Graviola (Annona muricata) Exerts Anti-Proliferative, Anti-Clonogenic and Pro-Apoptotic Effects in Human Non-Melanoma Skin Cancer UW-BCC1 and A431 Cells In Vitro: Involvement of Hedgehog Signaling
Abstract
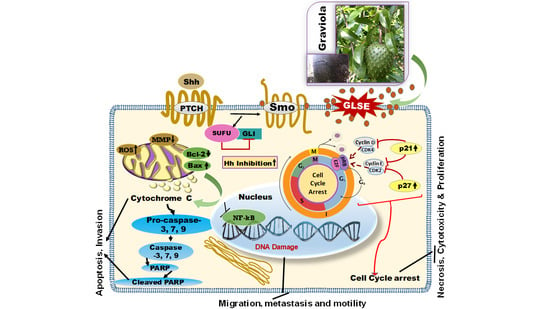
:1. Introduction
2. Results
2.1. GLSE Inhibits Cell Proliferation, Viability and Clonogenicity of UW-BCC1 and A431 Cell Lines
2.2. GLSE Suppresses Transwell Membrane Migration and Scratch Wound Healing
2.3. GLSE Induces G0/G1-Phase Cell Cycle Arrest in UW-BCC1 and A431 Cell Lines
2.4. GLSE Induces Apoptosis in UW-BCC1 and A431 Cell Lines
2.5. GLSE Modulates the Hedgehog Signaling Pathway Components in UW-BCC1 and A431 Cell Lines
2.6. Extraction of Graviola Aerial Parts Powder with Hexane, Dichloromethane or Methane Yields Fractions with Distinct Abilities to Inhibit UW-BCC1 and A431 Cell Viability
2.7. Chemical Characterization of Different Solvent-Extracted Fractions of Graviola Leaf and Stem Powder
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents and Antibodies
4.2. Preparation of Graviola Leaf and Stem Extract (GLSE) and Successive Extractions
4.3. Cell Lines, Culture and Treatment Conditions
4.4. Cell Growth/Proliferation and Viability Assays
4.5. Scratch Wound Healing Assay (SWHA)
4.6. Colony Formation Assay
4.7. Trans-Well Migration/Motility Assay
4.8. Protein Extract Preparation and Immunoblot Analysis
4.9. Detection of Caspase-3 and -7 Activity
4.10. Cell Cycle and Apoptosis Assessment by Flow Cytometry/Immunofluorescence Microscopy
4.11. Apoptosis Assessment by Immunocytochemistry/Immunofluorescence Microscopy
4.12. Nuclear Magnetic Resonance (NMR) Spectroscopic Analysis of Extracts
4.13. Mass Spectrometric (MS) Analysis of Graviola DCM Extract
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NMSC | Non-Melanoma Skin Cancer |
| BCC | Basal cell Carcinoma |
| CDK | Cyclin Dependent Kinase |
| PARP | Poly ADP Ribose Polymerase |
| SHH | Sonic Hedgehog |
| Smo | Smoothened |
| Gli 1 & 2 | Glioma-Associated Oncogene Homolog 1 & 2 |
| SCC | Squamous Cell Carcinoma |
| DCM | Dichloromethane |
| MeOH | Methanol |
| PBS | Phosphate Buffered Saline |
| GLSE | Graviola Leaf and Stem Extract |
| NHEK | Normal Human Epidermal Keratinocytes |
| SuFu | Suppressor of Fused |
References
- Fahradyan, A.; Howell, A.C.; Wolfswinkel, E.M.; Tsuha, M.; Sheth, P.; Wong, A.K. Updates on the Management of Non-Melanoma Skin Cancer (NMSC). Healthcare (Basel) 2017, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Eisemann, N.; Waldmann, A.; Geller, A.C.; Weinstock, M.A.; Volkmer, B.; Greinert, R.; Breitbart, E.W.; Katalinic, A. Non-melanoma skin cancer incidence and impact of skin cancer screening on incidence. J. Investig. Dermatol. 2014, 134, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Havighurst, T.; Kim, K.; Hebbring, S.J.; Ye, Z.; Aylward, J.; Keles, S.; Xu, Y.G.; Spiegelman, V.S. RNA-Binding Protein IGF2BP1 in Cutaneous Squamous Cell Carcinoma. J. Investig. Dermatol. 2017, 137, 772–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzuka, A.G.; Book, S.E. Basal cell carcinoma: Pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J. Biol. Med. 2015, 88, 167–179. [Google Scholar] [PubMed]
- Guy, G.P., Jr.; Machlin, S.R.; Ekwueme, D.U.; Yabroff, K.R. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am. J. Prev. Med. 2015, 48, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Guy, G.P., Jr.; Thomas, C.C.; Thompson, T.; Watson, M.; Massetti, G.M.; Richardson, L.C. Vital signs: Melanoma incidence and mortality trends and projections—United States, 1982–2030. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 591–596. [Google Scholar] [PubMed]
- Robinson, J.K. Sun exposure, sun protection, and vitamin D. JAMA 2005, 294, 1541–1543. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.S. Prevalence of a history of skin cancer in 2007: Results of an incidence-based model. Arch. Dermatol. 2010, 146, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Van der Geer, S.; Siemerink, M.; Reijers, H.A.; Verhaegh, M.E.; Ostertag, J.U.; Neumann, H.A.; Krekels, G.A. The incidence of skin cancer in dermatology. Clin. Exp. Dermatol. 2013, 38, 724–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarallo, M.; Cigna, E.; Frati, R.; Delfino, S.; Innocenzi, D.; Fama, U.; Corbianco, A.; Scuderi, N. Metatypical basal cell carcinoma: A clinical review. J. Exp. Clin. Cancer Res. 2008, 27, 65. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Li, C.; Kim, A.L.; Spiegelman, V.S.; Bickers, D.R. Sonic hedgehog signaling in Basal cell nevus syndrome. Cancer Res. 2014, 74, 4967–4975. [Google Scholar] [CrossRef] [PubMed]
- Noubissi, F.K.; Kim, T.; Kawahara, T.N.; Aughenbaugh, W.D.; Berg, E.; Longley, B.J.; Athar, M.; Spiegelman, V.S. Role of CRD-BP in the growth of human basal cell carcinoma cells. J. Investig. Dermatol. 2014, 134, 1718–1724. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.; Garbe, C.; Lebbe, C.; Malvehy, J.; del Marmol, V.; Pehamberger, H.; Peris, K.; Becker, J.C.; Zalaudek, I.; Saiag, P.; et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur. J. Cancer 2015, 51, 1989–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowotarski, S.L.; Feith, D.J.; Shantz, L.M. Skin Carcinogenesis Studies Using Mouse Models with Altered Polyamines. Cancer Growth Metastasis 2015, 8, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K.A.; Lake, A.J.; Scott, S.; Sherman, R.L.; Noone, A.M.; Howlader, N.; Henley, S.J.; Anderson, R.N.; Firth, A.U.; Ma, J.; et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018. [Google Scholar] [CrossRef] [PubMed]
- Pleasance, E.D.; Cheetham, R.K.; Stephens, P.J.; McBride, D.J.; Humphray, S.J.; Greenman, C.D.; Varela, I.; Lin, M.L.; Ordonez, G.R.; Bignell, G.R.; et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 2010, 463, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Rippey, J.J. Why classify basal cell carcinomas? Histopathology 1998, 32, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Lucas, R.; Hales, S.; Neale, R. Incidence of nonmelanoma skin cancer in relation to ambient UV radiation in white populations, 1978–2012: Empirical relationships. JAMA Dermatol. 2014, 150, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Ananthaswamy, H.N.; Pierceall, W.E. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem. Photobiol. 1990, 52, 1119–1136. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Takebe, N.; Lorusso, P. Targeting the Hedgehog pathway in cancer. Ther. Adv. Med. Oncol. 2010, 2, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Bale, A.E.; Yu, K.P. The hedgehog pathway and basal cell carcinomas. Hum. Mol. Genet. 2001, 10, 757–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciani, F.; Tafuri, S.; Troiano, A.; Cimmino, A.; Fioretto, B.S.; Guarino, A.M.; Pollice, A.; Vivo, M.; Evidente, A.; Carotenuto, D.; et al. Anti-proliferative and pro-apoptotic effects of Uncaria tomentosa aqueous extract in squamous carcinoma cells. J. Ethnopharmacol. 2018, 211, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Ribnicky, D.M.; Poulev, A.; Logendra, S.; Cefalu, W.T.; Raskin, I. A natural history of botanical therapeutics. Metabolism 2008, 57, 39. [Google Scholar] [CrossRef] [PubMed]
- Gavamukulya, Y.; Wamunyokoli, F.; El-Shemy, H.A. Annona muricata: Is the natural therapy to most disease conditions including cancer growing in our backyard? A systematic review of its research history and future prospects. Asian Pac. J. Trop. Med. 2017, 10, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.M.; Kadir, H.A. Annona muricata (Annonaceae): A Review of Its Traditional Uses, Isolated Acetogenins and Biological Activities. Int. J. Mol. Sci. 2015, 16, 15625–15658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coria-Tellez, A.V.; Montalvo-Gonzalez, E.; Yahia, E.M.; Obledo-Vazquez, E.N. Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab. J. Chem. 2017, 30. [Google Scholar] [CrossRef]
- Torres, M.P.; Rachagani, S.; Purohit, V.; Pandey, P.; Joshi, S.; Moore, E.D.; Johansson, S.L.; Singh, P.K.; Ganti, A.K.; Batra, S.K. Graviola: A novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Cancer Lett. 2012, 323, 29–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhakaran, K.; Ramasamy, G.; Doraisamy, U.; Mannu, J.K.R.; Murugesan, J.R. Polyketide Natural Products, Acetogenins from Graviola (Annona muricata L), its Biochemical, Cytotoxic Activity and Various Analyses Through Computational and Bio-Programming Methods. Curr. Pharm. Des. 2016, 22, 5204–5210. [Google Scholar] [CrossRef] [PubMed]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Huang, X.; Xue, Z.; Cao, D.; Huang, K.; Chen, J.; Pan, Y.; Gao, Y. The Role of p21 in Apoptosis, Proliferation, Cell Cycle Arrest, and Antioxidant Activity in UVB-Irradiated Human HaCaT Keratinocytes. Med. Sci. Monit. Basic Res. 2015, 21, 86–95. [Google Scholar] [PubMed] [Green Version]
- Soldani, C.; Scovassi, A.I. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: An update. Apoptosis Int. J. Progr. Cell Death 2002, 7, 321–328. [Google Scholar] [CrossRef]
- Ebrahim, H.Y.; Akl, M.R.; Elsayed, H.E.; Hill, R.A.; El Sayed, K.A. Usnic Acid Benzylidene Analogues as Potent Mechanistic Target of Rapamycin Inhibitors for the Control of Breast Malignancies. J. Nat. Prod. 2017, 80, 932–952. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, H.Y.; Mohyeldin, M.M.; Hailat, M.M.; El Sayed, K.A. (1S,2E,4S,7E,11E)-2,7,11-Cembratriene-4,6-diol semisynthetic analogs as novel c-Met inhibitors for the control of c-Met-dependent breast malignancies. Bioorg. Med. Chem. 2016, 24, 5748–5761. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, H.E.; Ebrahim, H.Y.; Haggag, E.G.; Kamal, A.M.; El Sayed, K.A. Rationally designed hecogenin thiosemicarbazone analogs as novel MEK inhibitors for the control of breast malignancies. Bioorg. Med. Chem. 2017, 25, 6297–6312. [Google Scholar] [CrossRef] [PubMed]
- Rady, I.; Bloch, M.B.; Chamcheu, R.-C.N.; Banang-Mbeumi, S.; Anwar, M.R.; Mohamed, H.; Babatunde, A.S.; Kuiate, J.R.; Noubissi, F.K.; El Sayed, K.A.; et al. Anti-cancer Properties of Graviola (Annona muricata): A Comprehensive Mechanistic Review. Oxidative Medicine and Cellular Longevity 2018. In press. [Google Scholar]
- Ekstrom, A.M.; Serafini, M.; Nyren, O.; Hansson, L.E.; Ye, W.; Wolk, A. Dietary antioxidant intake and the risk of cardia cancer and noncardia cancer of the intestinal and diffuse types: A population-based case-control study in Sweden. Int. J. Cancer 2000, 87, 133–140. [Google Scholar] [CrossRef]
- Guo, W.D.; Hsing, A.W.; Li, J.Y.; Chen, J.S.; Chow, W.H.; Blot, W.J. Correlation of cervical cancer mortality with reproductive and dietary factors, and serum markers in China. Int. J. Epidemiol. 1994, 23, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Kellen, E.; Zeegers, M.; Paulussen, A.; Van Dongen, M.; Buntinx, F. Fruit consumption reduces the effect of smoking on bladder cancer risk. The Belgian case control study on bladder cancer. Int. J. Cancer 2006, 118, 2572–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013, 78 (Suppl. 1), A18–A25. [Google Scholar] [CrossRef] [PubMed]
- Syed, D.N.; Mukhtar, H. Botanicals for the prevention and treatment of cutaneous melanoma. Pigment Cell. Melanoma Res. 2011, 24, 688–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed, D.N.; Chamcheu, J.C.; Adhami, V.M.; Mukhtar, H. Pomegranate extracts and cancer prevention: Molecular and cellular activities. Anti-Cancer Agents Med. Chem. 2013, 13, 1149–1161. [Google Scholar] [CrossRef]
- Kim, J. Protective effects of Asian dietary items on cancers—Soy and ginseng. Asian Pac. J. Cancer Prev. 2008, 9, 543–548. [Google Scholar] [PubMed]
- Lansky, E.P.; Jiang, W.; Mo, H.; Bravo, L.; Froom, P.; Yu, W.; Harris, N.M.; Neeman, I.; Campbell, M.J. Possible synergistic prostate cancer suppression by anatomically discrete pomegranate fractions. Investig. New Drugs 2005, 23, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Hardy, M.L.; Heber, D. Total cranberry extract versus its phytochemical constituents: Antiproliferative and synergistic effects against human tumor cell lines. J. Agric. Food Chem. 2004, 52, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Viladomiu, M.; Hontecillas, R.; Lu, P.; Bassaganya-Riera, J. Preventive and prophylactic mechanisms of action of pomegranate bioactive constituents. Evid.-Based Complement. Alternat. Med. 2013, 2013, 789764. [Google Scholar] [CrossRef] [PubMed]
- Baillon, L.; Basler, K. Reflections on cell competition. Semin. Cell Dev. Biol. 2014, 32, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, I.; Dynlacht, B.D. New insights into cyclins, CDKs, and cell cycle control. Semin. Cell Dev. Biol. 2005, 16, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, D.; Ortega, S. Cyclins and CDKS in development and cancer: Lessons from genetically modified mice. Front. Biosci. 2006, 11, 1164–1188. [Google Scholar] [CrossRef] [PubMed]
- Sola, S.; Morgado, A.L.; Rodrigues, C.M. Death receptors and mitochondria: Two prime triggers of neural apoptosis and differentiation. Biochim. Biophys. Acta 2013, 1830, 2160–2166. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, A.; Martin, A.; Symonds, C.E.; Odajima, J.; Dubus, P.; Barbacid, M.; Santamaria, D. Genetic characterization of the role of the Cip/Kip family of proteins as cyclin-dependent kinase inhibitors and assembly factors. Mol. Cell. Biol. 2014, 34, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Kasibhatla, S.; Tseng, B. Why target apoptosis in cancer treatment? Mol. Cancer Ther. 2003, 2, 573–580. [Google Scholar] [PubMed]
- Khan, K.H.; Blanco-Codesido, M.; Molife, L.R. Cancer therapeutics: Targeting the apoptotic pathway. Crit. Rev. Oncol. Hematol. 2014, 90, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Asare, G.A.; Afriyie, D.; Ngala, R.A.; Abutiate, H.; Doku, D.; Mahmood, S.A.; Rahman, H. Antiproliferative activity of aqueous leaf extract of Annona muricata L. on the prostate, BPH-1 cells, and some target genes. Integr. Cancer Ther. 2015, 14, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Zorofchian Moghadamtousi, S.; Karimian, H.; Rouhollahi, E.; Paydar, M.; Fadaeinasab, M.; Abdul Kadir, H. Annona muricata leaves induce G(1) cell cycle arrest and apoptosis through mitochondria-mediated pathway in human HCT-116 and HT-29 colon cancer cells. J. Ethnopharmacol. 2014, 156, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Syed Najmuddin, S.U.; Romli, M.F.; Hamid, M.; Alitheen, N.B.; Nik Abd Rahman, N.M. Anti-cancer effect of Annona Muricata Linn Leaves Crude Extract (AMCE) on breast cancer cell line. BMC Complement. Altern. Med. 2016, 16, 311. [Google Scholar] [CrossRef] [PubMed]
- Pieme, C.A.; Kumar, S.G.; Dongmo, M.S.; Moukette, B.M.; Boyoum, F.F.; Ngogang, J.Y.; Saxena, A.K. Antiproliferative activity and induction of apoptosis by Annona muricata (Annonaceae) extract on human cancer cells. BMC Complement. Altern. Med. 2014, 14, 516. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Inzunza, H.; Chang, H.; Qi, Z.; Hu, B.; Malone, D.; Cogswell, J. Mutations in the hedgehog pathway genes SMO and PTCH1 in human gastric tumors. PLoS ONE 2013, 8, e54415. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Song, R.; Xie, J. Sonidegib: Mechanism of action, pharmacology, and clinical utility for advanced basal cell carcinomas. Onco Targets Ther. 2017, 10, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Basset-Seguin, N.; Hauschild, A.; Grob, J.J.; Kunstfeld, R.; Dreno, B.; Mortier, L.; Ascierto, P.A.; Licitra, L.; Dutriaux, C.; Thomas, L.; et al. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): A pre-planned interim analysis of an international, open-label trial. Lancet Oncol. 2015, 16, 729–736. [Google Scholar] [CrossRef]
- Sekulic, A.; Migden, M.R.; Oro, A.E.; Dirix, L.; Lewis, K.D.; Hainsworth, J.D.; Solomon, J.A.; Yoo, S.; Arron, S.T.; Friedlander, P.A.; et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012, 366, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Fecher, L.A.; Sharfman, W.H. Advanced basal cell carcinoma, the hedgehog pathway, and treatment options—Role of smoothened inhibitors. Biologics 2015, 9, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Lacouture, M.E.; Dreno, B.; Ascierto, P.A.; Dummer, R.; Basset-Seguin, N.; Fife, K.; Ernst, S.; Licitra, L.; Neves, R.I.; Peris, K.; et al. Characterization and Management of Hedgehog Pathway Inhibitor-Related Adverse Events in Patients With Advanced Basal Cell Carcinoma. Oncologist 2016, 21, 1218–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kainu, K.; Kivinen, K.; Zucchelli, M.; Suomela, S.; Kere, J.; Inerot, A.; Baker, B.S.; Powles, A.V.; Fry, L.; Samuelsson, L.; et al. Association of psoriasis to PGLYRP and SPRR genes at PSORS4 locus on 1q shows heterogeneity between Finnish, Swedish and Irish families. Exp. Dermatol. 2009, 18, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, T.; Abrouk, M.; Sima, C.S.; Sadetsky, N.; Hou, J.; Caro, I.; Chren, M.M.; Arron, S.T. Risk of cutaneous squamous cell carcinoma after treatment of basal cell carcinoma with vismodegib. J. Am. Acad. Dermatol. 2017, 77, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Deep, G.; Kumar, R.; Jain, A.K.; Dhar, D.; Panigrahi, G.K.; Hussain, A.; Agarwal, C.; El-Elimat, T.; Sica, V.P.; Oberlies, N.H.; et al. Graviola inhibits hypoxia-induced NADPH oxidase activity in prostate cancer cells reducing their proliferation and clonogenicity. Sci. Rep. 2016, 6, 23135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Hogan, S.; Schmelz, E.M.; Ju, Y.H.; Canning, C.; Zhou, K. Selective growth inhibition of human breast cancer cells by graviola fruit extract in vitro and in vivo involving downregulation of EGFR expression. Nutr. Cancer 2011, 63, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Chamcheu, J.C.; Afaq, F.; Syed, D.N.; Siddiqui, I.A.; Adhami, V.M.; Khan, N.; Singh, S.; Boylan, B.T.; Wood, G.S.; Mukhtar, H. Delphinidin, a dietary antioxidant, induces human epidermal keratinocyte differentiation but not apoptosis: Studies in submerged and three-dimensional epidermal equivalent models. Exp. Dermatol. 2013, 22, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Chamcheu, J.C.; Lorie, E.P.; Akgul, B.; Bannbers, E.; Virtanen, M.; Gammon, L.; Moustakas, A.; Navsaria, H.; Vahlquist, A.; Torma, H. Characterization of immortalized human epidermolysis bullosa simplex (KRT5) cell lines: Trimethylamine N-oxide protects the keratin cytoskeleton against disruptive stress condition. J. Dermatol. Sci. 2009, 53, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Chamcheu, J.C.; Adhami, V.M.; Esnault, S.; Sechi, M.; Siddiqui, I.A.; Satyshur, K.A.; Syed, D.N.; Dodwad, S.M.; Chaves-Rodriquez, M.I.; Longley, B.J.; et al. Dual Inhibition of PI3K/Akt and mTOR by the Dietary Antioxidant, Delphinidin, Ameliorates Psoriatic Features In Vitro and in an Imiquimod-Induced Psoriasis-Like Disease in Mice. Antioxid. Redox Signal. 2017, 26, 49–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamcheu, J.C.; Rady, I.; Chamcheu, R.-C.N.; Siddique, A.B.; Bloch, M.B.; Banang Mbeumi, S.; Babatunde, A.S.; Uddin, M.B.; Noubissi, F.K.; Jurutka, P.W.; et al. Graviola (Annona muricata) Exerts Anti-Proliferative, Anti-Clonogenic and Pro-Apoptotic Effects in Human Non-Melanoma Skin Cancer UW-BCC1 and A431 Cells In Vitro: Involvement of Hedgehog Signaling. Int. J. Mol. Sci. 2018, 19, 1791. https://doi.org/10.3390/ijms19061791
Chamcheu JC, Rady I, Chamcheu R-CN, Siddique AB, Bloch MB, Banang Mbeumi S, Babatunde AS, Uddin MB, Noubissi FK, Jurutka PW, et al. Graviola (Annona muricata) Exerts Anti-Proliferative, Anti-Clonogenic and Pro-Apoptotic Effects in Human Non-Melanoma Skin Cancer UW-BCC1 and A431 Cells In Vitro: Involvement of Hedgehog Signaling. International Journal of Molecular Sciences. 2018; 19(6):1791. https://doi.org/10.3390/ijms19061791
Chicago/Turabian StyleChamcheu, Jean Christopher, Islam Rady, Roxane-Cherille N. Chamcheu, Abu Bakar Siddique, Melissa B. Bloch, Sergette Banang Mbeumi, Abiola S. Babatunde, Mohammad B. Uddin, Felicite K. Noubissi, Peter W. Jurutka, and et al. 2018. "Graviola (Annona muricata) Exerts Anti-Proliferative, Anti-Clonogenic and Pro-Apoptotic Effects in Human Non-Melanoma Skin Cancer UW-BCC1 and A431 Cells In Vitro: Involvement of Hedgehog Signaling" International Journal of Molecular Sciences 19, no. 6: 1791. https://doi.org/10.3390/ijms19061791
APA StyleChamcheu, J. C., Rady, I., Chamcheu, R.-C. N., Siddique, A. B., Bloch, M. B., Banang Mbeumi, S., Babatunde, A. S., Uddin, M. B., Noubissi, F. K., Jurutka, P. W., Liu, Y.-Y., Spiegelman, V. S., Whitfield, G. K., & El Sayed, K. A. (2018). Graviola (Annona muricata) Exerts Anti-Proliferative, Anti-Clonogenic and Pro-Apoptotic Effects in Human Non-Melanoma Skin Cancer UW-BCC1 and A431 Cells In Vitro: Involvement of Hedgehog Signaling. International Journal of Molecular Sciences, 19(6), 1791. https://doi.org/10.3390/ijms19061791