DNA Nanotechnology for Cancer Diagnosis and Therapy
Abstract
:1. Introduction
2. DNA Nanotechnologies for the Establishment of Theranostic Nanoplatforms
2.1. DNA Origami-Based Theranostic Nanoplatform
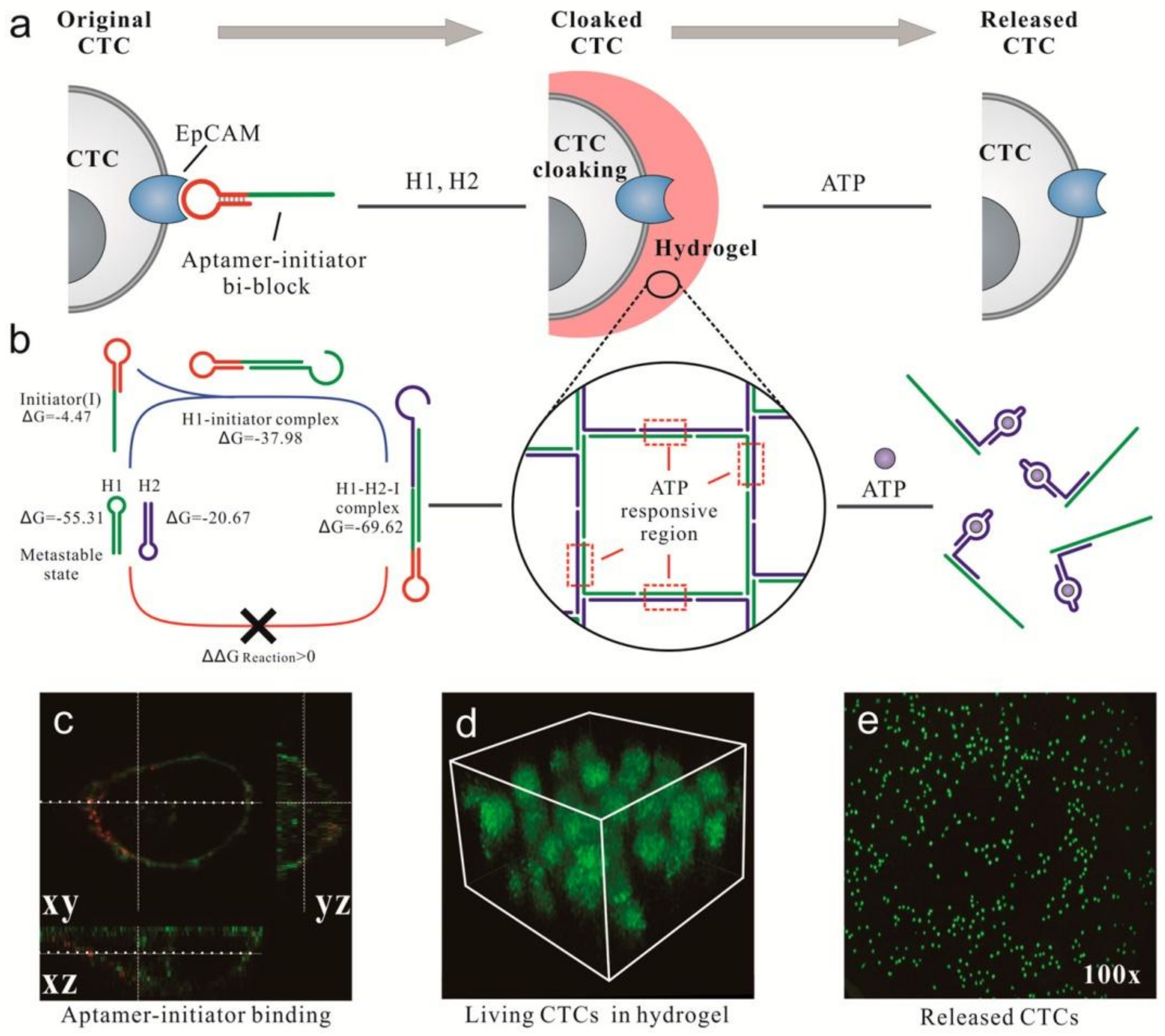
2.2. DNA Hydrogel-Based Theranostic Nanoplatform
2.3. DNA Signal Amplification-Based Theranostic Nanoplatform
2.4. Other DNA Nanotechnologies for the Establishment of Theranostic Nanoplatforms
3. DNA-Integrated Gold Nanomaterials for the Establishment of Theranostic Nanoplatforms
3.1. DNA-Integrated AuNPs
3.2. DNA-Integrated AuNRs
4. Other Materials Integrated with DNA
4.1. 2D Nanosheets Integrated with DNA
4.2. Fluorescent Nanoparticles Integrated with DNA
4.3. Magnetic Nanoparticles Integrated with DNA
5. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, X.; Chen, Z.; Yang, T.; Yang, D.; Liu, Q.; Du, K.; Li, B.; Wang, Z.; Li, S.; et al. Aptamer selection and applications for breast cancer diagnostics and therapy. J. Nanobiotechnol. 2017, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Hartshorn, C.M.; Bradbury, M.S.; Lanza, G.M.; Nel, A.E.; Rao, J.; Wang, A.Z.; Wiesner, U.B.; Yang, L.; Grodzinski, P. Nanotechnology strategies to advance outcomes in clinical cancer care. ACS Nano 2018, 12, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Prakash, G.; Bal Ozturk, A.; Saghazadeh, S.; Farhan Sohail, M.; Seo, J.; Remzi Dokmeci, M.; Zhang, Y.S.; Khademhosseini, A. Evolution and clinical translation of drug delivery nanomaterials. Nano Today 2017, 15, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, X.; Ban, F.; Cao, Y.; Zhao, J.; Chen, G.; Li, G. The design of a mechanical wave-like DNA nanomachine for the fabrication of a programmable and multifunctional molecular device. Chem. Commun. 2017, 53, 10504–10507. [Google Scholar] [CrossRef] [PubMed]
- Brodin, J.D.; Sprangers, A.J.; McMillan, J.R.; Mirkin, C.A. DNA-mediated cellular delivery of functional enzymes. J. Am. Chem. Soc. 2015, 137, 14838–14841. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Meyer, T.A.; Pan, V.; Dutta, P.K.; Ke, Y. The beauty and utility of DNA origami. Chem 2017, 2, 359–382. [Google Scholar] [CrossRef]
- Zhang, H.; Li, F.; Dever, B.; Li, X.F.; Le, X.C. DNA-mediated homogeneous binding assays for nucleic acids and proteins. Chem. Rev. 2013, 113, 2812–2841. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Groves, B.; Muscat, R.A.; Seelig, G. DNA nanotechnology from the test tube to the cell. Nat. Nanotechnol. 2015, 10, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Surana, S.; Shenoy, A.R.; Krishnan, Y. Designing DNA nanodevices for compatibility with the immune system of higher organisms. Nat. Nanotechnol. 2015, 10, 741–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linko, V.; Ora, A.; Kostiainen, M.A. DNA nanostructures as smart drug-delivery vehicles and molecular devices. Trends Biotechnol. 2015, 33, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Llevot, A.; Astruc, D. Applications of vectorized gold nanoparticles to the diagnosis and therapy of cancer. Chem. Soc. Rev. 2012, 41, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Rahman, M.A.; Zhao, Z.; Weiss, K.; Zhang, C.; Chen, Z.; Hurwitz, S.J.; Chen, Z.G.; Shin, D.M.; Ke, Y. Visualization of the cellular uptake and trafficking of DNA origami nanostructures in cancer cells. J. Am. Chem. Soc. 2018, 140, 2478–2484. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lytton-Jean, A.K.; Chen, Y.; Love, K.T.; Park, A.I.; Karagiannis, E.D.; Sehgal, A.; Querbes, W.; Zurenko, C.S.; Jayaraman, M.; et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo sirna delivery. Nat. Nanotechnol. 2012, 7, 389–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bathe, M.; Rothemund, P.W.K. DNA nanotechnology: A foundation for programmable nanoscale materials. MRS Bull. 2017, 42, 882–888. [Google Scholar] [CrossRef]
- Nummelin, S.; Kommeri, J.; Kostiainen, M.A.; Linko, V. Evolution of structural DNA nanotechnology. Adv. Mater. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Nangreave, J.; Liu, Y.; Yan, H. Structural DNA nanotechnology: State of the art and future perspective. J. Am. Chem. Soc. 2014, 136, 11198–11211. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xia, D.; Klausen, L.H.; Dong, M. The self-assembled behavior of DNA bases on the interface. Int. J. Mol. Sci. 2014, 15, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Han, D.; Chen, T.; Peng, L.; Zhu, G.; You, M.; Qiu, L.; Sefah, K.; Zhang, X.; Tan, W. Building a multifunctional aptamer-based DNA nanoassembly for targeted cancer therapy. J. Am. Chem. Soc. 2013, 135, 18644–18650. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, J.T.; Akhuetie-Oni, B.O.; Zhang, Z.; Lin, C. DNA origami rotaxanes: Tailored synthesis and controlled structure switching. Angew. Chem. Int. Ed. 2016, 55, 11412–11416. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Zhang, F.; Liu, Y.; Yan, H. DNA origami: Scaffolds for creating higher order structures. Chem. Rev. 2017, 117, 12584–12640. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xiao, M.; Yan, Q.; Ma, Y.; Xiao, S.J. Small circular DNA molecules act as rigid motifs to build DNA nanotubes. J. Am. Chem. Soc. 2014, 136, 10194–10197. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gao, Y.; Xu, Y.; Chao, J.; Liu, H.; Wang, L.; Li, D.; Fan, C. Real-time imaging of single-molecule enzyme cascade using a DNA origami raft. J. Am. Chem. Soc. 2017, 139, 17525–17532. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, X.; Lu, J.; Mao, X.; Xiang, Y.; Shu, Y.; Li, G. Design of DNA nanostructure-based interfacial probes for the electrochemical detection of nucleic acids directly in whole blood. Chem. Sci. 2018, 9, 979–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.; Li, J.; Li, Q.; Huang, Q.; Shi, J.; Yan, H.; Fan, C. Single-particle tracking and modulation of cell entry pathways of a tetrahedral DNA nanostructure in live cells. Angew. Chem. Int. Ed. 2014, 53, 7745–7750. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lu, D.Q.; Liang, H.; Xie, S.; Luo, C.; Hu, M.; Xu, L.; Zhang, X.; Tan, W. Fluorescence resonance energy transfer-based DNA tetrahedron nanotweezer for highly reliable detection of tumor-related mRNA in living cells. ACS Nano 2017, 11, 4060–4066. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.C.; Wang, A.; Ye, K.; Jin, S. A rna-DNA hybrid aptamer for nanoparticle-based prostate tumor targeted drug delivery. Int. J. Mol. Sci. 2016, 17, 380. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Lee, J.B.; Hong, J. Controlled release of an anti-cancer drug from DNA structured nano-films. Sci. Rep. 2014, 4, 4078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jiang, Q.; Li, N.; Dai, L.R.; Liu, Q.; Song, L.L.; Wang, J.Y.; Li, Y.Q.; Tian, J.; Ding, B.Q.; et al. DNA origami as an in vivo drug delivery vehicle for cancer therapy. ACS Nano 2014, 8, 6633–6643. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Hu, R.; Zhu, G.; Zhang, X.; Mei, L.; Liu, Q.; Qiu, L.; Wu, C.; Tan, W. Preparation and biomedical applications of programmable and multifunctional DNA nanoflowers. Nat. Protoc. 2015, 10, 1508–1524. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.M.; Bachelet, I.; Church, G.M. A logic-gated nanorobot for targeted transport of molecular payloads. Science 2012, 335, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, Q.; Liu, S.; Zhang, Y.; Tian, Y.; Song, C.; Wang, J.; Zou, Y.; Anderson, G.J.; Han, J.-Y.; et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 2018, 36, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Mao, X.; Wang, Z.; Feng, C.; Chen, G.; Li, G. Fabrication of nanozyme@DNA hydrogel and its application in biomedical analysis. Nano Res. 2016, 10, 959–970. [Google Scholar] [CrossRef]
- Mao, X.; Chen, G.; Wang, Z.; Zhang, Y.; Zhu, X.; Li, G. Surface-immobilized and self-shaped DNA hydrogels and their application in biosensing. Chem. Sci. 2018, 9, 811–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Mo, L.; Lu, C.H.; Fu, T.; Yang, H.H.; Tan, W. Functional nucleic acid-based hydrogels for bioanalytical and biomedical applications. Chem. Soc. Rev. 2016, 45, 1410–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, P.; Ye, D.; Zuo, X.; Li, J.; Wang, J.; Liu, H.; Hwang, M.T.; Chao, J.; Su, S.; Wang, L.; et al. DNA hydrogel with aptamer-toehold-based recognition, cloaking, and decloaking of circulating tumor cells for live cell analysis. Nano Lett. 2017, 17, 5193–5198. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, C.; Cansiz, S.; Wu, C.; Xu, J.; Cui, C.; Liu, Y.; Hou, W.; Wang, Y.; Zhang, L.; et al. Self-assembly of DNA nanohydrogels with controllable size and stimuli-responsive property for targeted gene regulation therapy. J. Am. Chem. Soc. 2015, 137, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Li, F.; Zhang, Z.; Zhang, K.; Kang, D.K.; Ankrum, J.A.; Le, X.C.; Zhao, W. Rolling circle amplification: A versatile tool for chemical biology, materials science and medicine. Chem. Soc. Rev. 2014, 43, 3324–3341. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, F.; Li, Q.; Wang, L.; Fan, C. Isothermal amplification of nucleic acids. Chem. Rev. 2015, 115, 12491–12545. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Mao, X.; Yang, Y.; Zhu, X.; Yin, Y.; Li, G. Rolling circle amplification in electrochemical biosensor with biomedical applications. J. Electroanal. Chem. 2016, 781, 223–232. [Google Scholar] [CrossRef]
- Bi, S.; Yue, S.; Zhang, S. Hybridization chain reaction: A versatile molecular tool for biosensing, bioimaging, and biomedicine. Chem. Soc. Rev. 2017, 46, 4281–4298. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, B.; Shi, L.; Zhu, X.; Xiang, Y.; Anzai, J.I.; Li, G. Ultrasensitive quantitation of plasma membrane proteins via isrta. Anal. Chem. 2017, 89, 10776–10782. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Feng, C.; Zhang, B.; Tong, H.; Gao, T.; Li, G. A netlike rolling circle nucleic acid amplification technique. Analyst 2015, 140, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Mao, X.; Shi, H.; Bo, B.; Chen, X.; Chen, T.; Zhu, X.; Li, G. Detection of microRNA: A point-of-care testing method based on a ph-responsive and highly efficient isothermal amplification. Anal. Chem. 2017, 89, 6631–6636. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Bo, B.; Mao, X.; Shi, H.; Zhu, X.; Li, G. From interface to solution: Integrating immunoassay with netlike rolling circle amplification for ultrasensitive detection of tumor biomarker. Theranostics 2017, 7, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yang, Z.; Liu, D. DNA nanotechnology based on i-motif structures. Acc. Chem. Res. 2014, 47, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Willner, I. Stimuli-responsive DNA-functionalized nano-/microcontainers for switchable and controlled release. Angew. Chem. Int. Ed. 2015, 54, 12212–12235. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, H.; Feng, C.; Ye, Z.; Li, G. A dual-colorimetric signal strategy for DNA detection based on graphene and DNAzyme. RSC Adv. 2014, 4, 2421–2426. [Google Scholar] [CrossRef]
- Wei, L.; Wang, X.; Wu, D.; Li, C.; Yin, Y.; Li, G. Proximity ligation-induced assembly of DNAzymes for simple and cost-effective colourimetric detection of proteins with high sensitivity. Chem. Commun. 2016, 52, 5633–5636. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tao, Y.; Yang, Y.; Xiang, Y.; Li, G. In vitro analysis of DNA-protein interactions in gene transcription using DNAzyme-based electrochemical assay. Anal. Chem. 2017, 89, 5003–5007. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, J.; Chen, T.; Gao, T.; Zhu, X.; Li, G. Nondestructive analysis of tumor-associated membrane protein integrating imaging and amplified detection in situ based on dual-labeled DNAzyme. Theranostics 2018, 8, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.M.; Kang, Y.; Kim, W.J. Tumor-homing, size-tunable clustered nanoparticles for anticancer therapeutics. ACS Nano 2014, 8, 9358–9367. [Google Scholar] [CrossRef] [PubMed]
- Rane, T.D.; Armani, A.M. Two-photon microscopy analysis of gold nanoparticle uptake in 3D cell spheroids. PLoS ONE 2016, 11, e0167548. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Hu, X.; Tao, Y.; Li, C.; Mao, X.; Li, G. Gold nanoparticle-based biosensors for the assay of tumor marker proteins with clinical applications. Adv. Mater. Lett. 2017, 8, 1125–1131. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Wang, L.; Mao, X.; Li, G. Highly sensitive protein detection based on smart hybrid nanocomposite-controlled switch of DNA polymerase activity. ACS Appl. Mater. Interfaces 2016, 8, 28202–28207. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, Z.; Chen, T.; Chen, X.; Mao, D.; Zhao, J.; Li, G. A dual-enzyme-assisted three-dimensional DNA walking machine using t4 polynucleotide kinase as activators and application in polynucleotide kinase assays. Anal. Chem. 2018, 90, 2810–2815. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Li, X.F.; Zhang, H.; Le, X.C. A microRNA-initiated DNAzyme motor operating in living cells. Nat. Commun. 2017, 8, 14378. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, G.Q.; Yang, X.L.; Jiang, J.H. Electrostatic nucleic acid nanoassembly enables hybridization chain reaction in living cells for ultrasensitive mRNA imaging. J. Am. Chem. Soc. 2015, 137, 6829–6836. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, Z.H.; Zhang, M.; Han, Z.H.; Chen, D.; Zhu, Q.Y.; Gao, W.D.; Qian, Z.Y.; Gu, Y.Q. A telomerase-specific doxorubicin-releasing molecular beacon for cancer theranostics. Angew. Chem. Int. Ed. 2016, 55, 3304–3308. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Ji, C.; Shi, J.; Pridgen, E.M.; Frieder, J.; Wu, J.; Farokhzad, O.C. DNA self-assembly of targeted near-infrared-responsive gold nanoparticles for cancer thermo-chemotherapy. Angew. Chem. Int. Ed. 2012, 51, 11853–11857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, Y.; Wang, H.; Wang, X.; Liu, Q.; Ye, M.; Tan, W. A smart, photocontrollable drug release nanosystem for multifunctional synergistic cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 5847–5854. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Dong, H.; Guo, K.; Zhang, X. Near-infrared triggered strand displacement amplification for microRNA quantitative detection in single living cells. Chem. Sci. 2018, 9, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, C.; Zhao, J.; Xiao, A.; Shen, Q.; Li, L.; Li, J.; Zhang, J.; Min, Q.; Chen, J.; et al. Near infrared-guided smart nanocarriers for microRNA-controlled release of doxorubicin/siRNA with intracellular ATP as fuel. ACS Nano 2016, 10, 3637–3647. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Yang, D.; Ning, L.; Lei, L.; Ye, Z.; Li, G. Ultrafine and well dispersed silver nanocrystals on 2d nanosheets: Synthesis and application as a multifunctional material for electrochemical catalysis and biosensing. Nanoscale 2014, 6, 14828–14835. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, B.; Ding, J.; Liu, J. Fluorescent sensors using DNA-functionalized graphene oxide. Anal. Bioanal. Chem. 2014, 406, 6885–6902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, C.; Kim, Y.K.; Shin, D.; Ryoo, S.R.; Hong, B.H.; Min, D.H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; He, S.; Shao, C.; Zhao, H.; Li, J.; Tian, L. A common anchor facilitated go-DNA nano-system for multiplex microRNA analysis in live cells. Nanoscale 2018, 10, 7067–7076. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhao, Z.; Yan, G.; Zhang, X.; Yang, C.; Meng, H.; Chen, Z.; Liu, H.; Tan, W. A smart DNAzyme-MNO(2) nanosystem for efficient gene silencing. Angew. Chem. Int. Ed. 2015, 54, 4801–4805. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Ishtiaq, S.; Rabia, S.; Khatoon, M.; Zeb, A.; Khan, G.M.; Ur Rehman, A.; Ud Din, F. Nanotechnology: From in vivo imaging system to controlled drug delivery. Nanoscale Res. Lett. 2017, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- He, X.W.; Zeng, T.; Li, Z.; Wang, G.L.; Ma, N. Catalytic molecular imaging of microRNA in living cells by DNA-programmed nanoparticle disassembly. Angew. Chem. Int. Ed. 2016, 55, 3073–3076. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, X.; Luo, X.; Wang, L.; Ma, N. DNA-programmed quantum dot polymerization for ultrasensitive molecular imaging of cancer cells. Anal. Chem. 2016, 88, 9355–9358. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Gao, Y.; Diefenbach, T.J.; Shen, J.K.; Hornicek, F.J.; Park, Y.I.; Xu, F.; Lu, T.J.; Amiji, M.; Duan, Z. Facial layer-by-layer engineering of upconversion nanoparticles for gene delivery: Near-infrared-initiated fluorescence resonance energy transfer tracking and overcoming drug resistance in ovarian cancer. ACS Appl. Mater. Interfaces 2017, 9, 7941–7949. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, L.; Ma, W.; Wu, X.; Sun, M.; Kuang, H.; Wang, L.; Kotov, N.A.; Xu, C. Dual-mode ultrasensitive quantification of microRNA in living cells by chiroplasmonic nanopyramids self-assembled from gold and upconversion nanoparticles. J. Am. Chem. Soc. 2016, 138, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Xing, R.; Xu, Z.; Hou, Y.; Gao, S.; Sun, S. Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. Adv. Mater. 2010, 22, 2729–2742. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, D.; Lopez, S.; Garcia-Martin, M.L.; Pozo, D. Iron oxide nanoparticles as magnetic relaxation switching (MRSW) sensors: Current applications in nanomedicine. Nanomedicine 2016, 12, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Li, F.; Baik, S.; Shao, W.; Ling, D.; Hyeon, T. Surface design of magnetic nanoparticles for stimuli-responsive cancer imaging and therapy. Biomaterials 2017, 136, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Dai, P.P.; Xu, J.J.; Chen, H.Y. Highly sensitive colorimetric cancer cell detection based on dual signal amplification. ACS Appl. Mater. Interfaces 2016, 8, 4434–4441. [Google Scholar] [CrossRef] [PubMed]










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Ren, L.; Liu, X.; Zhou, M.; Li, L.; Xu, J.; Zhu, X. DNA Nanotechnology for Cancer Diagnosis and Therapy. Int. J. Mol. Sci. 2018, 19, 1671. https://doi.org/10.3390/ijms19061671
Chen T, Ren L, Liu X, Zhou M, Li L, Xu J, Zhu X. DNA Nanotechnology for Cancer Diagnosis and Therapy. International Journal of Molecular Sciences. 2018; 19(6):1671. https://doi.org/10.3390/ijms19061671
Chicago/Turabian StyleChen, Tianshu, Lingjie Ren, Xiaohao Liu, Mengru Zhou, Lingling Li, Jingjing Xu, and Xiaoli Zhu. 2018. "DNA Nanotechnology for Cancer Diagnosis and Therapy" International Journal of Molecular Sciences 19, no. 6: 1671. https://doi.org/10.3390/ijms19061671
APA StyleChen, T., Ren, L., Liu, X., Zhou, M., Li, L., Xu, J., & Zhu, X. (2018). DNA Nanotechnology for Cancer Diagnosis and Therapy. International Journal of Molecular Sciences, 19(6), 1671. https://doi.org/10.3390/ijms19061671





