The Pattern of Signatures in Gastric Cancer Prognosis
Abstract
:1. Introduction
2. Screening for Epstein–Barr viruses
3. HER2 Status in Gastric Cancer
4. Gastric Cancers with Microsatellite Instability
5. CDX2 Expression in the Intestinal Type of Gastric Epithelial Dysplasia and Its Correlation with CD10
6. Abnormalities in Cell Cycle Regulators
7. Factors that Regulate Apoptosis Process
8. Multidrug Resistance Related Proteins
9. Mucins with Impact on Cell Membrane Properties
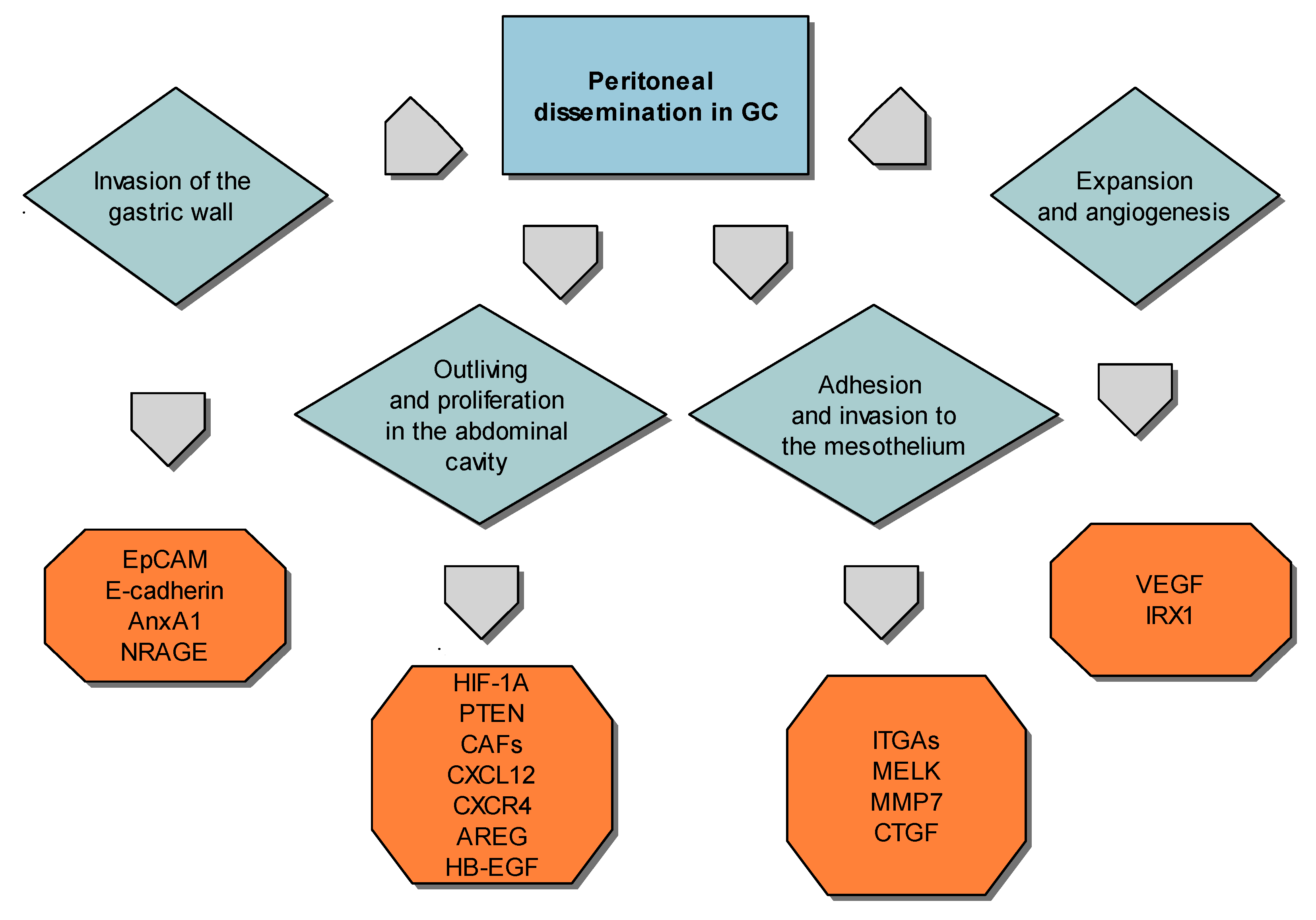
10. Factors that Influence High Progression of Gastric Cancer and Peritoneal Metastasis
10.1. Gastric Cancer Cells Invasiveness
10.2. Expansion of Peritoneal Dissemination
10.3. Adhesion of Gastric Carcinoma Cells to the Peritoneum
10.4. Peritoneal Spreading and Neovascularization
11. Conclusions
Conflicts of Interest
References
- Zhang, X.; Zhang, P. Gastric cancer: Somatic genetics as a guide to therapy. J. Med. Genet. 2017, 54, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Franz, J.L.; Cruz, A.B., Jr. The treatment of gastric cancer with combined surgical resection and chemotherapy. J. Surg. Oncol. 1977, 9, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Coburn, N.; Cosby, R.; Klein, L.; Knight, G.; Malthaner, R.; Mamazza, J.; Mercer, C.D.; Ringash, J. Staging and surgical approaches in gastric cancer: A systematic review. Cancer Treat. Rev. 2018, 63, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.; Preusser, P.; Fink, U.; Gunzer, U.; Meyer, H.J.; Meyer, J.; Siewert, J.R.; Achterrath, W.; Lenaz, L.; Knipp, H. Preoperative chemotherapy in locally advanced and nonresectable gastric cancer: A phase II study with etoposide, doxorubicin, and cisplatin. J. Clin. Oncol. 1989, 7, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Kelsen, D.; Karpeh, M.; Schwartz, G.; Gerdes, H.; Lightdale, C.; Botet, J.; Lauers, G.; Klimstra, D.; Huang, Y.; Saltz, L.; et al. Neoadjuvant therapy of high-risk gastric cancer: A phase II trial of preoperative FAMTX and postoperative intraperitoneal fluorouracil-cisplatin plus intravenous fluorouracil. J. Clin. Oncol. 1996, 14, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.; Marcus, S.G.; Potmesil, M.; Sewak, S.; Yee, H.; Sorich, J.; Hayek, M.; Muggia, F.; Hochster, H. Neoadjuvant chemotherapy with CPT-11 and cisplatin downstages locally advanced gastric cancer. J. Gastrointest. Surg. 2002, 6, 212–223. [Google Scholar] [CrossRef]
- Songun, I.; Keizer, H.J.; Hermans, J.; Klementschitsch, P.; de Vries, J.E.; Wils, J.A.; van der Bijl, J.; van Krieken, J.H.; van de Velde, C.J. Chemotherapy for operable gastric cancer: Results of the Dutch randomised FAMTX trial. The Dutch Gastric Cancer Group (DGCG). Eur. J. Cancer 1999, 35, 558–562. [Google Scholar] [CrossRef]
- Rosenberg, S. Lymphokine-activated killer cells: A new approach to immunotherapy of cancer. J. Natl. Cancer Inst. 1985, 75, 595–603. [Google Scholar] [PubMed]
- Rosenberg, S.A.; Spiess, P.; Lafreniere, R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 1986, 233, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.S.; Hargrove, M.E.; Ting, C.C. In vivo antitumor activity of anti-CD3-induced activated killer cells. Cancer Res. 1989, 49, 4770–4774. [Google Scholar] [PubMed]
- Kyte, J.A.; Gaudernack, G. Immuno-gene therapy of cancer with tumour-mRNA transfected dendritic cells. Cancer Immunol. Immunother. 2006, 55, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Kono, K.; Rongcun, Y.; Charo, J.; Ichihara, F.; Celis, E.; Sette, A.; Appella, E.; Sekikawa, T.; Matsumoto, Y.; Kiessling, R. Identification of HER2/neu-derived peptide epitopes recognized by gastric cancer-specific cytotoxic T lymphocytes. Int. J. Cancer 1998, 78, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Traversari, C.; van der Bruggen, P.; Luescher, I.F.; Lurquin, C.; Chomez, P.; Van Pel, A.; de Plaen, E.; Amar-Costesec, A.; Boon, T. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J. Exp. Med. 1992, 176, 1453–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulley, M.L.; Tang, W. Laboratory assays for Epstein–Barr virus-related disease. J. Mol. Diagn. 2008, 10, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Tsurumi, T.; Fujita, M.; Kudoh, A. Latent and lytic Epstein-Barr virus replication strategies. Rev. Med. Virol. 2005, 15, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Klutts, J.S.; Ford, B.A.; Perez, N.R.; Gronowski, A.M. Evidence-Based Approach for Interpretation of Epstein-Barr virus Serological Patterns. J. Clin. Microbiol. 2009, 47, 3204–3210. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.M.; Chen, Y.Y. EBER in situ hybridization for Epstein-Barr virus. Methods Mol. Biol. 2013, 999, 223–230. [Google Scholar] [PubMed]
- Zur Hausen, A.; van Rees, B.P.; van Beek, J.; Craanen, M.E.; Bloemena, E.; Offerhaus, G.J.; Meijer, C.J.; van den Brule, A.J. Epstein-Barr virus in gastric carcinomas and gastric stump carcinomas: A late event in gastric carcinogenesis. J. Clin. Pathol. 2004, 57, 487–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Lee, H.S.; Bae, S.I.; Lee, Y.M.; Kim, W.H. Silencing and CpG island methylation of GSTP1 is rare in ordinary gastric carcinomas but common in Epstein-Barr virus-associated gastric carcinomas. Anticancer Res. 2005, 25, 4013–4019. [Google Scholar] [PubMed]
- Sudo, M.; Chong, J.M.; Sakuma, K.; Ushiku, T.; Uozaki, H.; Nagai, H.; Funata, N.; Matsumoto, Y.; Fukayama, M. Promoter hypermethylation of E-cadherin and its abnormal expression in Epstein-Barr virus-associated gastric carcinoma. Int. J. Cancer 2004, 109, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liang, Q.; Cheung, K.F.; Kang, W.; Lung, R.W.; Tong, J.H.; To, K.F.; Sung, J.J.; Yu, J. Genome-wide identification of Epstein-Barr virus-driven promoter methylation profiles of human genes in gastric cancer cells. Cancer 2013, 119, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Bass, A.J.; Thorsson, V.; Shmulevich, I.; Reynolds, S.M.; Miller, M.; Bernard, B.; Hinoue, T.; Laird, P.W.; Curtis, C.; Shen, H.; et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, T.; Sudo, C.; Ogawara, H.; Toyoshima, K.; Yamamoto, T. The product of the human c-erbB-2 gene: A 185-kilodalton glycoprotein with tyrosine kinase activity. Science 1986, 232, 1644–1646. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.A.; Jung, E.J.; Lee, H.S.; Lee, H.E.; Jeon, Y.K.; Yang, H.K.; Kim, W.H. Evaluation of HER-2 gene status in gastric carcinoma using immunohistochemistry, fluorescence in situ hybridization, and real-time quantitative polymerase chain reaction. Hum. Pathol. 2007, 38, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Takehana, T.; Kunitomo, K.; Kono, K.; Kitahara, F.; Iizuka, H.; Matsumoto, Y.; Fujino, M.A.; Ooi, A. Status of c-erbB-2 in gastric adenocarcinoma: A comparative study of immunohistochemistry, fluorescence in situ hybridization and enzyme-linked immuno-sorbent assay. Int. J. Cancer 2002, 98, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Tsapralis, D.; Panayiotides, I.; Peros, G.; Liakakos, T.; Karamitopoulou, E. Human epidermal growth factor receptor-2 gene amplification in gastric cancer using tissue microarray technology. World J. Gastroenterol. 2012, 18, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Stoss, O.; Shi, D.; Büttner, R.; van de Vijver, M.; Kim, W.; Ochiai, A.; Rüschoff, J.; Henkel, T. Assessment of a HER2 scoring system for gastric cancer: Results from a validation study. Histopathology 2008, 52, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto-Ouchi, K.; Sekiguchi, F.; Yasuno, H.; Moriya, Y.; Mori, K.; Tanaka, Y. Antitumor activity of trastuzumab in combination with chemotherapy in human gastric cancer xenograft models. Cancer Chemother. Pharmacol. 2007, 59, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.; Hollmén, M.; Junttila, T.T.; Kapanen, A.I.; Tommola, S.; Soini, Y.; Helin, H.; Salo, J.; Joensuu, H.; Sihvo, E. Amplification of HER-2 in gastric carcinoma: Association with Topoisomerase IIα gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann. Oncol. 2005, 16, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk, P.G.; Song, S.U.; Di Fiore, P.P.; King, C.R. Therapy of an animal model of human gastric cancer using a combination of anti-erbB-2 monoclonal antibodies. Cancer Res. 1992, 52, 2771–2776. [Google Scholar] [PubMed]
- Nicholas, G.; Cripps, C.; Au, H.J.; et al. Early results of a trial of trastuzumab, cisplatin and docetaxel (TCD) for the treatment of metastatic gastric cancer overexpressing HER2. Ann. Oncol. 2006, 17, 316. [Google Scholar]
- Yuza, K.; Nagahashi, M.; Watanabe, S.; Takabe, K.; Wakai, T. Hypermutation and microsatellite instability in gastrointestinal cancers. Oncotarget 2017, 8, 112103–112115. [Google Scholar] [CrossRef] [PubMed]
- Hang, X.; Li, D.; Wang, J.; Wang, G. Prognostic significance of microsatellite instability-associated pathways and genes in gastric cancer. Int. J. Mol. Med. 2018, 42, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Shi, Y.; Li, A. Association between hMLH1 Promoter Methylation and Risk of Gastric Cancer: A Meta-Analysis. Front. Physiol. 2018, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Polom, K.; Marrelli, D.; Smyth, E.C.; Voglino, C.; Roviello, G.; Pascale, V.; Varas, J.; Vindigni, C.; Roviello, F. The Role of Microsatellite Instability in Positive Margin Gastric Cancer Patients. Surg. Innov. 2018, 25, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Beck, F.; Chawengsaksophak, K.; Waring, P.; Playford, R.J.; Furness, J.B. Reprogramming of intestinal differentiation and intercalary regeneration in Cdx2 mutant mice. Proc. Natl. Acad. Sci. USA 1999, 96, 7318–7323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freund, J.N.; Domon-Dell, C.; Kedinger, M.; Duluc, I. The Cdx-1 and Cdx-2 homeobox genes in the intestine. Biochem. Cell Biol. 1998, 76, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.Q.; Miyake, S.; Iwai, T.; Yuasa, Y. CDX2, a homeobox transcription factor, upregulates transcription of the p21/WAF1/CIP1 gene. Oncogene 2003, 22, 7942–7949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.Y.; Srivastava, A.; Kim, G.H.; Mino-Kenudson, M.; Deshpande, V.; Zukerberg, L.R.; Song, G.A.; Lauwers, G.Y. CDX2 expression in the intestinal-type gastric epithelial neoplasia: Frequency and significance. Mod. Pathol. 2010, 23, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.B.; Zhou, X.J.; Chen, J.Y.; Zhang, L.H.; Meng, K.; Ma, H.H.; Lu, Z.F. CD10-positive stromal cells in gastric carcinoma: Correlation with invasion and metastasis. Jpn. J. Clin. Oncol. 2005, 35, 245–250. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Bird, R.C. Selective induction of cell cycle regulatory genes cdk1 (p34cdc2), cyclins A/B, and the tumor suppressor gene Rb in transformed cells by okadaic acid. J. Cell. Physiol. 1995, 164, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, M.A.; Fenton, J.E.; Jones, A.S. An overview of the role and inter-relationship of epidermal growth factor receptor, cyclin D and retinoblastoma protein on the carcinogenesis of squamous cell carcinoma of the larynx. Clin. Otolaryngol. Allied Sci. 2001, 26, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Arici, D.S.; Tuncer, E.; Ozer, H.; Simek, G.; Koyuncu, A. Expression of retinoblastoma and cyclin D1 in gastric carcinoma. Neoplasma 2009, 56, 63–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, P.; Zhou, G.Y.; Liu, Y.; Li, J.S.; Zhen, J.H.; Yuan, Y.P. Alteration of cyclin D1 in gastric carcinoma and its clinicopathologic significance. World J. Gastroenterol. 2004, 10, 2936–2939. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Hannon, G.J.; Beach, D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 1993, 366, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Metzger, R.; Salonga, D.; Danenberg, K.; Leichman, L.P.; Fink, U.; Sendler, A.; Kelsen, D.; Schwartz, G.K.; Groshen, S.; et al. High frequency of simultaneous loss of p16 and p16beta gene expression in squamous cell carcinoma of the esophagus but not in adenocarcinoma of the esophagus or stomach. Oncogene 1997, 15, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.S.; Shun, C.T.; Sheu, J.C.; Wang, H.P.; Wang, J.T.; Lee, W.J.; Chen, C.J.; Wang, T.H.; Lin, J.T. Overexpression of mutant p53 and c-erbB-2 proteins and mutations of the p15 and p16 genes in human gastric carcinoma: With respect to histological subtypes and stages. J. Gastroenterol. Hepatol. 1998, 13, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Ficorella, C.; Cannita, K.; Ricevuto, E.; Toniato, E.; Fusco, C.; Sinopoli, N.T.; De Galitiis, F.; Di Rocco, Z.C.; Porzio, G.; Frati, L.; et al. P16 hypermethylation contributes to the characterization of gene inactivation profiles in primary gastric cancer. Oncol. Rep. 2003, 10, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, R.; Martinez-Sanchez, A.; Gebauer, F. miR-181a regulates cap-dependent translation of p27(kip1) mRNA in myeloid cells. Mol. Cell. Biol. 2009, 29, 2841–2851. [Google Scholar] [CrossRef] [PubMed]
- Nitti, D.; Belluco, C.; Mammano, E.; Marchet, A.; Ambrosi, A.; Mencarelli, R.; Segato, P.; Lise, M. Low level of p27(Kip1) protein expression in gastric adenocarcinoma is associated with disease progression and poor outcome. J. Surg. Oncol. 2002, 81, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.Y.; Wang, W.Z.; Li, K.Z.; Guan, W.X.; Yan, W. Effect of p27(KIP1) on cell cycle and apoptosis in gastric cancer cells. World J. Gastroenterol. 2005, 11, 7072–7077. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.H.; Harlow, E.; Meyerson, M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature 1991, 353, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.H.; Zhou, Q.; Hu, S.K.; Xia, Y.Q.; Xu, C.C.; Lin, T.S.; Pan, Y.T.; Wu, J.S.; Jin, R. Differential expression of Notch1 intracellular domain and p21 proteins, and their clinical significance in gastric cancer. Oncol. Lett. 2014, 7, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.R.; Carneiro, A.J.; Martins, I.; de Faria, P.A.; Ferreira, M.A.; de Mello, E.L.; Fogaça, H.S.; Elia, C.C.; de Souza, H.S. Prognostic significance of p53 protein expression in early gastric cancer. Pathol. Oncol. Res. 2011, 17, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Gomyo, Y.; Osaki, M.; Kaibara, N.; Ito, H. Numerical aberration and point mutation of p53 gene in human gastric intestinal metaplasia and well-differentiated adenocarcinoma: Analysis by fluorescence in situ hybridization (FISH) and PCR-SSCP. Int. J. Cancer 1996, 66, 594–599. [Google Scholar] [CrossRef]
- Hongyo, T.; Buzard, G.S.; Palli, D.; Weghorst, C.M.; Amorosi, A.; Galli, M.; Caporaso, N.E.; Fraumeni, J.F., Jr.; Rice, J.M. Mutations of the K-ras and p53 genes in gastric adenocarcinomas from a high-incidence region around Florence, Italy. Cancer Res. 1995, 55, 2665–2672. [Google Scholar] [PubMed]
- Kushima, R.; Müller, W.; Stolte, M.; Borchard, F. Differential p53 protein expression in stomach adenomas of gastric and intestinal phenotypes: Possible sequences of p53 alteration in stomach carcinogenesis. Virchows Arch. 1996, 428, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Ohira, M.; Tanaka, H.; Muguruma, K.; Toyokawa, T.; Kubo, N.; Sakurai, K.; Amano, R.; Kimura, K.; Shibutani, M.; et al. Programmed Death-1 Ligand-1 (PDL1) Expression Is Associated with the Prognosis of Patients with Stage II/III Gastric Cancer. Anticancer Res. 2015, 35, 5369–5376. [Google Scholar] [PubMed]
- Xing, X.; Guo, J.; Wen, X.; Ding, G.; Li, B.; Dong, B.; Feng, Q.; Li, S.; Zhang, J.; Cheng, X.; et al. Analysis of PD1, PDL1, PDL2 expression and T cells infiltration in 1014 gastric cancer patients. Oncoimmunology 2017, 7, e1356144. [Google Scholar] [CrossRef] [PubMed]
- Böger, C.; Behrens, H.M.; Mathiak, M.; Krüger, S.; Kalthoff, H.; Röcken, C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget 2016, 7, 24269–24283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.J.; Park, B.J.; Byun, D.S.; Park, J.I.; Kim, H.J.; Park, J.H.; Chi, S.G. Loss of imprinting and elevated expression of wild-type p73 in human gastric adenocarcinoma. Clin. Cancer Res. 2000, 1767–1771. [Google Scholar]
- Oliner, J.D.; Kinzler, K.W.; Meltzer, P.S.; George, D.L.; Vogelstein, B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature 1992, 358, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Günther, T.; Schneider-Stock, R.; Häckel, C.; Kasper, H.U.; Pross, M.; Hackelsberger, A.; Lippert, H.; Roessner, A. Mdm2 gene amplification in gastric cancer correlation with expression of Mdm2 protein and p53 alterations. Mod. Pathol. 2000, 13, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, N.; Ito, Y.; Yokoyama, K.; Uno, A.; Kinukawa, N.; Nemoto, N.; Moriyama, M. The expression of murine double minute 2 (MDM2) on helicobacter pylori-infected intestinal metaplasia and gastric cancer. J. Clin. Biochem. Nutr. 2009, 44, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, X.Y.; Xu, L.; Liu, W.J.; Zhang, J.; Zhang, J.P. Expression and significance of p53 and mdm2 in atypical intestinal metaplasia and gastric carcinoma. Oncol. Lett. 2011, 2, 707–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Liu, X.; Cai, H.; Wang, Y. Prediction of tumor recurrence after curative resection in gastric carcinoma based on bcl-2 expression. World J. Surg. Oncol. 2014, 12, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugitani, N.; Sivley, R.M.; Perry, K.E.; Capra, J.A.; Chazin, W.J. XPA: A key scaffold for human nucleotide excision repair. DNA Repair 2016, 44, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Z.; Guo, W.; Zhou, R.; Wan, L.; Li, Y.; Wang, N.; Kuang, G.; Wang, S. Polymorphisms of the DNA repair gene XPA and XPC and its correlation with gastric cardiac adenocarcinoma in a high incidence population in North China. J. Clin. Gastroenterol. 2008, 42, 910–915. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, M.; Parlanti, E.; Teson, M.; de Jesus, B.M.; Degan, P.; Calcagnile, A.; Jaruga, P.; Bjørås, M.; Crescenzi, M.; Pedrini, A.M.; et al. New functions of XPC in the protection of human skin cells from oxidative damage. EMBO J. 2006, 25, 4305–4315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dusinská, M.; Dzupinková, Z.; Wsólová, L.; Harrington, V.; Collins, A.R. Possible involvement of XPA in repair of oxidative DNA damage deduced from analysis of damage, repair and genotype in a human population study. Mutagenesis 2006, 21, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandusky, G.E.; Mintze, K.S.; Pratt, S.E.; Dantzig, A.H. Expression of multidrug resistance-associated protein 2 (MRP2) in normal human tissues and carcinomas using tissue microarrays. Histopathology 2002, 41, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Toffoli, G.; Cecchin, E. Pharmacogenetics of stomach cancer. Suppl. Tumori 2003, 2, S19–S22. [Google Scholar] [PubMed]
- Qiao, W.; Wang, T.; Zhang, L.; Tang, Q.; Wang, D.; Sun, H. Association between single genetic polymorphisms of MDR1 gene and gastric cancer susceptibility in Chinese. Med. Oncol. 2013, 30, 643. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Y.; Lv, Y.P.; Yan, D.F.; Gao, F.L. Knockdown of MDR1 increases the sensitivity to adriamycin in drug resistant gastric cancer cells. Asian Pac. J. Cancer Prev. 2013, 14, 6757–6760. [Google Scholar] [CrossRef] [PubMed]
- Hayes, P.C.; Bouchier, I.A.; Beckett, G.J. Glutathione S-transferase in humans in health and disease. Gut 1991, 32, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Du, Y.; Cheng, X.; Yu, Q.; Huang, L.; Dong, R. Expression of multidrug resistance-associated proteins and their relation to postoperative individualized chemotherapy in gastric cancer. World J. Surg. Oncol. 2014, 12, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekker, J.; Rossen, J.W.; Büller, H.A.; Einerhand, A.W. The MUC family: An obituary. Trends Biochem. Sci. 2002, 27, 126–131. [Google Scholar] [CrossRef]
- Marin, F.; Luquet, G.; Marie, B.; Medakovic, D. Molluscan shell proteins: Primary structure, origin, and evolution. Curr. Top. Dev. Biol. 2008, 80, 209–276. [Google Scholar] [PubMed]
- Lee, H.S.; Lee, H.K.; Kim, H.S.; Yang, H.K.; Kim, Y.I.; Kim, W.H. MUC1, MUC2, MUC5AC, and MUC6 expressions in gastric carcinomas: Their roles as prognostic indicators. Cancer 2001, 92, 1427–1434. [Google Scholar] [CrossRef]
- Wang, X.T.; Kong, F.B.; Mai, W.; Li, L.; Pang, L.M. MUC1 Immunohistochemical Expression as a Prognostic Factor in Gastric Cancer: Meta-Analysis. Dis. Mark. 2016, 2016, 9421571. [Google Scholar] [CrossRef] [PubMed]
- Park, K.K.; Yang, S.I.; Seo, K.W.; Yoon, K.Y.; Lee, S.H.; Jang, H.K.; Shin, Y.M. Correlations of Human Epithelial Growth Factor Receptor 2 Overexpression with MUC2, MUC5AC, MUC6, p53, and Clinicopathological Characteristics in Gastric Cancer Patients with Curative Resection. Gastroenterol. Res. Pract. 2015, 2015, 946359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.T.; He, K.C.; Pan, F.; Li, Y.; Wu, J. Prognostic value of Muc5AC in gastric cancer: A meta-analysis. World J. Gastroenterol. 2015, 21, 10453–10460. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Shin, N.; Kim, G.H.; Song, G.A.; Jeon, T.Y.; Kim, D.H.; Lauwers, G.Y.; Park, D.Y. Mucin expression in gastric cancer: Reappraisal of its clinicopathologic and prognostic significance. Arch. Pathol. Lab. Med. 2013, 137, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Shan, Y.S.; Hu, H.M.; Price, T.J.; Sirohi, B.; Yeh, K.H.; Yang, Y.H.; Sano, T.; Yang, H.K.; Zhang, X.; et al. Management of gastric cancer in Asia: Resource-stratified guidelines. Lancet Oncol. 2013, 14, e535–e547. [Google Scholar] [CrossRef]
- Trzpis, M.; McLaughlin, P.M.; de Leij, L.M.; Harmsen, M.C. Epithelial cell adhesion molecule: More than a carcinoma marker and adhesion molecule. Am. J. Pathol. 2007, 171, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Imano, M.; Itoh, T.; Satou, T.; Yasuda, A.; Nishiki, K.; Kato, H.; Shiraishi, O.; Peng, Y.F.; Shinkai, M.; Tsubaki, M.; et al. High expression of epithelial cellular adhesion molecule in peritoneal metastasis of gastric cancer. Target. Oncol. 2013, 8, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Van Roy, F.; Berx, G. The cell-cell adhesion molecule E-cadherin. Cell. Mol. Life Sci. 2008, 65, 3756–3788. [Google Scholar] [CrossRef] [PubMed]
- Till, J.E.; Yoon, S.S.; Ryeom, S. E-cadherin and K-ras: Implications of a newly developed model of gastric cancer. Oncoscience 2017, 4, 162–163. [Google Scholar] [PubMed]
- Lee, H.S.; Choi, S.I.; Lee, H.K.; Kim, H.S.; Yang, H.K.; Kang, G.H.; Kim, Y.I.; Lee, B.L.; Kim, W.H. Distinct clinical features and outcomes of gastric cancers with microsatellite instability. Mod. Pathol. 2002, 15, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Perri, F.; Cotugno, R.; Piepoli, A.; Merla, A.; Quitadamo, M.; Gentile, A.; Pilotto, A.; Annese, V.; Andriulli, A. Aberrant DNA methylation in non-neoplastic gastric mucosa of H. Pylori infected patients and effect of eradication. Am. J. Gastroenterol. 2007, 102, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.O.; Peng, J.Z.; Lam, S.K.; Lai, K.C.; Yuen, M.F.; Cheung, H.K.; Kwong, Y.L.; Rashid, A.; Chan, C.K.; Wong, B.C. Eradication of Helicobacter pylori infection reverses E-cadherin promoter hypermethylation. Gut 2006, 55, 463–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilford, P.; Hopkins, J.; Harraway, J.; McLeod, M.; McLeod, N.; Harawira, P.; Taite, H.; Scoular, R.; Miller, A.; Reeve, A.E. E-cadherin germline mutations in familial gastric cancer. Nature 1998, 392, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Van der Post, R.S.; Vogelaar, I.P.; Carneiro, F.; Guilford, P.; Huntsman, D.; Hoogerbrugge, N.; Caldas, C.; Schreiber, K.E.; Hardwick, R.H.; Ausems, M.G.; et al. Hereditary diffuse gastric cancer: Updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J. Med. Genet. 2015, 52, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Perretti, M.; D’Acquisto, F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 2009, 9, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.Y.; Wu, M.S.; Lin, J.T.; Lin, M.T.; Shun, C.T.; Huang, H.Y.; Hua, K.T.; Kuo, M.L. Annexin A1 is associated with gastric cancer survival and promotes gastric cancer cell invasiveness through the formyl peptide receptor/extracellular signal-regulated kinase/integrin beta-1-binding protein 1 pathway. Cancer 2012, 118, 5757–5767. [Google Scholar] [PubMed] [Green Version]
- Zhang, G.; Zhou, H.; Xue, X. Complex roles of NRAGE on tumor. Tumour Biol. 2016, 37, 11535–11540. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Shimizu, D.; Fujii, T.; Tanaka, H.; Tanaka, Y.; Ezaka, K.; Shibata, M.; Takami, H.; Hashimoto, R.; Sueoka, S.; et al. Neurotrophin Receptor-Interacting Melanoma Antigen-Encoding Gene Homolog is Associated with Malignant Phenotype of Gastric Cancer. Ann. Surg. Oncol. 2016, 23 (Suppl. S4), 532–539. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.D.; Black, D.; Sugarbaker, P.H.; Zhu, J.; Yonemura, Y.; Petrou, G.; Morris, D.L. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann. Surg. Oncol. 2007, 14, 2702–2713. [Google Scholar] [CrossRef] [PubMed]
- Mebius, R.E. Lymphoid organs for peritoneal cavity immune response: Milky spots. Immunity 2009, 30, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.F.; Wang, Z.N.; Zhao, T.T.; Xu, Y.Y.; Gao, J.; Miao, F.; Xu, H.M. Peritoneal milky spots serve as a hypoxic niche and favor gastric cancer stem/progenitor cell peritoneal dissemination through hypoxia-inducible factor 1α. Stem Cells 2014, 32, 3062–3074. [Google Scholar] [CrossRef] [PubMed]
- Chu, E.C.; Tarnawski, A.S. PTEN regulatory functions in tumor suppression and cell biology. Med. Sci. Monit. 2004, 10, RA235–RA241. [Google Scholar] [PubMed]
- Zhang, L.L.; Liu, J.; Lei, S.; Zhang, J.; Zhou, W.; Yu, H.G. PTEN inhibits the invasion and metastasis of gastric cancer via downregulation of FAK expression. Cell Signal. 2014, 26, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Guo, X.; Zhang, J.; Wu, D.; Hu, X.; Li, J.; Lan, Q.; Liu, Y.; Dong, W. PTEN Gene Induces Cell Invasion and Migration via Regulating AKT/GSK-3β/β-Catenin Signaling Pathway in Human Gastric Cancer. Dig. Dis. Sci. 2017, 62, 3415–3425. [Google Scholar] [CrossRef] [PubMed]
- Izumi, D.; Ishimoto, T.; Miyake, K.; Sugihara, H.; Eto, K.; Sawayama, H.; Yasuda, T.; Kiyozumi, Y.; Kaida, T.; Kurashige, J.; et al. CXCL12/CXCR4 activation by cancer-associated fibroblasts promotes integrin β1 clustering and invasiveness in gastric cancer. Int. J. Cancer 2016, 138, 1207–1219. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, K.; Koizumi, K.; Kawashima, A.; Saitoh, Y.; Arita, Y.; Shinohara, K.; Minami, T.; Nakayama, T.; Sakurai, H.; Takahashi, Y.; et al. Role of the CXCL12/CXCR4 axis in peritoneal carcinomatosis of gastric cancer. Cancer Res. 2006, 66, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, K.; Yamada, T.; Kawashima, A.; Wang, W.; Li, Q.; Donev, I.S.; Tacheuchi, S.; Mouri, H.; Yamashita, K.; Ohtsubo, K.; et al. The EGFR ligands amphiregulin and heparin-binding egf-like growth factor promote peritoneal carcinomatosis in CXCR4-expressing gastric cancer. Clin. Cancer Res. 2011, 17, 3619–3630. [Google Scholar] [CrossRef] [PubMed]
- Takatsuki, H.; Komatsu, S.; Sano, R.; Takada, Y.; Tsuji, T. Adhesion of gastric carcinoma cells to peritoneum mediated by alpha3beta1 integrin (VLA-3). Cancer Res. 2004, 64, 6065–6070. [Google Scholar] [CrossRef] [PubMed]
- Nakano, I.; Paucar, A.A.; Bajpai, R.; Dougherty, J.D.; Zewail, A.; Kelly, T.K.; Kim, K.J.; Ou, J.; Groszer, M.; Imura, T.; et al. Maternal embryonic leucine zipper kinase (MELK) regulates multipotent neural progenitor proliferation. J. Cell Biol. 2005, 170, 413–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, T.; Qu, Y.; Li, J.; Li, H.; Su, L.; Zhou, Q.; Yan, M.; Li, C.; Zhu, Z.; Liu, B. Maternal embryonic leucine zipper kinase enhances gastric cancer progression via the FAK/Paxillin pathway. Mol. Cancer 2014, 13, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitoh, T.; Yanai, H.; Saitoh, Y.; Nakamura, Y.; Matsubara, Y.; Kitoh, H.; Yoshida, T.; Okita, K. Increased expression of matrix metalloproteinase-7 in invasive early gastric cancer. J. Gastroenterol. 2004, 39, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, Y.; Endou, Y.; Fujita, H.; Fushida, S.; Bandou, E.; Taniguchi, K.; Miwa, K.; Sugiyama, K.; Sasaki, T. Role of MMP-7 in the formation of peritoneal dissemination in gastric cancer. Gastric Cancer 2000, 3, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.G.; Lv, L.; Liu, F.R.; Wang, Z.N.; Liu, F.N.; Li, Y.S.; Wang, C.Y.; Zhang, H.Y.; Sun, Z.; Xu, H.M. Downregulation of connective tissue growth factor inhibits the growth and invasion of gastric cancer cells and attenuates peritoneal dissemination. Mol. Cancer 2011, 10, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.N.; Chang, C.C.; Lai, H.S.; Jeng, Y.M.; Chen, C.I.; Chang, K.J.; Lee, P.H.; Lee, H. Connective tissue growth factor inhibits gastric cancer peritoneal metastasis by blocking integrin α3β1-dependent adhesion. Gastric Cancer 2015, 18, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liu, W.; Guo, X.; Zhang, R.; Zhi, Q.; Ji, J.; Zhang, J.; Chen, X.; Li, J.; Zhang, J.; et al. IRX1 influences peritoneal spreading and metastasis via inhibiting BDKRB2-dependent neovascularization on gastric cancer. Oncogene 2011, 30, 4498–4508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, D.W.; Cachianes, G.; Kuang, W.J.; Goeddel, D.V.; Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Kowanetz, M.; Ferrara, N. Vascular endothelial growth factor signaling pathways: Therapeutic perspective. Clin. Cancer Res. 2006, 12, 5018–5022. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Smyth, E.C.; Chau, I. Ramucirumab: Successfully targeting angiogenesis in gastric cancer. Clin. Cancer Res. 2014, 20, 5875–5881. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Polom, K.; Roviello, F.; Marrelli, D.; Multari, A.G.; Paganini, G.; Pacifico, C.; Generali, D. Targeting VEGFR-2 in Metastatic Gastric Cancer: Results from a Literature-Based Meta-Analysis. Cancer Investig. 2017, 35, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Yamac, D.; Ayyildiz, T.; Coşkun, U.; Akyürek, N.; Dursun, A.; Seckin, S.; Koybasioglu, F. Cyclooxygenase-2 expression and its association with angiogenesis, Helicobacter pylori, and clinicopathologic characteristics of gastric carcinoma. Pathol. Res. Pract. 2008, 204, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.J.; Zhao, S.; Zhao, E.H.; Zheng, X.; Gou, W.F.; Takano, Y.; Zheng, H.C. Clinicopathological and prognostic significance of Ki-67, caspase-3 and p53 expression in gastric carcinomas. Oncol. Lett. 2013, 6, 1277–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| MUC Type | Function | Expression Patterns in Gastric Cancer | Authors |
|---|---|---|---|
| MUC1 | Protective role by binding to pathogens, functions in a cell signalling capacity, presented in the nucleus regulation the activity of transcription factor complexes, which take part in tumor-induced changes of host immunity system | MUC1 positive cases highly overexpressed in intestinal-type carcinomas, increased rate of vascular invasion and lymph node metastasis, lower 5-year survival rate | Wang et al., 2016 [81] |
| MUC2 | The major intestinal mucin, expressed by goblet cells of the small intestine and colon, important role in organizing the intestinal mucus layers at the epithelial surface; forming trimers that crosslink with TFF3 and Fcγbp, allow to highly viscous extracellular layer | Strongly correlated with the intestinal histological type, the correlation between MUC2 and HER2 expression is possible to demonstrate the connection between the intestinal differentiation of cancer cells and HER2 expression | Park et al., 2015 [82] |
| MUC5AC | Glycoprotein of gastric and respiratory tract epithelium guards the mucosa from infection and chemical damage, thanks to binding to inhaled microorganisms and particles, which are later removed by the mucociliary system | Decreased Muc5AC expression was importantly correlated with poor overall survival, additionally, decreased Muc5AC expression was also meaningfully reported to the tumour invasion depth and lymph node metastasis | Zhang et al., 2015 [83] |
| MUC6 | Might deliver a mechanism for modulation of the composition of the protective mucus layer connected to acid secretion or the presence of bacteria and noxious agents in the lumen, involvement in the cytoprotection of epithelial surfaces | MUC6 is a marker of gastric foveolar cells and antral/cardiac mucous glandular cells, reflect to gastric phenotypes | Kim et al., 2013 [84] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machlowska, J.; Maciejewski, R.; Sitarz, R. The Pattern of Signatures in Gastric Cancer Prognosis. Int. J. Mol. Sci. 2018, 19, 1658. https://doi.org/10.3390/ijms19061658
Machlowska J, Maciejewski R, Sitarz R. The Pattern of Signatures in Gastric Cancer Prognosis. International Journal of Molecular Sciences. 2018; 19(6):1658. https://doi.org/10.3390/ijms19061658
Chicago/Turabian StyleMachlowska, Julita, Ryszard Maciejewski, and Robert Sitarz. 2018. "The Pattern of Signatures in Gastric Cancer Prognosis" International Journal of Molecular Sciences 19, no. 6: 1658. https://doi.org/10.3390/ijms19061658
APA StyleMachlowska, J., Maciejewski, R., & Sitarz, R. (2018). The Pattern of Signatures in Gastric Cancer Prognosis. International Journal of Molecular Sciences, 19(6), 1658. https://doi.org/10.3390/ijms19061658





