Detection of Dystrophin Dp71 in Human Skeletal Muscle Using an Automated Capillary Western Assay System
Abstract
:1. Introduction
2. Results
2.1. Detection of Dp71 mRNA in Human Skeletal Muscle
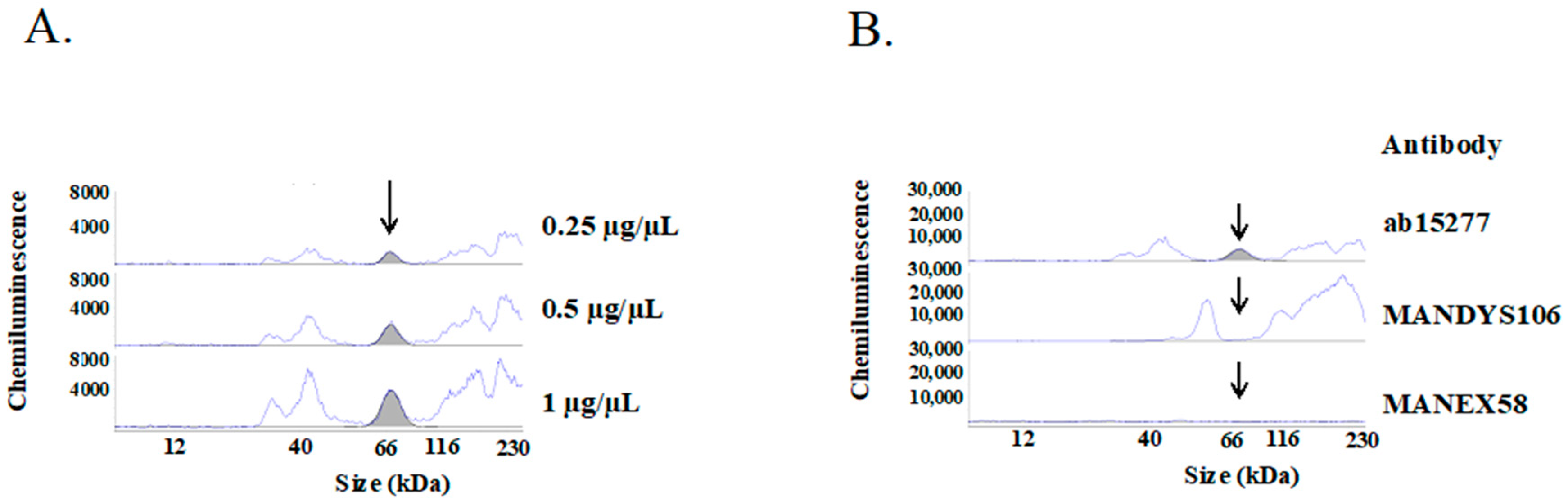
2.2. Detection of Over-Expressed Dp71 Protein by the Simple Western
2.3. Detection and Suppression of Dp71 in Rhabdomyosarcoma Cells
2.4. Detection of Dp71 Protein in Skeletal Muscles from Normal and DMDmdx Mice
2.5. Detection of Dp71 in Human Skeletal Muscle
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Muscle Samples
4.3. mRNA Analysis
4.4. Over-Expression of Dp71
4.5. Protein Sample Preparation
4.6. Simple Western Analysis
4.7. Antisense Oligonucleotide to Induce Exon 75 Skipping
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahn, A.H.; Kunkel, L.M. The structural and functional diversity of dystrophin. Nat. Genet. 1993, 3, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Whitehead, N.P.; Froehner, S.C. Absence of Dystrophin Disrupts Skeletal Muscle Signaling: Roles of Ca2+, Reactive Oxygen Species, and Nitric Oxide in the Development of Muscular Dystrophy. Physiol. Rev. 2016, 96, 253–305. [Google Scholar] [CrossRef] [PubMed]
- Muntoni, F.; Torelli, S.; Ferlini, A. Dystrophin and mutations: One gene, several proteins, multiple phenotypes. Lancet Neurol. 2003, 2, 731–740. [Google Scholar] [CrossRef]
- Massourides, E.; Polentes, J.; Mangeot, P.E.; Mournetas, V.; Nectoux, J.; Deburgrave, N.; Nusbaum, P.; Leturcq, F.; Popplewell, L.; Dickson, G. Dp412e: A novel human embryonic dystrophin isoform induced by BMP4 in early differentiated cells. Skelet Muscle 2015, 5, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar, S.; Barnea, E.; Levy, Z.; Neuman, S.; Yaffe, D.; Nudel, U. A novel product of the Duchenne muscular dystrophy gene which greatly differs from the known isoforms in its structure and tissue distribution. Biochem. J. 1990, 272, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, D.; Lederfein, D.; den Dunnen, J.; Grootscholten, P.; van Ommen, G.; Fuchs, O.; Nudel, U.; Yaffe, D. Characterization and cell type distribution of a novel, major transcript of the Duchenne muscular dystrophy gene. Differentiation 1992, 49, 187–193. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, V.N.; Sigesmund, D.A.; Morris, G.E.; Pillers, D.M.; Weleber, R.G.; Ray, P.N. Identification and characterization of a novel retinal isoform od dystrophin. Am. J. Hum. Genet. 1994, 55, A131. [Google Scholar]
- Lederfein, D.; Levy, Z.; Augier, N.; Mornet, D.; Morris, G.; Fuchs, O.; Yaffe, D.; Nudel, U. A 71-kilodalton protein is a major product of the Duchenne muscular dystrophy gene in brain and other nonmuscle tissues. Proc. Natl. Acad. Sci. USA 1992, 89, 5346–5350. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, S.A.; Duncan, N.M.; Rash, S.M.; Sadeghi, A.; Dewan, A.K.; Pillers, D.A. Redefinition of dystrophin isoform distribution in mouse tissue by RT-PCR implies role in nonmuscle manifestations of duchenne muscular dystrophy. Mol. Genet. Metab. 1998, 65, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Tadayoni, R.; Rendon, A.; Soria-Jasso, L.E.; Cisneros, B. Dystrophin Dp71: The smallest but multifunctional product of the Duchenne muscular dystrophy gene. Mol. Neurobiol. 2012, 45, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Marquez, F.G.; Cisneros, B.; Garcia, F.; Ceja, V.; Velazquez, F.; Depardon, F.; Cervantes, L.; Rendon, A.; Mornet, D.; Rosas-vargas, H.; et al. Differential expression and subcellular distribution of dystrophin Dp71 isoforms during differentiation process. Neuroscience 2003, 118, 957–966. [Google Scholar] [CrossRef]
- Sarig, R.; Mezger-Lallemand, V.; Gitelman, I.; Davis, C.; Fuchs, O.; Yaffe, D.; Nudel, U. Targeted inactivation of Dp71, the major non-muscle product of the DMD gene: Differential activity of the Dp71 promoter during development. Hum. Mol. Genet. 1999, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Silva, M.; Centeno-Cruz, F.; Suarez-Sanchez, R.; Garrido, E.; Cisneros, B. Knockdown of dystrophin Dp71 impairs PC12 cells cycle: Localization in the spindle and cytokinesis structures implies a role for Dp71 in cell division. PLoS ONE 2011, 6, e23504. [Google Scholar] [CrossRef] [PubMed]
- Ceccarini, M.; Rizzo, G.; Rosa, G.; Chelucci, C.; Macioce, P.; Petrucci, T.C. A splice variant of Dp71 lacking the syntrophin binding site is expressed in early stages of human neural development. Brain Res. Dev. Brain Res. 1997, 103, 77–82. [Google Scholar] [CrossRef]
- Gonzalez, E.; Montanez, C.; Ray, P.N.; Howard, P.L.; Garcia-Sierra, F.; Mornet, D.; Cisneros, B. Alternative splicing regulates the nuclear or cytoplasmic localization of dystrophin Dp71. FEBS Lett. 2000, 482, 209–214. [Google Scholar] [CrossRef]
- Nishida, A.; Yasuno, S.; Takeuchi, A.; Awano, H.; Lee, T.; Niba, E.T.; Fujimoto, T.; Itoh, K.; Takeshima, Y.; Nishio, H.; et al. HEK293 cells express dystrophin Dp71 with nucleus-specific localization of Dp71ab. Histochem. Cell Biol. 2016, 146, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Mera, L.; Rodriguez-Munoz, R.; Gonzalez-Ramirez, R.; Garcia-Sierra, F.; Gonzalez, E.; Mornet, D.; Cisneros, B. Characterization of a novel Dp71 dystrophin-associated protein complex (DAPC) present in the nucleus of HeLa cells: Members of the nuclear DAPC associate with the nuclear matrix. Exp. Cell Res. 2006, 312, 3023–3035. [Google Scholar] [CrossRef] [PubMed]
- Moizard, M.P.; Toutain, A.; Fournier, D.; Berret, F.; Raynaud, M.; Billard, C.; Andres, C.; Moraine, C. Severe cognitive impairment in DMD: Obvious clinical indication for Dp71 isoform point mutation screening. Eur. J. Hum. Genet. 2000, 8, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Claudepierre, T.; Mornet, D.; Pannicke, T.; Forster, V.; Dalloz, C.; Bolanos, F.; Sahel, J.; Reichenbach, A.; Rendon, A. Expression of Dp71 in Muller glial cells: A comparison with utrophin- and dystrophin-associated proteins. Investig. Ophthalmol. Vis. Sci. 2000, 41, 294–304. [Google Scholar]
- Matsumoto, M.; Awano, H.; Lee, T.; Takeshima, Y.; Matsuo, M.; Iijima, K. Patients with Duchenne muscular dystrophy are significantly shorter than those with Becker muscular dystrophy, with the higher incidence of short stature in Dp71 mutated subgroup. Neuromuscul. Disord. 2017, 27, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Sánchez, R.; Bulmaro, C. DystrophinDp71, a novel tumor suppressor? J. Xiangya Med. 2016, 1, 40. [Google Scholar] [CrossRef]
- Tan, S.; Tan, J.; Tan, S.; Zhao, S.; Cao, X.; Chen, Z.; Weng, Q.; Zhang, H.; Wang, K.K.; Zhou, J.; et al. Decreased Dp71 expression is associated with gastric adenocarcinoma prognosis. Oncotarget 2016, 7, 53702–53711. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Chen, Z.; Cheng, K.; Wang, W.; Wen, Q.; Zhang, W. Knocking down Dp71 expression in A549 cells reduces its malignancy in vivo and in vitro. Cancer Investig. 2015, 34, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.M. Protein detection by Simple Western analysis. Methods Mol. Biol. 2015, 1312, 465–468. [Google Scholar] [PubMed]
- Chen, J.Q.; Wakefield, L.M.; Goldstein, D.J. Capillary nano-immunoassays: Advancing quantitative proteomics analysis, biomarker assessment, and molecular diagnostics. J. Transl. Med. 2015, 13, 182. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.G.; Barby, T.F.; Manners, E.; Bobrow, M.; Bentley, D.R. Direct detection of dystrophin gene rearrangements by analysis of dystrophin mRNA in peripheral blood lymphocytes. Am. J. Hum. Genet. 1991, 49, 298–310. [Google Scholar] [PubMed]
- Takeshima, Y.; Yagi, M.; Okizuka, Y.; Awano, H.; Zhang, Z.; Yamauchi, Y.; Nishio, H.; Matsuo, M. Mutation spectrum of the dystrophin gene in 442 Duchenne/Becker muscular dystrophy cases from one Japanese referral center. J. Hum. Genet. 2010, 55, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Gitterman, D.P.; Wilson, J.; Randall, A.D. Functional properties and pharmacological inhibition of ASIC channels in the human SJ-RH30 skeletal muscle cell line. J. Physiol. 2005, 562, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Takeshima, Y.; Nishio, H. Contributions of Japanese patients to development of antisense therapy for DMD. Brain Dev. 2016, 38, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, T.; Yagi, M.; Matsuo, M. Optimizing RNA/ENA chimeric antisense oligonucleotides using in vitro splicing. In Exon skipping: Methods and Protocols; Aartsma-Rus, A., Ed.; Human Press: New York, NY, USA, 2012; Volume 867, pp. 131–141. [Google Scholar]
- Lee, T.; Awano, H.; Yagi, M.; Matsumoto, M.; Watanabe, N.; Goda, R.; Koizumi, M.; Takeshima, Y.; Matsuo, M. 2′-O-Methyl RNA/ethylene-bridged nucleic acid chimera antisense oligonucleotides to induce dystrophin Exon 45 skipping. Genes 2017, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Lederfein, D.; Yaffe, D.; Nudel, U. A housekeeping type promoter, located in the 3′ region of the Duchenne muscular dystrophy gene, controls the expression of Dp71, a major product of the gene. Hum. Mol. Genet. 1993, 2, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Niba, E.T.E.; Yamanaka, R.; Rani, A.Q.M.; Awano, H.; Matsumoto, M.; Nishio, H.; Matsuo, M. DMD transcripts in CRL-2061 rhabdomyosarcoma cells show high levels of intron retention by intron-specific PCR amplification. Cancer Cell Int. 2017, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Fourier, A.; Dorey, A.; Perret-Liaudet, A.; Quadrio, I. Detection of CSF 14-3-3 protein in sporadic Creutzfeldt-Jakob disease patients using a new automated capillary western assay. Mol. Neurobiol. 2017, 55, 3537–3545. [Google Scholar] [CrossRef] [PubMed]
- Yahiaoui, A.; Meadows, S.A.; Sorensen, R.A.; Cui, Z.H.; Keegan, K.S.; Brockett, R.; Chen, G.; Queva, C.; Li, L.; Tannheimer, S.L. PI3Kdelta inhibitor idelalisib in combination with BTK inhibitor ONO/GS-4059 in diffuse large B cell lymphoma with acquired resistance to PI3Kdelta and BTK inhibitors. PLoS ONE 2017, 12, e0171221. [Google Scholar] [CrossRef] [PubMed]
- Arahata, K.; Ishiura, S.; Ishiguro, T.; Tsukahara, T.; Suhara, Y.; Eguchi, C.; Ishihara, T.; Nonaka, I.; Ozawa, E.; Sugita, H. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature 1988, 333, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Emery, A.E. Duchenne muscylar dsytrophy or Meryon’s disease. In The Muscular Dystrophies; Emery, A.E., Ed.; Oxford University Press: Oxford, UK, 2001; p. 5571. [Google Scholar]
- Neef, D.W.; Jaeger, A.M.; Thiele, D.J. Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases. Nat. Rev. Drug Discov. 2011, 10, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Tan, S.; Zheng, H.; Liu, M.; Chen, G.; Zhang, H.; Wang, K.; Tan, S.; Zhou, J.; Xiao, X.Z. HSF1 functions as a transcription regulator for Dp71 expression. Cell Stress Chaperones 2015, 20, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Yaoi, T.; Fushiki, S.; Itoh, K. Dp71 is regulated by phosphorylation and ubiquitin-proteasome system in neuronal cells. Biochem. Biophys. Res. Commun. 2017, 492, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M. Duchenne/Becker muscular dystrophy: From molecular diagnosis to gene therapy. Brain Dev. 1996, 18, 167–172. [Google Scholar] [CrossRef]
- Beekman, C.; Janson, A.A.; Baghat, A.; van Deutekom, J.C.; Datson, N.A. Use of capillary Western immunoassay (Wes) for quantification of dystrophin levels in skeletal muscle of healthy controls and individuals with Becker and Duchenne muscular dystrophy. PLoS ONE 2018, 13, e0195850. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Minegishi, M.; Takeuchi, A.; Awano, H.; Niba, E.T.; Matsuo, M. Neuronal SH-SY5Y cells use the C-dystrophin promoter coupled with exon 78 skipping and display multiple patterns of alternative splicing including two intronic insertion events. Hum. Genet. 2015, 134, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Minegishi, M.; Takeuchi, A.; Niba, E.T.; Awano, H.; Lee, T.; Iijima, K.; Takeshima, Y.; Matsuo, M. Tissue and case-specific retention of intron 40 in mature dystrophin mRNA. J. Hum. Genet. 2015, 60, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.K.; Zhang, Z.; Yagi, M.; Nishiyama, A.; Habara, Y.; Takeshima, Y.; Matsuo, M. A novel cryptic exon identified in the 3' region of intron 2 of the human dystrophin gene. J. Hum. Genet. 2005, 50, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Kataoka, N.; Takeshima, Y.; Yagi, M.; Awano, H.; Ota, M.; Itoh, K.; Hagiwara, M.; Matsuo, M. Chemical treatment enhances skipping of a mutated exon in the dystrophin gene. Nat. Commun. 2011, 2, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawaguchi, T.; Niba, E.T.E.; Rani, A.Q.M.; Onishi, Y.; Koizumi, M.; Awano, H.; Matsumoto, M.; Nagai, M.; Yoshida, S.; Sakakibara, S.; et al. Detection of Dystrophin Dp71 in Human Skeletal Muscle Using an Automated Capillary Western Assay System. Int. J. Mol. Sci. 2018, 19, 1546. https://doi.org/10.3390/ijms19061546
Kawaguchi T, Niba ETE, Rani AQM, Onishi Y, Koizumi M, Awano H, Matsumoto M, Nagai M, Yoshida S, Sakakibara S, et al. Detection of Dystrophin Dp71 in Human Skeletal Muscle Using an Automated Capillary Western Assay System. International Journal of Molecular Sciences. 2018; 19(6):1546. https://doi.org/10.3390/ijms19061546
Chicago/Turabian StyleKawaguchi, Tatsuya, Emma Tabe Eko Niba, Abdul Qawee Mahyoob Rani, Yoshiyuki Onishi, Makoto Koizumi, Hiroyuki Awano, Masaaki Matsumoto, Masashi Nagai, Shinobu Yoshida, Sachiko Sakakibara, and et al. 2018. "Detection of Dystrophin Dp71 in Human Skeletal Muscle Using an Automated Capillary Western Assay System" International Journal of Molecular Sciences 19, no. 6: 1546. https://doi.org/10.3390/ijms19061546





