
| Cys | Cysteine |
| CSE | Cystathionine γ Lyase |
| CBS | Cystathionine β Synthase |
| E | Ethanol |
| ED | Embryonic Day |
| PCNs | Primary Cortical Neurons |
| PN7 | Post-Natal Day 7 |
| GSH-NEM | Glutathione-N-ethylmaleimide |
| TSP | Transulfuration Pathway |
| DAPI | 4′,6-Diamidino-2-Phenylindole, Dihydrochloride |
References
- Heaton, M.B.; Paiva, M.; Madorsky, I.; Shaw, G. Ethanol effects on neonatal rat cortex: Comparative analyses of neurotrophic factors, apoptosis-related proteins, and oxidative processes during vulnerable and resistant periods. Brain Res. Dev. Brain Res. 2003, 145, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.I.; Devi, B.G.; Perez, A.; Schenker, S. In utero ethanol exposure elicits oxidative stress in the rat fetus. Alcohol. Clin. Exp. Res. 1995, 19, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Maffi, S.K.; Rathinam, M.L.; Cherian, P.P.; Pate, W.; Hamby-Mason, R.; Schenker, S.; Henderson, G.I. Glutathione content as a potential mediator of the vulnerability of cultured fetal cortical neurons to ethanol-induced apoptosis. J. Neurosci. Res. 2008, 86, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, M.; Mahimainathan, L.; Rathinam, M.L.; Riar, A.K.; Henderson, G.I. Overexpression of Nrf2 protects cerebral cortical neurons from ethanol-induced apoptotic death. Mol. Pharmacol. 2011, 80, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Heaton, M.B.; Paiva, M.; Mayer, J.; Miller, R. Ethanol-mediated generation of reactive oxygen species in developing rat cerebellum. Neurosci. Lett. 2002, 334, 83–86. [Google Scholar] [CrossRef]
- Narasimhan, M.; Rathinam, M.; Patel, D.; Henderson, G.; Mahimainathan, L. Astrocytes Prevent Ethanol Induced Apoptosis of Nrf2 Depleted Neurons by Maintaining GSH Homeostasis. Open J. Apoptosis 2012, 1. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Harris, C. Redox control of teratogenesis. Reprod. Toxicol. 2013, 35, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.; Shuster, D.Z.; Roman Gomez, R.; Sant, K.E.; Reed, M.S.; Pohl, J.; Hansen, J.M. Inhibition of glutathione biosynthesis alters compartmental redox status and the thiol proteome in organogenesis-stage rat conceptuses. Free Radic. Biol. Med. 2013, 63, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008, 295, C849–C868. [Google Scholar] [CrossRef] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Hamby-Mason, R.L.; Mason, P.A.; Schenker, S.; Henderson, G.I. Histochemical method for localization of hydrogen peroxide and oxygen radicals in the intact neonatal brain. Methods Find. Exp. Clin. Pharmacol. 1998, 20, 743–748. [Google Scholar] [PubMed]
- Watts, L.T.; Rathinam, M.L.; Schenker, S.; Henderson, G.I. Astrocytes protect neurons from ethanol-induced oxidative stress and apoptotic death. J. Neurosci. Res. 2005, 80, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A. Effects of oxidative stress on embryonic development. Birth Defects Res. C Embryo Today 2007, 81, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Fantel, A.G. Reactive oxygen species in developmental toxicity: Review and hypothesis. Teratology 1996, 53, 196–217. [Google Scholar] [CrossRef]
- Wells, P.G.; Kim, P.M.; Laposa, R.R.; Nicol, C.J.; Parman, T.; Winn, L.M. Oxidative damage in chemical teratogenesis. Mutat. Res. 1997, 396, 65–78. [Google Scholar] [CrossRef]
- Wells, P.G.; McCallum, G.P.; Chen, C.S.; Henderson, J.T.; Lee, C.J.; Perstin, J.; Preston, T.J.; Wiley, M.J.; Wong, A.W. Oxidative stress in developmental origins of disease: Teratogenesis, neurodevelopmental deficits, and cancer. Toxicol. Sci. 2009, 108, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K.; Watabe, M.; Nakaki, T. Regulation of neuronal glutathione synthesis. J. Pharmacol. Sci. 2008, 108, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.S.; Moore, W.R.; Meister, A. On the active site thiol of gamma-glutamylcysteine synthetase: Relationships to catalysis, inhibition, and regulation. Proc. Natl. Acad. Sci. USA 1988, 85, 2464–2468. [Google Scholar] [CrossRef] [PubMed]
- Toroser, D.; Yarian, C.S.; Orr, W.C.; Sohal, R.S. Mechanisms of gamma-glutamylcysteine ligase regulation. Biochim. Biophys. Acta 2006, 1760, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.M.; Jones, D.P. Thiol/disulfide redox states in signaling and sensing. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 173–181. [Google Scholar] [CrossRef] [PubMed]
- McBean, G.J. The transsulfuration pathway: A source of cysteine for glutathione in astrocytes. Amino Acids 2012, 42, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Yang, G.; Wang, R. A critical life-supporting role for cystathionine gamma-lyase in the absence of dietary cysteine supply. Free Radic. Biol. Med. 2011, 50, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Regnier, V.; Billard, J.M.; Gupta, S.; Potier, B.; Woerner, S.; Paly, E.; Ledru, A.; David, S.; Luilier, S.; Bizot, J.C.; et al. Brain phenotype of transgenic mice overexpressing cystathionine beta-synthase. PLoS ONE 2012, 7, e29056. [Google Scholar] [CrossRef] [PubMed]
- Vitvitsky, V.; Thomas, M.; Ghorpade, A.; Gendelman, H.E.; Banerjee, R. A functional transsulfuration pathway in the brain links to glutathione homeostasis. J. Biol. Chem. 2006, 281, 35785–35793. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Mahimainathan, L.; Narasimhan, M.; Rathinam, M.; Henderson, G. Ethanol (E) Impairs Fetal Brain GSH Homeostasis by Inhibiting Excitatory Amino-Acid Carrier 1 (EAAC1)-Mediated Cysteine Transport. Int. J. Mol. Sci. 2017, 18, 2596. [Google Scholar] [CrossRef] [PubMed]
- Kery, V.; Bukovska, G.; Kraus, J.P. Transsulfuration depends on heme in addition to pyridoxal 5'-phosphate. Cystathionine beta-synthase is a heme protein. J. Biol. Chem. 1994, 269, 25283–25288. [Google Scholar] [PubMed]
- Narasimhan, M.; Rathinam, M.; Riar, A.; Patel, D.; Mummidi, S.; Yang, H.S.; Colburn, N.H.; Henderson, G.I.; Mahimainathan, L. Programmed cell death 4 (PDCD4): A novel player in ethanol-mediated suppression of protein translation in primary cortical neurons and developing cerebral cortex. Alcohol. Clin. Exp. Res. 2013, 37, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Sarnat, H.B. Clinical neuropathology practice guide 5-2013: Markers of neuronal maturation. Clin. Neuropathol. 2013, 32, 340–369. [Google Scholar] [CrossRef] [PubMed]
- Riar, A.K.; Narasimhan, M.; Rathinam, M.L.; Henderson, G.I.; Mahimainathan, L. Ethanol induces cytostasis of cortical basal progenitors. J. Biomed. Sci. 2016, 23, 6. [Google Scholar] [CrossRef] [PubMed]
- Mullen, R.J.; Buck, C.R.; Smith, A.M. NeuN, a neuronal specific nuclear protein in vertebrates. Development 1992, 116, 201–211. [Google Scholar] [PubMed]
- Kurki, P.; Vanderlaan, M.; Dolbeare, F.; Gray, J.; Tan, E.M. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp. Cell Res. 1986, 166, 209–219. [Google Scholar] [CrossRef]
- Shanmugam, G.; Narasimhan, M.; Conley, R.L.; Sairam, T.; Kumar, A.; Mason, R.P.; Sankaran, R.; Hoidal, J.R.; Rajasekaran, N.S. Chronic Endurance Exercise Impairs Cardiac Structure and Function in Middle-Aged Mice with Impaired Nrf2 Signaling. Front. Physiol. 2017, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.M.; Lawrence, D.A.; Mondal, T.K.; Seegal, R.F. Reduced glutathione is highly expressed in white matter and neurons in the unperturbed mouse brain—Implications for oxidative stress associated with neurodegeneration. Brain Res. 2009, 1276, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Yang, X. Proteases for cell suicide: Functions and regulation of caspases. Microbiol. Mol. Biol. Rev. 2000, 64, 821–846. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000, 62, 649–671. [Google Scholar] [CrossRef]
- Sun, X.; Shih, A.Y.; Johannssen, H.C.; Erb, H.; Li, P.; Murphy, T.H. Two-photon imaging of glutathione levels in intact brain indicates enhanced redox buffering in developing neurons and cells at the cerebrospinal fluid and blood-brain interface. J. Biol. Chem. 2006, 281, 17420–17431. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Sbodio, J.I.; Xu, R.; Vandiver, M.S.; Cha, J.Y.; Snowman, A.M.; Snyder, S.H. Cystathionine gamma-lyase deficiency mediates neurodegeneration in Huntington's disease. Nature 2014, 509, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhang, W.; Wu, L.; Yang, G.; Li, H.; Wang, R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad. Sci. USA 2012, 109, 2943–2948. [Google Scholar] [CrossRef] [PubMed]
- Diwakar, L.; Ravindranath, V. Inhibition of cystathionine-gamma-lyase leads to loss of glutathione and aggravation of mitochondrial dysfunction mediated by excitatory amino acid in the CNS. Neurochem. Int. 2007, 50, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Denton, T.T. Alternative functions of the brain transsulfuration pathway represent an underappreciated aspect of brain redox biochemistry with significant potential for therapeutic engagement. Free Radic. Biol. Med. 2015, 78, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Kandil, S.; Brennan, L.; McBean, G.J. Glutathione depletion causes a JNK and p38MAPK-mediated increase in expression of cystathionine-gamma-lyase and upregulation of the transsulfuration pathway in C6 glioma cells. Neurochem. Int. 2010, 56, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Espinos, C.; Garcia-Cazorla, A.; Martinez-Rubio, D.; Martinez-Martinez, E.; Vilaseca, M.A.; Perez-Duenas, B.; Kozich, V.; Palau, F.; Artuch, R. Ancient origin of the CTH alelle carrying the c.200C>T (p.T67I) variant in patients with cystathioninuria. Clin. Genet. 2010, 78, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.; Penrose, L.S.; Thomas, D.H. Cystathioniuria. Ann. Hum. Genet. 1959, 23, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Vina, J.; Vento, M.; Garcia-Sala, F.; Puertes, I.R.; Gasco, E.; Sastre, J.; Asensi, M.; Pallardo, F.V. L-cysteine and glutathione metabolism are impaired in premature infants due to cystathionase deficiency. Am. J. Clin. Nutr. 1995, 61, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hegele, R.A. Genomic basis of cystathioninuria (MIM 219500) revealed by multiple mutations in cystathionine gamma-lyase (CTH). Hum. Genet. 2003, 112, 404–408. [Google Scholar] [PubMed]
- Zhu, X.Y.; Liu, S.J.; Liu, Y.J.; Wang, S.; Ni, X. Glucocorticoids suppress cystathionine gamma-lyase expression and H2S production in lipopolysaccharide-treated macrophages. Cell. Mol. Life Sci. 2010, 67, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shen, Z.; Miao, L.; Xin, X.; Lin, S.; Zhu, Y.; Guo, W.; Zhu, Y.Z. miRNA-30 family inhibition protects against cardiac ischemic injury by regulating cystathionine-gamma-lyase expression. Antioxid. Redox Signal. 2015, 22, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, K.K.; Goggin, S.L.; Tyler, C.R.; Allan, A.M. Prenatal alcohol exposure is associated with altered subcellular distribution of glucocorticoid and mineralocorticoid receptors in the adolescent mouse hippocampal formation. Alcohol. Clin. Exp. Res. 2014, 38, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Zhang, Z.; Li, Q.; Yang, R.; Pei, X.; Xu, Y.; Wang, J.; Zhou, S.F.; Li, Y. Ethanol exposure induces differential microRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation. Hum. Reprod. 2009, 24, 562–579. [Google Scholar] [CrossRef] [PubMed]
- Hazelton, G.A.; Hjelle, J.J.; Klaassen, C.D. Effects of cysteine pro-drugs on acetaminophen-induced hepatotoxicity. J. Pharmacol. Exp. Ther. 1986, 237, 341–349. [Google Scholar] [PubMed]
- Maclean, K.N.; Greiner, L.S.; Evans, J.R.; Sood, S.K.; Lhotak, S.; Markham, N.E.; Stabler, S.P.; Allen, R.H.; Austin, R.C.; Balasubramaniam, V.; et al. Cystathionine protects against endoplasmic reticulum stress-induced lipid accumulation, tissue injury, and apoptotic cell death. J. Biol. Chem. 2012, 287, 31994–32005. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J.; Laster, L. Transsulfuration in fetal and postnatal mammalian liver and brain. Cystathionine synthase, its relation to hormonal influences, and cystathionine. Biol. Neonate 1972, 20, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.H.; Stabler, S.P.; Lindenbaum, J. Serum betaine, N,N-dimethylglycine and N-methylglycine levels in patients with cobalamin and folate deficiency and related inborn errors of metabolism. Metabolism 1993, 42, 1448–1460. [Google Scholar] [CrossRef]
- Aziz, N.A.; Onkenhout, W.; Kerstens, H.J.; Roos, R.A. Cystathionine Levels in Patients With Huntington Disease. PLoS Curr. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P.; Lindenbaum, J.; Savage, D.G.; Allen, R.H. Elevation of serum cystathionine levels in patients with cobalamin and folate deficiency. Blood 1993, 81, 3404–3413. [Google Scholar] [PubMed]
- Lee, Z.W.; Low, Y.L.; Huang, S.; Wang, T.; Deng, L.W. The cystathionine gamma-lyase/hydrogen sulfide system maintains cellular glutathione status. Biochem. J. 2014, 460, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Li, H.; Untereiner, A.; Wu, L.; Yang, G.; Austin, R.C.; Dickhout, J.G.; Lhotak, S.; Meng, Q.H.; Wang, R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation 2013, 127, 2523–2534. [Google Scholar] [CrossRef] [PubMed]
- Sriram, K.; Shankar, S.K.; Boyd, M.R.; Ravindranath, V. Thiol oxidation and loss of mitochondrial complex I precede excitatory amino acid-mediated neurodegeneration. J. Neurosci. 1998, 18, 10287–10296. [Google Scholar] [CrossRef] [PubMed]
- Bos, E.M.; Wang, R.; Snijder, P.M.; Boersema, M.; Damman, J.; Fu, M.; Moser, J.; Hillebrands, J.L.; Ploeg, R.J.; Yang, G.; et al. Cystathionine gamma-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J. Am. Soc. Nephrol. 2013, 24, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Ang, A.D.; Rivers-Auty, J.; Hegde, A.; Ishii, I.; Bhatia, M. The effect of CSE gene deletion in caerulein-induced acute pancreatitis in the mouse. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G712–G721. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, R.R.; Fraser, R.; Badiei, A.; Chambers, S.; Cogger, V.C.; Le Couteur, D.G.; Ishii, I.; Bhatia, M. Cystathionine-Gamma-Lyase Gene Deletion Protects Mice against Inflammation and Liver Sieve Injury following Polymicrobial Sepsis. PLoS ONE 2016, 11, e0160521. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, M.L.; Watts, L.T.; Stark, A.A.; Mahimainathan, L.; Stewart, J.; Schenker, S.; Henderson, G.I. Astrocyte control of fetal cortical neuron glutathione homeostasis: Up-regulation by ethanol. J. Neurochem. 2006, 96, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.W. The drunkest drinking driver in Sweden: Blood alcohol concentration 0.545% w/v. J. Stud. Alcohol. 1999, 60, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Page, A.; Paoli, P.P.; Hill, S.J.; Howarth, R.; Wu, R.; Kweon, S.M.; French, J.; White, S.; Tsukamoto, H.; Mann, D.A.; et al. Alcohol directly stimulates epigenetic modifications in hepatic stellate cells. J. Hepatol. 2015, 62, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Sulik, K.K.; Chen, S.Y. Nrf2-mediated transcriptional induction of antioxidant response in mouse embryos exposed to ethanol in vivo: Implications for the prevention of fetal alcohol spectrum disorders. Antioxid. Redox Signal. 2008, 10, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Munoz, A.; Wrighton, C.; Seliger, B.; Bernal, J.; Beug, H. Thyroid hormone receptor/c-erbA: Control of commitment and differentiation in the neuronal/chromaffin progenitor line PC12. J. Cell Biol. 1993, 121, 423–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riar, A.K.; Narasimhan, M.; Rathinam, M.L.; Vedpathak, D.; Mummidi, S.; Henderson, G.I.; Mahimainathan, L. Ethanol-induced transcriptional activation of programmed cell death 4 (Pdcd4) is mediated by GSK-3beta signaling in rat cortical neuroblasts. PLoS ONE 2014, 9, e98080. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.J.; Lawrence, C.R. Intragastric intubation of alcohol during the perinatal period. Methods Mol. Biol. 2008, 447, 101–110. [Google Scholar] [PubMed]
- West, J.R.; Hamre, K.M.; Pierce, D.R. Delay in brain growth induced by alcohol in artificially reared rat pups. Alcohol 1984, 1, 213–222. [Google Scholar] [CrossRef]
- Clancy, B.; Finlay, B.L.; Darlington, R.B.; Anand, K.J. Extrapolating brain development from experimental species to humans. Neurotoxicology 2007, 28, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Dobbing, J.; Sands, J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979, 3, 79–83. [Google Scholar] [CrossRef]
- Alvarez, M.P.; Jimenez, V.; Cano, P.; Rebollar, P.; Cardinali, D.P.; Esquifino, A.I. Circadian rhythms of prolactin secretion in neonatal female rabbits after acute separation from their mothers. Gen. Comp. Endocrinol. 2006, 146, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Chiku, T.; Padovani, D.; Zhu, W.; Singh, S.; Vitvitsky, V.; Banerjee, R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 2009, 284, 11601–11612. [Google Scholar] [CrossRef] [PubMed]






 (green curved arrow) denotes the yielding reaction, ↓ (black down arrow) represents the downstream event(s) and ⊥ indicates inhibition/blockade.
(green curved arrow) denotes the yielding reaction, ↓ (black down arrow) represents the downstream event(s) and ⊥ indicates inhibition/blockade.
 (green curved arrow) denotes the yielding reaction, ↓ (black down arrow) represents the downstream event(s) and ⊥ indicates inhibition/blockade.
(green curved arrow) denotes the yielding reaction, ↓ (black down arrow) represents the downstream event(s) and ⊥ indicates inhibition/blockade.
| S.No. | Antibody | Catalog # | Source |
|---|---|---|---|
| 1 | CSE | 12217-1-AP | Proteintech, Rosemont, IL, USA |
| 2 | CBS | 14782 | Cell Signaling Technology, Beverly, MA, USA |
| 3 | PCNA | 13110 | |
| 4 | Cl-Caspase-3 | 9664 | |
| 5 | Anti-rabbit IgG-HRP | 7074 | |
| 6 | GAPDH | sc25778 | Santa Cruz Biotechnologies, Santa Cruz, CA, USA |
| 7 | NF-200 | N4142 | Sigma-Aldrich, St. Louis, MO, USA |
| 8 | Actin | A2066 | |
| 9 | NeuN | MAB377 | Chemicon, Temecula, CA, USA |
| 10 | GSH-NEM | MAB3194 | Millipore Sigma, Burlington, MA, USA |
| 11 | FL-Caspase 3 | ab4051 | Abcam, Cambridge, MA, USA |
| 12 | Alexa flour 488 rabbit | A11008 | Invitrogen, Carlsbad, CA, USA |
| 13 | Alexa flour 555 rabbit | A21428 | |
| 14 | Alexa flour 555 mouse | A21422 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, D.; Rathinam, M.; Jarvis, C.; Mahimainathan, L.; Henderson, G.; Narasimhan, M. Role for Cystathionine γ Lyase (CSE) in an Ethanol (E)-Induced Lesion in Fetal Brain GSH Homeostasis. Int. J. Mol. Sci. 2018, 19, 1537. https://doi.org/10.3390/ijms19051537
Patel D, Rathinam M, Jarvis C, Mahimainathan L, Henderson G, Narasimhan M. Role for Cystathionine γ Lyase (CSE) in an Ethanol (E)-Induced Lesion in Fetal Brain GSH Homeostasis. International Journal of Molecular Sciences. 2018; 19(5):1537. https://doi.org/10.3390/ijms19051537
Chicago/Turabian StylePatel, Dhyanesh, Marylatha Rathinam, Courtney Jarvis, Lenin Mahimainathan, George Henderson, and Madhusudhanan Narasimhan. 2018. "Role for Cystathionine γ Lyase (CSE) in an Ethanol (E)-Induced Lesion in Fetal Brain GSH Homeostasis" International Journal of Molecular Sciences 19, no. 5: 1537. https://doi.org/10.3390/ijms19051537
APA StylePatel, D., Rathinam, M., Jarvis, C., Mahimainathan, L., Henderson, G., & Narasimhan, M. (2018). Role for Cystathionine γ Lyase (CSE) in an Ethanol (E)-Induced Lesion in Fetal Brain GSH Homeostasis. International Journal of Molecular Sciences, 19(5), 1537. https://doi.org/10.3390/ijms19051537