Supplementation with IL-6 and Muscle Cell Culture Conditioned Media Enhances Myogenic Differentiation of Adipose Tissue-Derived Stem Cells through STAT3 Activation
Abstract
:1. Introduction
2. Results
2.1. Combination of IL-6 and C2C12 CM Promoted Myogenic Differentiation
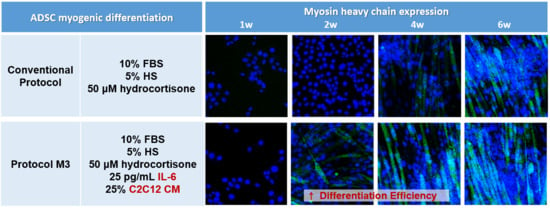
2.2. Combination of IL-6 and C2C12 CM Reduced the Myogenic Differentiation Period
2.3. Addition of STAT3 Activator Promoted Myogenic Differentiation
3. Discussion
4. Materials and Methods
4.1. Differentiation and Tissue Culture Reagents
4.2. Myogenic Differentiation of hADSCs
4.3. Measurement of IL-6 Levels
4.4. Quantitative Real-Time-PCR (qRT-PCR) Analysis
4.5. Immunofluorescence Staining
4.6. Western Blotting
4.7. Statistical Analyses
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| hADSCs | human adipose tissue-derived stem cells |
| FBS | fetal bovine serum |
| HS | horse serum |
| DMEM | Dulbecco’s Modified Eagle Medium |
| IL | interleukin |
| CM | conditioned medium |
| MyoG | myogenine |
| MYH | myosin heavy chain |
| MSC | mesenchymal stem cells |
References
- Diz, J.B.; Leopoldino, A.A.; Moreira, B.S.; Henschke, N.; Dias, R.C.; Pereira, L.S.; Oliveira, V.C. Prevalence of sarcopenia in older brazilians: A systematic review and meta-analysis. Geriatr. Gerontol. Int. 2016, 17, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Cederholm, T.; Muscaritoli, M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: From sarcopenic obesity to cachexia. Clin. Nutr. 2014, 33, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J. Skeletal muscle loss: Cachexia, sarcopenia, and inactivity. Am. J. Clin. Nutr. 2010, 91, 1123s–1127s. [Google Scholar] [CrossRef] [PubMed]
- Charge, S.B.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Chang, H.; Umeda, K.; Niwa, A.; Iwasa, T.; Awaya, T.; Fukada, S.; Yamamoto, H.; Yamanaka, S.; Nakahata, T.; et al. Generation of skeletal muscle stem/progenitor cells from murine induced pluripotent stem cells. FASEB J. 2010, 24, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Perniconi, B.; Coletti, D.; Aulino, P.; Costa, A.; Aprile, P.; Santacroce, L.; Chiaravalloti, E.; Coquelin, L.; Chevallier, N.; Teodori, L.; et al. Muscle acellular scaffold as a biomaterial: Effects on C2C12 cell differentiation and interaction with the murine host environment. Front. Physiol. 2014, 5, 354. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, N.; Asakura, Y.; Asakura, A. Isolation, culture, and transplantation of muscle satellite cells. J. Vis. Exp. JoVE 2014. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Krauss, R.S. Muscle stem cells in developmental and regenerative myogenesis. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Kobolak, J.; Dinnyes, A.; Memic, A.; Khademhosseini, A.; Mobasheri, A. Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods 2016, 99, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Galli, D.; Vitale, M.; Vaccarezza, M. Bone marrow-derived mesenchymal cell differentiation toward myogenic lineages: Facts and perspectives. BioMed Res. Int. 2014, 2014, 762695. [Google Scholar] [CrossRef] [PubMed]
- Stern-Straeter, J.; Bonaterra, G.A.; Juritz, S.; Birk, R.; Goessler, U.R.; Bieback, K.; Bugert, P.; Schultz, J.; Hormann, K.; Kinscherf, R.; et al. Evaluation of the effects of different culture media on the myogenic differentiation potential of adipose tissue- or bone marrow-derived human mesenchymal stem cells. Int. J. Mol. Med. 2014, 33, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Jazedje, T.; Secco, M.; Vieira, N.M.; Zucconi, E.; Gollop, T.R.; Vainzof, M.; Zatz, M. Stem cells from umbilical cord blood do have myogenic potential, with and without differentiation induction in vitro. J. Transl. Med. 2009, 7, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuno, H.; Hyakusoku, H. Mesengenic potential and future clinical perspective of human processed lipoaspirate cells. J. Nippon Med. 2003, 70, 300–306. [Google Scholar] [CrossRef]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef]
- Gang, E.J.; Jeong, J.A.; Hong, S.H.; Hwang, S.H.; Kim, S.W.; Yang, I.H.; Ahn, C.; Han, H.; Kim, H. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells 2004, 22, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kosinski, P.A.; Kemp, D.M. Contribution of human bone marrow stem cells to individual skeletal myotubes followed by myogenic gene activation. Exp. Cell Res. 2005, 307, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Ramkisoensing, A.A.; Pijnappels, D.A.; Askar, S.F.; Passier, R.; Swildens, J.; Goumans, M.J.; Schutte, C.I.; de Vries, A.A.; Scherjon, S.; Mummery, C.L.; et al. Human embryonic and fetal mesenchymal stem cells differentiate toward three different cardiac lineages in contrast to their adult counterparts. PLoS ONE 2011, 6, e24164. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, J.W.; Hakim, C.H.; McIntosh, M.A.; Duan, D. Animal models of duchenne muscular dystrophy: From basic mechanisms to gene therapy. Dis. Models Mech. 2015, 8, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.; Banks, G.B.; Hall, J.K.; Muir, L.A.; Ramos, J.N.; Wicki, J.; Odom, G.L.; Konieczny, P.; Seto, J.; Chamberlain, J.R.; et al. Animal models of muscular dystrophy. Prog. Mol. Biol. Transl. Sci. 2012, 105, 83–111. [Google Scholar] [PubMed]
- Martic-Kehl, M.I.; Schibli, R.; Schubiger, P.A. Can animal data predict human outcome? Problems and pitfalls of translational animal research. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1492–1496. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.S.T.; Davis, J.; Lee, G.; Mack, D.L.; Kim, D.H. Muscular dystrophy in a dish: Engineered human skeletal muscle mimetics for disease modeling and drug discovery. Drug Discov. Today 2016, 21, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Heslop, L.; Morgan, J.E.; Partridge, T.A. Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J. Cell Sci. 2000, 113, 2299–2308. [Google Scholar] [PubMed]
- Yablonka-Reuveni, Z.; Anderson, J.E. Satellite cells from dystrophic (mdx) mice display accelerated differentiation in primary cultures and in isolated myofibers. Dev. Dyn. 2006, 235, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Abujarour, R.; Bennett, M.; Valamehr, B.; Lee, T.T.; Robinson, M.; Robbins, D.; Le, T.; Lai, K.; Flynn, P. Myogenic differentiation of muscular dystrophy-specific induced pluripotent stem cells for use in drug discovery. Stem Cells Transl. Med. 2014, 3, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Darabi, R.; Perlingeiro, R.C. Derivation of skeletal myogenic precursors from human pluripotent stem cells using conditional expression of PAX7. Methods Mol. Biol. 2016, 1357, 423–439. [Google Scholar] [PubMed]
- Sakurai, H. Modeling muscular diseases by patient-derived iPS cells. Folia Pharmacol. Jpn. 2016, 147, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Shoji, E.; Sakurai, H.; Nishino, T.; Nakahata, T.; Heike, T.; Awaya, T.; Fujii, N.; Manabe, Y.; Matsuo, M.; Sehara-Fujisawa, A. Early pathogenesis of duchenne muscular dystrophy modelled in patient-derived human induced pluripotent stem cells. Sci. Rep. 2015, 5, 12831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heffernan, C.; Sumer, H.; Verma, P.J. Generation of clinically relevant “induced pluripotent stem” (iPS) cells. J. Stem Cells 2011, 6, 109–127. [Google Scholar] [PubMed]
- Medvedev, S.P.; Shevchenko, A.I.; Zakian, S.M. Induced pluripotent stem cells: Problems and advantages when applying them in regenerative medicine. Acta Nat. 2010, 2, 18–28. [Google Scholar]
- English, K.; Mahon, B.P.; Wood, K.J. Mesenchymal stromal cells; role in tissue repair, drug discovery and immune modulation. Curr. Drug Deliv. 2014, 11, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Bajek, A.; Gurtowska, N.; Olkowska, J.; Kazmierski, L.; Maj, M.; Drewa, T. Adipose-derived stem cells as a tool in cell-based therapies. Arch. Immunol. Ther. Exp. 2016, 64, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.I.; Kim, K. Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int. 2016, 2016, 6737345. [Google Scholar] [CrossRef] [PubMed]
- Mattar, P.; Bieback, K. Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth-associated tissue mesenchymal stromal cells. Front. Immunol. 2015, 6, 560. [Google Scholar] [CrossRef] [PubMed]
- Forcales, S.V. Potential of adipose-derived stem cells in muscular regenerative therapies. Front. Aging Neurosci. 2015, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.; Razavi, M.R.; Ahmadi, N.; Kazemi, M. Estrogen treatment enhances neurogenic differentiation of human adipose derived stem cells in vitro. Iran. J. Basic Med. Sci. 2015, 18, 799–804. [Google Scholar] [PubMed]
- Saulnier, N.; Piscaglia, A.C.; Puglisi, M.A.; Barba, M.; Arena, V.; Pani, G.; Alfieri, S.; Gasbarrini, A. Molecular mechanisms underlying human adipose tissue-derived stromal cells differentiation into a hepatocyte-like phenotype. Dig. Liver Dis. 2010, 42, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Canoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardi, M.; Munoz-Canoves, P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008, 7, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Baeza-Raja, B.; Munoz-Canoves, P. P38 MAPK-induced nuclear factor-kappab activity is required for skeletal muscle differentiation: Role of interleukin-6. Mol. Biol. Cell 2004, 15, 2013–2026. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, G.; Iachininoto, M.G.; Tritarelli, A.; Straino, S.; Zacheo, A.; Germani, A.; Crea, F.; Capogrossi, M.C. Myogenic potential of adipose-tissue-derived cells. J. Cell Sci. 2006, 119, 2945–2952. [Google Scholar] [CrossRef] [PubMed]
- Barberi, T.; Bradbury, M.; Dincer, Z.; Panagiotakos, G.; Socci, N.D.; Studer, L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat. Med. 2007, 13, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; O’Donoghue, K.; de la Fuente, J.; Roberts, I.A.; Kumar, S.; Morgan, J.E.; Fisk, N.M. Human fetal mesenchymal stem cells as vehicles for gene delivery. Stem Cells 2005, 23, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Hoene, M.; Runge, H.; Haring, H.U.; Schleicher, E.D.; Weigert, C. Interleukin-6 promotes myogenic differentiation of mouse skeletal muscle cells: Role of the stat3 pathway. Am. J. Physiol. Cell Physiol. 2013, 304, C128–C136. [Google Scholar] [CrossRef] [PubMed]
- Aulino, P.; Costa, A.; Chiaravalloti, E.; Perniconi, B.; Adamo, S.; Coletti, D.; Marrelli, M.; Tatullo, M.; Teodori, L. Muscle extracellular matrix scaffold is a multipotent environment. Int. J. Med. Sci. 2015, 12, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, T.; Green, C.; Pedersen, B.K. Myokines in myogenesis and health. Recent Pat. Biotechnol. 2012, 6, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Horsley, V.; Jansen, K.M.; Mills, S.T.; Pavlath, G.K. Il-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 2003, 113, 483–494. [Google Scholar] [CrossRef]
- Carson, J.A.; Baltgalvis, K.A. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc. Sport Sci. Rev. 2010, 38, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.N. Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc. Soc. Exp. Biol. Med. 1994, 205, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, Y.; Jung, N.; Yu, Y.; Ryu, K.H.; Kim, H.S.; Jo, I.; Choi, B.O.; Jung, S.C. Myogenic differentiation potential of human tonsil-derived mesenchymal stem cells and their potential for use to promote skeletal muscle regeneration. Int. J. Mol. Med. 2016, 37, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Pisciotta, A.; Riccio, M.; Carnevale, G.; Lu, A.; De Biasi, S.; Gibellini, L.; La Sala, G.B.; Bruzzesi, G.; Ferrari, A.; Huard, J.; et al. Stem cells isolated from human dental pulp and amniotic fluid improve skeletal muscle histopathology in mdx/SCID mice. Stem Cell Res. Ther. 2015, 6, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajput, B.S.; Chakrabarti, S.K.; Dongare, V.S.; Ramirez, C.M.; Deb, K.D. Human umbilical cord mesenchymal stem cells in the treatment of duchenne muscular dystrophy: Safety and feasibility study in india. J. Stem Cells 2015, 10, 141–156. [Google Scholar] [PubMed]
- Gerli, M.F.; Maffioletti, S.M.; Millet, Q.; Tedesco, F.S. Transplantation of induced pluripotent stem cell-derived mesoangioblast-like myogenic progenitors in mouse models of muscle regeneration. J. Vis. Exp. JoVE 2014, e50532. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.E.; Hwang, M.; Kim, A.Y.; Lee, E.M.; Lee, E.J.; Hwang, S.K.; Kim, S.Y.; Kim, H.K.; Jeong, K.S. Myod overexpressed equine adipose-derived stem cells enhanced myogenic differentiation potential. Cell Transplant. 2016, 25, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Shon, Y.H.; Lim, J.O.; Yoo, J.J.; Shin, H.I.; Park, E.K. Myod mediates skeletal myogenic differentiation of human amniotic fluid stem cells and regeneration of muscle injury. Stem Cell Res. Ther. 2013, 4, 147. [Google Scholar] [CrossRef] [PubMed]
- Higashioka, K.; Koizumi, N.; Sakurai, H.; Sotozono, C.; Sato, T. Myogenic differentiation from MYOGENIN-mutated human iPS cells by CRISPR/Cas9. Stem Cells Int. 2017, 2017, 9210494. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Yamada, M.; Aiso, S. Targeting the JAK2/STAT3 axis in alzheimer’s disease. Expert Opin. Ther. Targets 2009, 13, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.C.; Lee, Y.C.; Su, Y.H.; Wang, Y.Y.; Tsai, C.H.; Hou, Y.A.; Wang, C.H.; Huang, Y.F.; Huang, C.J.; Chou, S.H.; et al. The synthetic beta-nitrostyrene derivative CYT-Rx20 inhibits esophageal tumor growth and metastasis via PI3K/AKT and STAT3 pathways. PLoS ONE 2016, 11, e0166453. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Pujia, A.; Palmieri, F.; Gatto, R.; Falisi, G.; Gargari, M.; Caruso, S.; Apicella, D.; Rastelli, C.; Nardi, G.M.; et al. Innovative approach for the in vitro research on biomedical scaffolds designed and customized with CAD-CAM technology. Int. J. Immunopathol. Pharmacol. 2016, 29, 778–783. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, E.; Kang, H.; Lim, O.-K.; Jun, H.-S. Supplementation with IL-6 and Muscle Cell Culture Conditioned Media Enhances Myogenic Differentiation of Adipose Tissue-Derived Stem Cells through STAT3 Activation. Int. J. Mol. Sci. 2018, 19, 1557. https://doi.org/10.3390/ijms19061557
Seo E, Kang H, Lim O-K, Jun H-S. Supplementation with IL-6 and Muscle Cell Culture Conditioned Media Enhances Myogenic Differentiation of Adipose Tissue-Derived Stem Cells through STAT3 Activation. International Journal of Molecular Sciences. 2018; 19(6):1557. https://doi.org/10.3390/ijms19061557
Chicago/Turabian StyleSeo, Eunhui, Hwansu Kang, Oh-Kyung Lim, and Hee-Sook Jun. 2018. "Supplementation with IL-6 and Muscle Cell Culture Conditioned Media Enhances Myogenic Differentiation of Adipose Tissue-Derived Stem Cells through STAT3 Activation" International Journal of Molecular Sciences 19, no. 6: 1557. https://doi.org/10.3390/ijms19061557
APA StyleSeo, E., Kang, H., Lim, O.-K., & Jun, H.-S. (2018). Supplementation with IL-6 and Muscle Cell Culture Conditioned Media Enhances Myogenic Differentiation of Adipose Tissue-Derived Stem Cells through STAT3 Activation. International Journal of Molecular Sciences, 19(6), 1557. https://doi.org/10.3390/ijms19061557