IL-6 and IL-8 Serum Levels Predict Tumor Response and Overall Survival after TACE for Primary and Secondary Hepatic Malignancies
Abstract
:1. Introduction
2. Results
2.1. Serum Levels of IL-8 and IL-6 Are Elevated in Patients with Primary or Secondary Liver Cancer
2.2. Pre-Interventional Serum Levels of IL-8 and IL-6 Predict an Objective Response to TACE Therapy
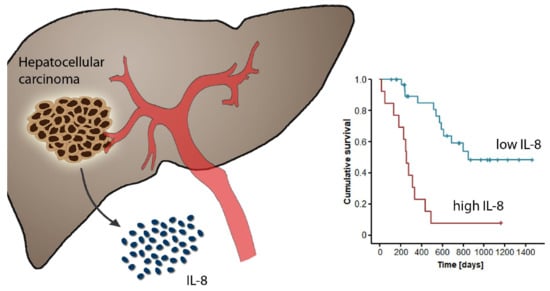
2.3. Elevated Pre-Interventional Serum Levels of IL-8 and IL-6 Are Associated with a Reduced Overall Survival after TACE
2.4. Post-Interventional Serum Levels of CCL22, IL-8, and IL-6 Are Unsuitable for the Prediction of TACE Response for 41 and 16 Patients
3. Discussion
4. Patients and Methods
4.1. Study Design and Patient Characteristics
4.2. Measurement of Serum Cytokine Level
4.3. Transarterial Chemoembolization (TACE)
4.4. Evaluation of TACE Response
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AFP | Alpha-fetoprotein |
| Akt | v-Akt Murine Thymoma Viral Oncogene |
| ALT | alanine transaminase |
| ART | Assessment for Retreatment with TACE |
| AUC | area under the curve |
| BCLC | Barcelona Clinic Liver Cancer |
| CCL | CC-chemokine ligand |
| CRC | colorectal carcinoma |
| CRP | C-reactive protein |
| CXCR | CXC chemokine receptors |
| DEN | Diethylnitrosamine |
| ECLIA | electrochemiluminescence immunoassay |
| GGT | γ-Glutamyl transpeptidase |
| HAP | Hepatoma arterial-embolisation prognostic |
| HCC | hepatocellular carcinoma |
| HcPCs | HCC progenitor cells |
| HFD | high-fat diet |
| HR | hazard ratio |
| IL | interleukin |
| JAK | janus kinase |
| LDH | Lactate dehydrogenase |
| MAPK | mitogen-activated protein kinase |
| OR | objective response |
| OS | overall survival |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| RFA | radiofrequency ablation |
| ROC | receiver operator characteristics |
| SNACOR | tumour Size and Number, baseline Alpha-fetoprotein, Child-Pugh and Objective radiological Response |
| STAT3 | signal transducer and activator of transcription 3 |
| TACE | transarterial chemoembolization |
References
- Tang, A.; Hallouch, O.; Chernyak, V.; Kamaya, A.; Sirlin, C.B. Epidemiology of hepatocellular carcinoma: Target population for surveillance and diagnosis. Abdom. Radiol. 2018, 43, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, G.; Sarti, D.; Aliberti, C.; Carandina, R.; Mambrini, A.; Guadagni, S. Multidisciplinary approach of colorectal cancer liver metastases. World J. Clin. Oncol. 2017, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Shamimi-Noori, S.; Gonsalves, C.; Shaw, C. Metastatic Liver Disease: Indications for Locoregional Therapy and Supporting Data. Semin. Intervent. Radiol. 2017, 34, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.; Tapete, G.; Simonetti, N.; Sellitri, R.; Natali, V.; Melissari, S.; Cabibbo, G.; Biscaglia, L.; Bresci, G.; Giacomelli, L. Transarterial chemoembolization for the treatment of hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma. 2017, 4, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Massmann, A.; Rodt, T.; Marquardt, S.; Seidel, R.; Thomas, K.; Wacker, F.; Richter, G.M.; Kauczor, H.U.; Bücker, A.; Pereira, P.L.; et al. Transarterial chemoembolization (TACE) for colorectal liver metastases—Current status and critical review. Langenbecks Arch. Surg. 2015, 400, 641–659. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Zhu, A.X.; Wigdan, F.; Almasri, J.; Zaiem, F.; Prokop, L.J.; Murad, M.H.; Mohammed, K. Therapies for Advanced Stage Hepatocellular Carcinoma with Macrovascular invasion or Metastatic Disease: A Systematic Review and Meta-analysis. Hepatology 2018, 67, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.Y.; Liu, P.H.; Hsu, C.Y.; Hsia, C.Y.; Lee, Y.H.; Lee, R.C.; Huang, Y.H.; Lee, F.Y.; Hou, M.C.; Tsai, Y.J.; et al. Prognostic role of noninvasive liver reserve markers in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. PLoS ONE 2017, 12, e0180408. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Shim, J.H.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, K.M.; Lim, Y.S.; Han, K.H.; Lee, H.C. Risk prediction for patients with hepatocellular carcinoma undergoing chemoembolization: Development of a prediction model. Liver Int. 2016, 36, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Zhou, Y.; Iribarren, P.; Wang, J. Chemokines and chemokine receptors: Their manifold roles in homeostasis and disease. Cell. Mol. Immunol. 2004, 1, 95–104. [Google Scholar] [PubMed]
- Ha, H.; Debnath, B.; Neamati, N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics 2017, 7, 1543–1588. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, T.; Song, H.; Tian, L.; Lyu, G.; Zhao, L.; Xue, Y. C-C motif chemokine 22 ligand (CCL22) concentrations in sera of gastric cancer patients are related to peritoneal metastasis and predict recurrence within one year after radical gastrectomy. J. Surg. Res. 2017, 211, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Fooladseresht, H.; Minaee, K.; Bazrafshani, M.R.; Khosravimashizi, A.; Nemati, M.; Mohammadizadeh, M.; Mohammadi, M.M.; Ghaderi, A. Higher circulating levels of chemokine CCL22 in patients with breast cancer: Evaluation of the influences of tumor stage and chemokine gene polymorphism. Tumor Biol. 2015, 36, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.J.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.W.; Zhou, Z.G.; Wang, M.; Dong, J.Q.; Du, K.P.; Li, S.; Liu, Y.L.; Lv, P.J.; Gao, J.B. Combination of IL-6, IL-10, and MCP-1 with traditional serum tumor markers in lung cancer diagnosis and prognosis. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Lee, J.; Paik, K.; Kang, J.; Kim, J.; Hwang, J.-H. Serum interleukin-6 is associated with pancreatic ductal adenocarcinoma progression pattern. Medicine 2017, 96, e5926. [Google Scholar] [CrossRef] [PubMed]
- Edeline, J.; Boucher, E.; Rolland, Y.; Vauléon, E.; Pracht, M.; Perrin, C.; Le Roux, C.; Raoul, J.L. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer 2012, 118, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Budczies, J.; Klauschen, F.; Sinn, B.V.; Győrffy, B.; Schmitt, W.D.; Darb-Esfahani, S.; Denkert, C. Cutoff Finder: A comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE 2012, 7, e51862. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; Heimbach, J.K.; Zaiem, F.; Almasri, J.; Prokop, L.J.; Wang, Z.; Murad, M.H.; Mohammed, K. Therapies for Patients with Hepatocellular Carcinoma Awaiting for Liver Transplantation: A Systematic Review and Meta-analysis. Hepatology 2018, 67, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Chen, M.; Colombo, M.; Roberts, L.R.; Schwartz, M.; Chen, P.J.; Kudo, M.; Johnson, P.; Wagner, S.; Orsini, L.S.; et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study. Liver Int. 2015, 35, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Kadalayil, L.; Benini, R.; Pallan, L.; O’Beirne, J.; Marelli, L.; Yu, D.; Hackshaw, A.; Fox, R.; Johnson, P.; Burroughs, A.K.; et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann. Oncol. 2013, 24, 2565–2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt-Arras, D.; Rose-John, S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Hammerich, L. Interleukins in chronic liver disease: Lessons learned from experimental mouse models. Clin. Exp. Gastroenterol. 2014, 7, 297. [Google Scholar] [CrossRef] [PubMed]
- Hatting, M.; Spannbauer, M.; Peng, J.; Al Masaoudi, M.; Sellge, G.; Nevzorova, Y.A.; Gassler, N.; Liedtke, C.; Cubero, F.J.; Trautwein, C. Lack of gp130 expression in hepatocytes attenuates tumor progression in the DEN model. Cell Death Dis. 2015, 6, e1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, G.; Dhar, D.; Nakagawa, H.; Font-Burgada, J.; Ogata, H.; Jiang, Y.; Shalapour, S.; Seki, E.; Yost, S.E.; Jepsen, K.; et al. Identification of Liver Cancer Progenitors Whose Malignant Progression Depends on Autocrine IL-6 Signaling. Cell 2013, 155, 384–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, J.T.; Feng, C.L.; Yu, C.J.; Tsai, S.M.; Hsu, P.N.; Chen, Y.L.; Wu, Y.Y. IL-6, through p-STAT3 rather than p-STAT1, activates hepatocarcinogenesis and affects survival of hepatocellular carcinoma patients: A cohort study. BMC Gastroenterol. 2015, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, J.; Couchy, G.; Imbeaud, S.; Amaddeo, G.; Letouzé, E.; Blanc, J.F.; Laurent, C.; Hajji, Y.; Azoulay, D.; Bioulac-Sage, P.; et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J. Hepatol. 2017, 67, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Poon, R.T.; Tsui, H.T.; Chen, W.H.; Li, Z.; Lau, C.; Yu, W.C.; Fan, S.T. Interleukin-8 serum levels in patients with hepatocellular carcinoma: Correlations with clinicopathological features and prognosis. Clin. Cancer Res. 2003, 9, 5996–6001. [Google Scholar] [PubMed]
- Wang, H.; Shao, Q.; Sun, J.; Ma, C.; Gao, W.; Wang, Q.; Zhao, L.; Qu, X. Interactions between colon cancer cells and tumor-infiltrated macrophages depending on cancer cell-derived colony stimulating factor 1. Oncoimmunology 2016, 5, e1122157. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Lenz, H.-J. Targeting IL-8 in colorectal cancer. Expert Opin. Ther. Targets 2012, 16, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Oppermann, E.; Qian, J.; Imlau, U.; Tran, A.; Hamidavi, Y.; Korkusuz, H.; Bechstein, W.O.; Nour-Eldin, N.E.; Gruber-Rouh, T.; et al. Transarterial chemoembolization of hepatocellular carcinoma in a rat model: The effect of additional injection of survivin siRNA to the treatment protocol. BMC Cancer 2016, 16, 325. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Loosen, S.H.; Roderburg, C.; Kauertz, K.L.; Pombeiro, I.; Leyh, C.; Benz, F.; Vucur, M.; Longerich, T.; Koch, A.; Braunschweig, T.; et al. Elevated levels of circulating osteopontin are associated with a poor survival after resection of cholangiocarcinoma. J. Hepatol. 2017, 67, 749–757. [Google Scholar] [CrossRef] [PubMed]





| Parameter | TACE Patients Median (Range) | Healthy Controls Median (Range) |
|---|---|---|
| Interleukin (IL)-8 pre-TACE (pg/mL) | 35.96 (7.69–381.77) | 7.78 (0–158.05) |
| IL-8 day 1 post-TACE (pg/mL) | 40.17 (12.48–323.73) | - |
| IL-8 day 2 post-TACE (pg/mL) | 39.76 (12.39–332.86) | - |
| IL-6 pre-TACE (pg/mL) | 12.17 (3.49–187.88) | 3.59 (0–17.57) |
| IL-6 day 1 post-TACE (pg/mL) | 31.67 (7.90–164.59) | - |
| IL-6 day 2 post-TACE (pg/mL) | 31.29 (10.34–566.03) | - |
| CC-chemokine ligand (CCL) 22 pre-TACE (pg/mL) | 835.12 (101.57–1872.29) | 934.48 (101.57–1925.91) |
| CCL22 day 1 post-TACE (pg/mL) | 780.66 (110.19–1516.91) | - |
| CCL22 day 2 post-TACE (pg/mL) | 644.16 (203.83–1167.72) | - |
| Bilirubin (mg/dL) | 0.73 (0.26–2.43) | 0.41 (0.1–1.46) |
| Parameter | Univariate Cox Regression | Multivariate Cox Regression | ||
|---|---|---|---|---|
| p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | |
| IL-6 > 21.19 pg/mL | 0.010 | 2.919 (1.290–6.605) | 0.009 | 3.446 (1.355–8.768) |
| IL-8 > 44.96 pg/mL | <0.001 | 5.977 (2.548–14.023) | 0.009 | 4.679 (1.471–14.889) |
| Size of target lesion | 0.277 | 1.006 (0.995–1.017) | ||
| Age | 0.444 | 1.014 (0.978–1.052) | ||
| Sex | 0.898 | 1.062 (0.424–2.662) | ||
| Creatinine | 0.238 | 1.426 (0.791–2.573) | 0.181 | 1.515 (0.824–2.787) |
| Potassium | 0.325 | 1.420 (0.706–2.858) | ||
| Leucocytes | 0.270 | 1.102 (0.928–1.308) | ||
| Alanine transaminase (ALT) | 0.807 | 0.999 (0.992–1.006) | ||
| Lactate dehydrogenase (LDH) | 0.943 | 1.000 (0.997–1.003) | ||
| Bilirubin | 0.857 | 1.075 (0.490–2.357) | ||
| γ-Glutamyl transpeptidase (GGT) | 0.043 | 1.001 (1.000–1.002) | 0.654 | 1.000 (0.999–1.002) |
| C-reactive protein (CRP) | 0.019 | 1.021 (1.003–1.040) | 0.487 | 0.991 (0.965–1.017) |
| Patient Characteristics | Study Cohort |
|---|---|
| TACE patients | 54 |
| Sex (%): | |
| male–female | 79.6–20.4 |
| Age (years, median and range) | 66.5 (37–89) |
| Hepatic malignancy (%): | |
| HCC | 81.5 |
| Liver metastasis (CRC) | 9.3 |
| Liver metastasis (gastric cancer) | 1.8 |
| Liver metastasis (pancreatic) | 3.7 |
| Liver metastasis (CCA) | 3.7 |
| Size of target lesion (mm, median and range) | 29 (10–129) |
| OR to TACE therapy (%): | |
| Yes–No | 43.2–56.8 |
| Deceased during follow-up (%): | |
| Yes–No | 56.6–43.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loosen, S.H.; Schulze-Hagen, M.; Leyh, C.; Benz, F.; Vucur, M.; Kuhl, C.; Trautwein, C.; Tacke, F.; Bruners, P.; Roderburg, C.; et al. IL-6 and IL-8 Serum Levels Predict Tumor Response and Overall Survival after TACE for Primary and Secondary Hepatic Malignancies. Int. J. Mol. Sci. 2018, 19, 1766. https://doi.org/10.3390/ijms19061766
Loosen SH, Schulze-Hagen M, Leyh C, Benz F, Vucur M, Kuhl C, Trautwein C, Tacke F, Bruners P, Roderburg C, et al. IL-6 and IL-8 Serum Levels Predict Tumor Response and Overall Survival after TACE for Primary and Secondary Hepatic Malignancies. International Journal of Molecular Sciences. 2018; 19(6):1766. https://doi.org/10.3390/ijms19061766
Chicago/Turabian StyleLoosen, Sven H., Maximilian Schulze-Hagen, Catherine Leyh, Fabian Benz, Mihael Vucur, Christiane Kuhl, Christian Trautwein, Frank Tacke, Philipp Bruners, Christoph Roderburg, and et al. 2018. "IL-6 and IL-8 Serum Levels Predict Tumor Response and Overall Survival after TACE for Primary and Secondary Hepatic Malignancies" International Journal of Molecular Sciences 19, no. 6: 1766. https://doi.org/10.3390/ijms19061766
APA StyleLoosen, S. H., Schulze-Hagen, M., Leyh, C., Benz, F., Vucur, M., Kuhl, C., Trautwein, C., Tacke, F., Bruners, P., Roderburg, C., & Luedde, T. (2018). IL-6 and IL-8 Serum Levels Predict Tumor Response and Overall Survival after TACE for Primary and Secondary Hepatic Malignancies. International Journal of Molecular Sciences, 19(6), 1766. https://doi.org/10.3390/ijms19061766