Novel Safranin-Tinted Candida rugosa Lipase Nanoconjugates Reagent for Visualizing Latent Fingerprints on Stainless Steel Knives Immersed in a Natural Outdoor Pond
Abstract
1. Introduction
2. Results and Discussion
2.1. Proposed Chemical Interactions
2.2. Quality of the Visualized Fingerprints Using the Safranin-Tinted Candida rugosa Lipase Nanoconjugates Reagent
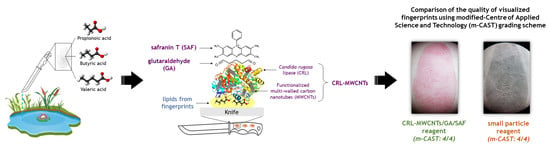
2.3. Comparison on the Quality of Visualized Fingerprints Using the Safranin-Tinted CRL Nanoconjugates Reagent with That of Small Particle Reagent
2.4. Characterization of Visualized Fingerprints Using the Safranin-Tinted CRL Nanoconjugates Reagent
2.4.1. Characterizations of Visualized Fingerprints Using Field Emission Scanning Electron Microscope (FESEM)
2.4.2. Computational Chemistry
2.5. Variations in the Physico-Chemical Parameters of the Natural Outdoor Pond Water
3. Materials and Methods
3.1. Experimental Design
3.2. Preparation of the CRL-Multiwalled Carbon Nanotubes Solution
3.2.1. Purification and Acid-Functionalization of MWCNTs
3.2.2. Immobilization of CRL onto the F-MWCNTs
3.3. Application of the Safranin-Tinted CRL Nanoconjugates Reagent for Visualizing Wet Latent Fingerprints
3.4. Assessment of Fingerprint Quality
3.5. Characterization of the Visualized Fingerprints Using the Safranin-Tinted Nanoconjugates Reagent
3.6. Computational Chemistry for Identifying the Chemical Interactions
3.7. Statistical Analysis
4. Conclusions
5. Patent
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| SPR | Small particle reagent |
| CRL | Candida rugosa lipase |
| MWCNTs | Multi-walled carbon nanotubes |
| GA | Glutaraldehyde |
| SAF | Safranin T dye |
| m-CAST | Modified-Centre for Applied Science and Technology |
| BOD | Biochemical oxygen demand |
| RMP | Royal Malaysia Police |
| RSM | Response surface methodology |
| FESEM | Field emission scanning electron microscopy |
| F-MWCNTs | Functionalized-multi-walled carbon nanotubes |
| UTM | Universiti Teknologi Malaysia |
| IFRG | International Fingerprint Research Group |
References
- Saferstein, R. Fingerprints. In Criminalistics, 10th ed.; Pearson: Upper Saddle River, NJ, USA, 2011; pp. 389–415. ISBN 0131391879. [Google Scholar]
- Arshad, A.; Farrukh, M.A.; Ali, S.; Khaleeq-ur-Rahman, M.; Tahir, M.A. Development of latent fingerprints on various surfaces using ZnO-SiO2 nanopowder. J. Forensic Sci. 2015, 60, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Ramotowski, R.S. Composition of latent print residue. In Advances in Fingerprint Technology, 2nd ed.; Lee, H.C., Gaensslen, R.E., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 63–104. ISBN 1420041347. [Google Scholar]
- Croxton, R.S.; Baron, M.G.; Butler, D.; Kent, T.; Sears, V.G. Variation in amino acid and lipid composition of latent fingerprints. Forensic Sci. Int. 2010, 199, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Girod, A.; Ramotowski, R.; Weyermann, C. Composition of fingerprint residue: A qualitative and quantitative review. Forensic Sci. Int. 2012, 223, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.F. Underwater Forensic Investigation, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9781466507517. [Google Scholar]
- Beresford, A.L.; Hillman, A.R. Electrochromic enhancement of latent fingerprints on stainless steel surfaces. Anal. Chem. 2010, 82, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Sapstead, R.M.; Corden, N.; Hillman, A.R. Latent fingerprint enhancement via conducting electrochromic copolymer films of pyrrole and 3,4-ethylenedioxythiophene on stainless steel. Electrochim. Acta 2015, 162, 119–128. [Google Scholar] [CrossRef]
- Rohatgi, R.; Sodhi, G.S.; Kapoor, A.K. Small particle reagent based on crystal violet dye for developing latent fingerprints on non-porous wet surfaces. Egypt. J. Forensic Sci. 2015, 5, 162–165. [Google Scholar] [CrossRef]
- SPR200 Small Particle Reagent—White. In Sirchie Safety Data Sheet. Available online: http://d1zh4ok0q8k7dm.cloudfront.net/media/resourcecenter/item/s/p/spr200_3.pdf (accessed on 16 March 2017).
- SPR100 Small Particle Reagent—Dark. In Sirchie Safety Data Sheet. Available online: http://d1zh4ok0q8k7dm.cloudfront.net/media/resourcecenter/item/s/p/spr100_1.pdf (accessed on 16 March 2017).
- IARC Working Group. Carbon black, titanium dioxide and talc. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2010; Volume 93, pp. 193–276. ISBN 9789283212935. [Google Scholar]
- Gao, X.; Wang, Y.; Peng, S.; Yue, B.; Fan, C.; Chen, W.; Li, X. Comparative toxicities of bismuth oxybromide and titanium dioxide exposure on human skin keratinocyte cells. Chemosphere 2015, 135, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Peng, R.; Li, X.; Ren, J.; Liu, T.; Wang, X. Effect of titanium dioxide nanoparticles on copper toxicity to Daphnia magna in water: Role of organic matter. Water Res. 2016, 105, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Molybdenum. Available online: https://www.cdc.gov/niosh/pel88/7439-98.html (accessed on 5 January 2017).
- Reid, S.D. Physiological impact of acute molybdenum exposure in juvenile kokanee salmon (Oncorhynchus nerka). Comp. Biochem. Phys. C 2002, 133, 355–367. [Google Scholar] [CrossRef]
- Van Dam, J.W.; Trenfield, M.A.; Harries, S.J.; Streten, C.; Harford, A.J.; Parry, D.; van Dam, R.A. A novel bioassay using the barnacle Amphibalanus amphitrite to evaluate chronic effects of aluminium, gallium and molybdenum in tropical marine receiving environments. Mar. Pollut. Bull. 2016, 112, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, G.; Jiang, X.; Ma, J.; Zhang, H.; Song, H. Active biocatalysts based on Candida rugosa lipase immobilised in vesicular silica. Process Biochem. 2012, 47, 953–959. [Google Scholar] [CrossRef]
- Joseph, B.; Ramteke, P.W.; Thomas, G.; Shrivastava, N. Standard review cold-active microbial lipases: A versatile tool for industrial applications. Biotechnol. Mol. Biol. Rev. 2007, 2, 39–48. [Google Scholar]
- Che Marzuki, N.H.; Mahat, N.A.; Huyop, F.; Aboul-Enein, H.Y.; Abdul Wahab, R. Sustainable production of the emulsifier methyl oleate by Candida rugosa lipase nanoconjugates. Food Bioprod. Process. 2015, 96, 211–220. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Buang, N.A.; Mahat, N.A.; Lok, Y.Y.; Huyop, F.; Aboul-Enein, H.Y.; Wahab, R.A. A facile enzymatic synthesis of geranyl propionate by physically adsorbed Candida rugosa lipase onto multi-walled carbon nanotubes. Enzym. Microb. Technol. 2015, 72, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kanwar, S.S. Organic solvent tolerant lipases and applications. Sci. World J. 2014, 2014, 625258. [Google Scholar] [CrossRef] [PubMed]
- Bandey, H.L.; Gibson, A.P. The Powders Process, Study 2: Evaluation of fingerprint powders on smooth surfaces. In Fingerprint Development and Imaging Newsletter; Publication No. 08/06; Home Office Scientific Development Branch (HOSDB): Sandridge, UK, 2006; pp. 1–16. [Google Scholar]
- Stancu, M.; Ruxanda, G.; Ciuparu, D.; Dinescu, A. Purification of multiwall carbon nanotubes obtained by AC arc discharge method. Optoelectron. Adv. Mater. 2011, 5, 846–850. [Google Scholar]
- Hamilton, R.F., Jr.; Xiang, C.; Li, M.; Ka, I.; Yang, F.; Ma, D.; Porter, D.W.; Wu, N.; Holian, A. Purification and sidewall functionalisation of multiwalled carbon nanotubes and resulting bioactivity in two macrophage models. Inhal. Toxicol. 2013, 25, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Guncheva, M.; Tashev, E.; Zhiryakova, D.; Tosheva, T.; Tzokova, N. Immobilisation of lipase from Candida rugosa on novel phosphorous-containing polyurethanes: Application in wax ester synthesis. Process Biochem. 2011, 46, 923–930. [Google Scholar] [CrossRef]
- Rehm, S.; Trodler, P.; Pleiss, J. Solvent-induced lid opening in lipases: A molecular dynamics study. Protein Sci. 2010, 19, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilisation techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Zhang, P.; Henthorn, D.B. Synthesis of PEGylated single wall carbon nanotubes by a photoinitiated graft from polymerization. AlChE J. 2010, 56, 1610–1615. [Google Scholar] [CrossRef]
- Castelló, A.; Francés, F.; Verdú, F. Solving underwater crimes: Development of latent prints made on submerged objects. Sci. Justice 2013, 53, 28–331. [Google Scholar] [CrossRef] [PubMed]
- Abdul Wahab, R.; Basri, M.; Abdul Rahman, M.B.; Raja Abdul Rahman, R.N.Z.; Salleh, A.B.; Leow, T.C. Engineering catalytic efficiency of thermophilic lipase from Geobacillus zalihae by hydrophobic residue mutation near the catalytic pocket. Adv. Biosci. Biotechnol. 2012, 3, 158–167. [Google Scholar] [CrossRef]
- Abd Manan, F.M.; Attan, N.; Zakaria, Z.; Abdul Keyon, A.E.; Abdul Wahab, R. Enzymatic esterification of eugenol and benzoic acid by a novel chitosan-chitin nanowhiskers supported Rhizomucor miehei lipase: Process optimization and kinetic assessments. Enzym. Microb. Technol. 2018, 108, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Abdul Wahab, R.; Basri, M.; Abdul Rahman, M.B.; Raja Abdul Rahman, R.N.Z.; Salleh, A.B.; Leow, T.C. Combination of oxyanion Gln114 mutation and medium engineering to influence the enantioselectivity of thermophilic lipase from Geobacillus zalihae. Int. J. Mol. Sci. 2012, 13, 11666–11680. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Sun, Y.; Feng, W.; Wang, P.; Yang, P.; Li, J.; Huang, Z.; Chen, Y.-J.; Liu, C.; Sun, L.; et al. Association of urinary metal levels with human semen quality: A cross-sectional study in China. Environ. Int. 2016, 91, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Norgate, T.E.; Jahanshahi, S.; Rankin, W.J. Assessing the environmental impact of metal production processes. J. Clean. Prod. 2007, 15, 838–848. [Google Scholar] [CrossRef]
- Shah, K.M. Synthethic Dyes. In Handbook of Synthetic Dyes and Pigments, 2nd ed.; Multitech Publishing Company: Mumbai, India, 1998; Volume 1, pp. 271–273. ISBN 8187070005. [Google Scholar]
- Derayea, S.M.; Ahmed, H.M.; Abdelmageed, O.H.; Haredy, A.M. New valid spectrofluorimetric method for determination of selected cephalosporins in different pharmaceutical formulations using safranin as fluorophore. Spectrochim. Acta A 2016, 153, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Safranin Stain. In Fisher Scientific Material Safety Data Sheet. Available online: http://www.westliberty.edu/health-and-safety/files/2012/08/Safranin-Stain.pdf (accessed on 4 December 2016).
- Hamzah, H.H. Persistence and Recovery of Latent Fingerprints on Fear Knob Holder of Automobile. Master’s Thesis, Universiti Sains Malaysia, Kubang Kerian, Kelantan, 16 November 2014. [Google Scholar]
- Shah, S.; Solanki, K.; Gupta, M.N. Enhancement of lipase activity in non-aqueous media upon immobilisation on multi-walled carbon nanotubes. Chem. Cent. J. 2007, 1, 30. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M.; Ortiz, C.; Fernández-Lorente, G.; Fuentes, M.; Guisán, J.M.; Fernández-Lorente, R. Lipase-lipase interactions as a new tool to immobilise and modulate the lipase properties. Enzym. Microb. Technol. 2005, 36, 447–454. [Google Scholar] [CrossRef]
- Mariappan, G.; Sundaraganesan, N.; Manoharan, S. The spectroscopic properties of anticancer drug Apigenin investigated by using DFT calculations, FT-IR, FT-Raman and NMR analysis. Spectrochim. Acta A 2012, 95, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 3, 1637–1641. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry, 3rd ed.; Cornell University Press: New York, NY, USA, 1960; ISBN 0801403332. [Google Scholar]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: Oxford, UK, 1997; ISBN 0195095499. [Google Scholar]
- Boyd, C.E. Water Quality: An Introduction, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2015; ISBN 9783319174457. [Google Scholar]
- Monthly Weather Bulletin. Available online: http://www.met.gov.my/web/metmalaysia/publications/bulletinpreview/monthlyweather (accessed on 6 December 2016).
- Cho, H.J. Effects of prevailing winds on turbidity of a shallow estuary. Int. J. Environ. Res. Public Health 2007, 4, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Hulyal, S.B.; Kaliwal, B.B. Seasonal variations in physico-chemical characteristics of Almatti reservoir of Bijapur district, Karnataka state. Int. J. Environ. Prot. 2011, 1, 58–67. [Google Scholar]
- International Fingerprint Research Group (IFRG). Guidelines for the assessment of fingermark detection techniques. J. Forensic Ident. 2014, 64, 174–200. [Google Scholar]








| Visualization Methods | Immersion Periods (Days) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | |
| safranin-tinted CRL nanoconjugates reagent | 4 | 4 | 4 | 4 | 3 * | 3 | 3 |
| Small particle reagent | 4 | 4 | 4 | 4 | 4 | 3 | 3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azman, A.R.; Mahat, N.A.; Abdul Wahab, R.; Abdul Razak, F.I.; Hamzah, H.H. Novel Safranin-Tinted Candida rugosa Lipase Nanoconjugates Reagent for Visualizing Latent Fingerprints on Stainless Steel Knives Immersed in a Natural Outdoor Pond. Int. J. Mol. Sci. 2018, 19, 1576. https://doi.org/10.3390/ijms19061576
Azman AR, Mahat NA, Abdul Wahab R, Abdul Razak FI, Hamzah HH. Novel Safranin-Tinted Candida rugosa Lipase Nanoconjugates Reagent for Visualizing Latent Fingerprints on Stainless Steel Knives Immersed in a Natural Outdoor Pond. International Journal of Molecular Sciences. 2018; 19(6):1576. https://doi.org/10.3390/ijms19061576
Chicago/Turabian StyleAzman, Aida Rasyidah, Naji Arafat Mahat, Roswanira Abdul Wahab, Fazira Ilyana Abdul Razak, and Hafezul Helmi Hamzah. 2018. "Novel Safranin-Tinted Candida rugosa Lipase Nanoconjugates Reagent for Visualizing Latent Fingerprints on Stainless Steel Knives Immersed in a Natural Outdoor Pond" International Journal of Molecular Sciences 19, no. 6: 1576. https://doi.org/10.3390/ijms19061576
APA StyleAzman, A. R., Mahat, N. A., Abdul Wahab, R., Abdul Razak, F. I., & Hamzah, H. H. (2018). Novel Safranin-Tinted Candida rugosa Lipase Nanoconjugates Reagent for Visualizing Latent Fingerprints on Stainless Steel Knives Immersed in a Natural Outdoor Pond. International Journal of Molecular Sciences, 19(6), 1576. https://doi.org/10.3390/ijms19061576