Lipase Immobilization on Silica Xerogel Treated with Protic Ionic Liquid and its Application in Biodiesel Production from Different Oils
Abstract
:1. Introduction
2. Results and Discussion
2.1. Lipase Immobilization
2.2. Biochemical Characterization
2.2.1. Temperature and pH Effect on Lipase Activity
2.2.2. Determination of Kinetic Parameters
2.2.3. Operational Stability
2.3. Morphological and Physicochemical Characterization
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Specific Surface Area and Porosity
2.3.3. Thermogravimetric Analysis
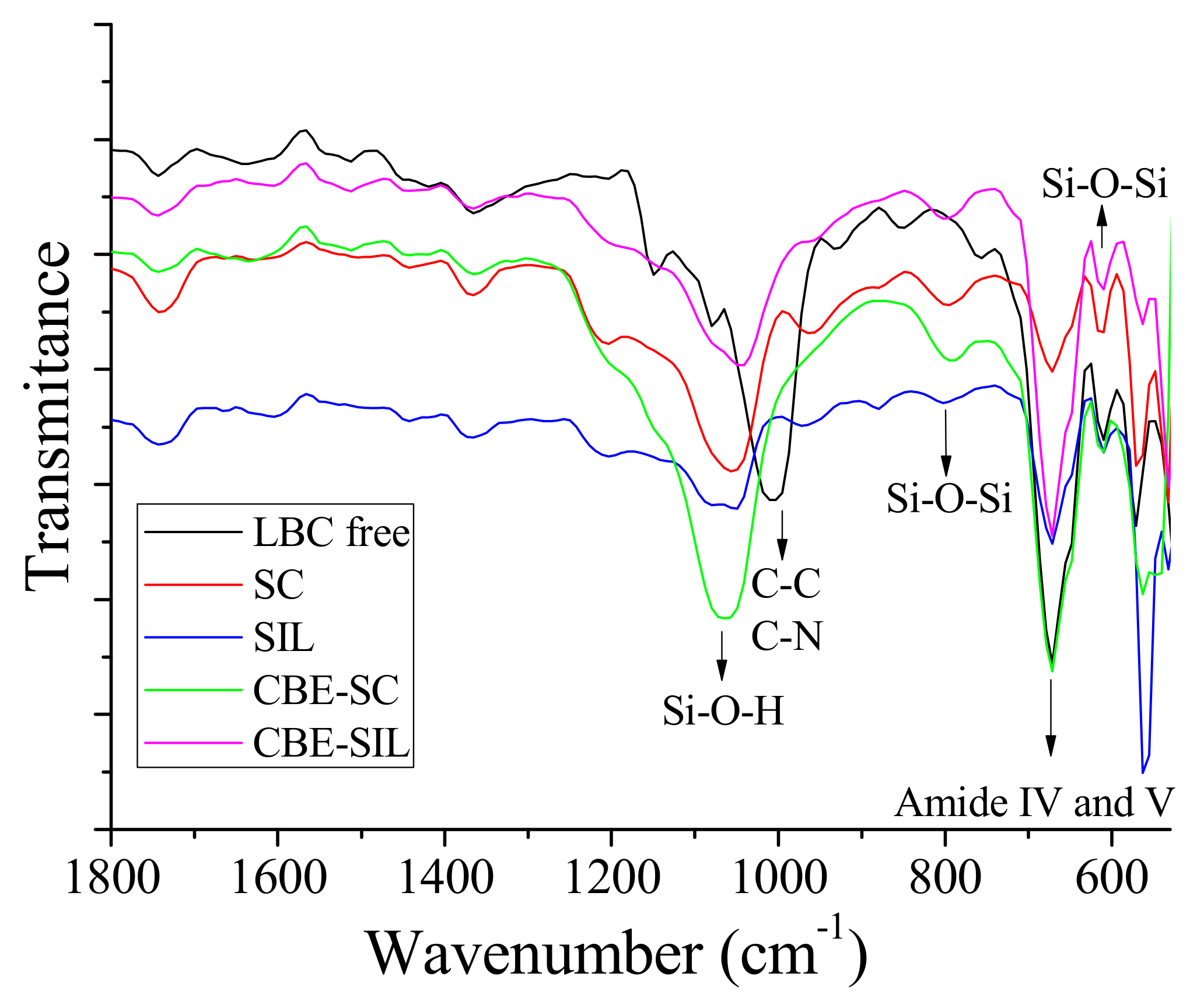
2.3.4. Fourier Transform Infrared Spectroscopy
2.4. Transesterification Activity
3. Material and Methods
3.1. Material
3.2. Xerogel Silica Preparation
3.3. Lipase Immobilization
3.4. Biochemical Characterization
3.4.1. Enzymatic Hydrolysis
3.4.2. Temperature and pH Effect on Lipase Activity in the Hydrolysis Reaction
3.4.3. Kinetic Parameters in the Hydrolysis Reaction
3.4.4. Operational Stability
3.5. Morphological and Physicochemical Characterization
3.6. Transesterification Reaction
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| LBC | Burkholderia cepacia lipase |
| CB | Covalent binding |
| CBE-SC | LBC immobilized by covalent binding onto xerogel silica control and with epichlorohydrin |
| CBE-SIL | LBC immobilized by covalent binding onto xerogel silica treated and with epichlorohydrin |
| CBG-SC | LBC immobilized by covalent binding onto xerogel silica control and with glutaraldehyde |
| CBG-SIL | LBC immobilized by covalent binding onto xerogel silica treated and with glutaraldehyde |
| CG-FID | Gas chromatography–Flame Ionization Detector |
| FTIR | Fourier-transform infrared spectroscopy |
| IL | Ionic liquids |
| PIL | Protic ionic liquid |
| SC | Xerogel silica control |
| SEM | Scanning electron microscopy |
| SIL | Xerogel silica treated with ionic liquid |
| TEOS | Tetraethoxysilane |
| TG | Thermogravimetric analysis |
| Ya | Total activity recovery yield |
References
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.C.; Yang, P.M.; Chen, S.C.; Lin, J.F. Improving biodiesel yields from waste cooking oil using ionic liquids as catalysts with a microwave heating system. Fuel Process. Technol. 2013, 115, 57–62. [Google Scholar] [CrossRef]
- Bernal, C.; Illanes, A.; Wilson, L. Heterofunctional Hydrophilic–Hydrophobic Porous Silica as Support for Multipoint Covalent Immobilization of Lipases: Application to Lactulose Palmitate Synthesis. Langmuir 2014, 30, 3557–3566. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Xiang, X.; Wang, S.; Shi, J.; Deng, Q.; Huang, F.; Cong, R. Lipase immobilized in ordered mesoporous silica: A powerful biocatalyst for ultrafast kinetic resolution of racemic secondary alcohols. Process Biochem. 2017, 53, 102–108. [Google Scholar] [CrossRef]
- Liu, L.; Shih, Y.; Liu, W.; Lin, C.; Huang, H. Enzyme Immobilized Nanoporous Carbons Derived from Metal Organic Framework: A New Support for Biodiesel Synthesis. ChemSusChem 2017, 10, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Galeano, J.D.; Mitchell, D.A.; Krieger, N. Biodiesel production by solvent-free ethanolysis of palm oil catalyzed by fermented solids containing lipases of Burkholderia contaminans. Biochem. Eng. J. 2017, 127, 77–86. [Google Scholar] [CrossRef]
- Li, Y.X.; Dong, B.X. Optimization of Lipase-Catalyzed Transesterification of Lard for Biodiesel Production Using Response Surface Methodology. Braz. Arch. Biol. Technol. 2016, 59, 504–515. [Google Scholar] [CrossRef]
- Abdulla, R.; Ravindra, P. Immobilized Burkholderia cepacia lipase for biodiesel production from crude Jatropha curcas L. oil. Biomass Bioenergy 2013, 56, 8–13. [Google Scholar] [CrossRef]
- Tran, D.T.; Chen, C.L.; Chang, J.S. Continuous biodiesel conversion via enzymatic transesterification catalyzed by immobilized Burkholderia lipase in a packed-bed bioreactor. Appl. Energy 2016, 168, 340–350. [Google Scholar] [CrossRef]
- Babaki, M.; Yousefi, M.; Habibi, Z.; Brask, J.; Mohammadi, M. Preparation of highly reusable biocatalysts by immobilization of lipases on epoxy-functionalized silica for production of biodiesel from canola oil. Biochem. Eng. J. 2015, 101, 23–31. [Google Scholar] [CrossRef]
- Carvalho, N.B.; Lima, Á.S.; Soares, C.M.F. Uso de sílicas modificadas para imobilização de lipases. Quim. Nova 2015, 38, 399–409. [Google Scholar] [CrossRef]
- Barbosa, A.S.; Lisboa, J.A.; Silva, M.A.O.; Carvalho, N.B.; Pereira, M.M.; Fricks, A.T.; Mattedi, S.; Lima, Á.S.; Franceschi, E.; Soares, C.M.F. The novel Mesoporous silica aerogel modified with protic ionic liquid for lipase immobilization. Quim. Nova 2016, 39, 415–422. [Google Scholar] [CrossRef]
- de Souza, R.L.; de Faria, E.L.P.; Figueiredo, R.T.; Freitas, L.d.S.; Iglesias, M.; Mattedi, S.; Zanin, G.M.; dos Santos, O.A.A.; Coutinho, J.A.P.; Lima, Á.S.; et al. Protic ionic liquid as additive on lipase immobilization using silica sol–gel. Enzyme Microb. Technol. 2013, 52, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.C.; Barbosa, A.S.; Fricks, A.T.; Freitas, L.S.; Lima, Á.S.; Soares, C.M.F. Use of conventional or non-conventional treatments of biochar for lipase immobilization. Process Biochem. 2017, 61, 124–129. [Google Scholar] [CrossRef]
- Zou, B.; Song, C.; Xu, X.; Xia, J.; Huo, S.; Cui, F. Enhancing stabilities of lipase by enzyme aggregate coating immobilized onto ionic liquid modified mesoporous materials. Appl. Surf. Sci. 2014, 311, 62–67. [Google Scholar] [CrossRef]
- Hu, Y.; Tang, S.; Jiang, L.; Zou, B.; Yang, J.; Huang, H. Immobilization of Burkholderia cepacia lipase on functionalized ionic liquids modified mesoporous silica SBA-15. Process Biochem. 2012, 47, 2291–2299. [Google Scholar] [CrossRef]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Hu, Y.; Jiang, L.; Zou, B.; Jia, R.; Huang, H. Enhancing the catalytic properties of porcine pancreatic lipase by immobilization on SBA-15 modified by functionalized ionic liquid. Biochem. Eng. J. 2013, 70, 46–54. [Google Scholar] [CrossRef]
- Brito, M.J.P.; Veloso, C.M.; Bonomo, R.C.F.; Fontan, R.d.C.I.; Santos, L.S.; Monteiro, K.A. Activated carbons preparation from yellow mombin fruit stones for lipase immobilization. Fuel Process. Technol. 2017, 156, 421–428. [Google Scholar] [CrossRef]
- Martins, S.R.; dos Santos, A.; Fricks, A.T.; Lima, Á.S.; Mattedi, S.; Silva, D.P.; Soares, C.M.; Cabrera-Padilla, R.Y. Protic ionic liquids influence on immobilization of Lipase Burkholderia cepacia on hybrid supports. J. Chem. Technol. Biotechnol. 2016, 92, 633–641. [Google Scholar] [CrossRef]
- Barbosa, A.d.S.; Silva, M.A.d.O.; Carvalho, N.B.; Mattedi, S.; Iglesias, M.A.; Fricks, A.T.; Lima, Á.S.; Franceschi, E.; Soares, C.M.F. Immobilization of Lipase By Encapsulation in Silica Aerogel. Quim. Nova 2014, 37, 969–976. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Zaidan, U.H.; Abdul Rahman, M.B.; Othman, S.S.; Basri, M.; Abdulmalek, E.; Abdul Rahman, R.N.Z. R.; Salleh, A.B. Biocatalytic production of lactose ester catalysed by mica-based immobilised lipase. Food Chem. 2012, 131, 199–205. [Google Scholar] [CrossRef]
- Zhou, Z.; Hartmann, M. Progress in enzyme immobilization in ordered mesoporous materials and related applications. Chem. Soc. Rev. 2013, 42, 3894–3912. [Google Scholar] [CrossRef] [PubMed]
- Talbert, J.N.; Goddard, J.M. Enzymes on material surfaces. Colloids Surfaces B Biointerfaces 2012, 93, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.V.S.; Da Rós, P.C.M.; Mattedi, S.; Castro, H.F.; Soares, C.M.F.; Lima, Á.S. Transesterification of babassu oil catalyzed by Burkholderia cepacia encapsulated in sol-gel matrix employing protic ionic liquid as an additive. Acta Sci. Technol. 2014, 36, 445. [Google Scholar] [CrossRef] [Green Version]
- Cabrera-Padilla, R.Y.; Albuquerque, M.; Figueiredo, R.T.; Fricks, A.T.; Franceschi, E.; Lima, Á.S.; A Dos Santos, O.A.; Silva, D.P.; Soares, C.M.F. Immobilization and characterisation of a lipase from a new source, Bacillus sp. ITP-001. Bioprocess Biosyst. Eng. 2013, 36, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.B.; Barbosa, J.M.P.; Oliveira, M.V.S.; Fricks, A.T.; Lima, Á.S.; Soares, C.M.F. Biochemical properties of Bacillus sp. ITP-001 lipase immobilized with a sol gel process. Quim. Nova 2013, 36, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Forde, J.; Vakurov, A.; Gibson, T.D.; Millner, P.; Whelehan, M.; Marison, I.W.; Ó’Fágáin, C. Chemical modification and immobilisation of lipase B from Candida antarctica onto mesoporous silicates. J. Mol. Catal. B Enzym. 2010, 66, 203–209. [Google Scholar] [CrossRef]
- Bastida, A.; Sabuquillo, P.; Armisen, P.; Fernández-Lafuente, R.; Huguet, J.; Guisán, J.M. A single step purification, immobilization, and hyperactivation of lipases via interfacial adsorption on strongly hydrophobic supports. Biotechnol. Bioeng. 1998, 58, 486–493. [Google Scholar] [CrossRef]
- Palomo, J.M.; Muñoz, G.; Fernández-Lorente, G.; Mateo, C.; Fernández-Lafuente, R.; Guisán, J.M. Interfacial adsorption of lipases on very hydrophobic support (octadecyl–Sepabeads): immobilization, hyperactivation and stabilization of the open form of lipases. J. Mol. Catal. B Enzym. 2002, 19–20, 279–286. [Google Scholar] [CrossRef]
- Minovska, V.; Winkelhausen, E.; Kuzmanova, S. Lipase immobilized by different techniques on various support materials applied in oil hydrolysis. J. Serb. Chem. Soc. 2005, 70, 609–624. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Paula, A.; Urioste, D.; Santos, J.; de Castro, H. Porcine pancreatic lipase immobilized on polysiloxane–polyvinyl alcohol hybrid matrix: catalytic properties and feasibility to mediate synthesis of surfactants and biodiesel. J. Chem. Technol. Biotechnol. 2007, 82, 281–288. [Google Scholar] [CrossRef]
- Betancor, L.; López-Gallego, F.; Hidalgo, A.; Alonso-Morales, N.; Mateo, G.D.-O.C.; Fernández-Lafuente, R.; Guisán, J.M. Different mechanisms of protein immobilization on glutaraldehyde activated supports: Effect of support activation and immobilization conditions. Enzyme Microb. Technol. 2006, 39, 877–882. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Monsan, P. Optimization of glutaraldehyde activation of a support for enzyme immobilization. J. Mol. Catal. 1978, 3, 371–384. [Google Scholar] [CrossRef]
- Mendes, A.A.; De Castro, H.F.; De L. C. Giordano, R. Triagem de suportes orgânicos e protocolos de ativação na imobilização e estabilização de lipase de Thermomyces lanuginosus. Quim. Nova 2013, 36, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-lafuente, R. Versatility of glutaraldehyde to immobilize lipases: Effect of the immobilization protocol on the properties of lipase B from Candida antarctica. Process Biochem. 2012, 47, 1220–1227. [Google Scholar] [CrossRef]
- Porath, I.; Fornstedt, N. Group fractionation of plasma proteins on dipolar ion exchangers. J. Chromatogr. A 1970, 51, 479–489. [Google Scholar] [CrossRef]
- Garcia-galan, C.; Fernandez-lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Wiley 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Paula, A.V; Moreira, A.B.R.; Braga, L.P.; De Castro, H.F.; Bruno, L.M. Comparação do desempenho da lipase de Candida rugosa imobilizada em suporte híbrido de polissiloxanopolivinilálcool empregando diferentes metodologias. Quim. Nova 2008, 31, 35–40. [Google Scholar] [CrossRef]
- Soares, C.M.F.; dos Santos, O.A.; Olivo, J.E.; de Castro, H.F.; de Moraes, F.F.; Zanin, G.M. Influence of the alkyl-substituted silane precursor on sol–gel encapsulated lipase activity. J. Mol. Catal. B Enzym. 2004, 29, 69–79. [Google Scholar] [CrossRef]
- Da Rós, P.C.M.; Silva, G.A.M.; Mendes, A.A.; Santos, J.C.; de Castro, H.F. Evaluation of the catalytic properties of Burkholderia cepacia lipase immobilized on non-commercial matrices to be used in biodiesel synthesis from different feedstocks. Bioresour. Technol. 2010, 101, 5508–5516. [Google Scholar] [CrossRef] [PubMed]
- Dhake, K.P.; Karoyo, A.H.; Mohamed, M.H.; Wilson, L.D.; Bhanage, B.M. Enzymatic activity studies of Pseudomonas cepacia lipase adsorbed onto copolymer supports containing β-cyclodextrin. J. Mol. Catal. B Enzym. 2013, 87, 105–112. [Google Scholar] [CrossRef]
- Zhou, Z.; Piepenbreier, F.; Marthala, V.R.R.; Karbacher, K.; Hartmann, M. Immobilization of lipase in cage-type mesoporous organosilicas via covalent bonding and crosslinking. Catal. Today 2015, 243, 173–183. [Google Scholar] [CrossRef]
- Hartmann, M.; Kostrov, X. Immobilization of enzymes on porous silicas—benefits and challenges. Chem. Soc. Rev. 2013, 42, 6277–6289. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, R.; Ravindra, P. Characterization of cross linked Burkholderia cepacia lipase in alginate and κ-carrageenan hybrid matrix. J. Taiwan Inst. Chem. Eng. 2013, 44, 545–551. [Google Scholar] [CrossRef]
- de Carvalho, L.M.; de Carvalho, H.W.L.; Carvalho, C.G.P.; de Oliveira, I.R.; Lira, M.A.; de Ferreira, F.M.B.; Tabosa, J.N.; Menezes, V.M.M.; dos Santos, D.L.; Moitinho, A.C.; et al. dos Desempenho de Cultivares de Girassol em Monocultivo e em Consórcio, nos Estados de Sergipe e Bahia, nos Anos Agrícolas de 2012–2013; Embrapa Tabuleiros Costeiros: Aracaju, Brazil, 2014. [Google Scholar]
- Gog, A.; Roman, M.; Toşa, M.; Paizs, C.; Irimie, F.D. Biodiesel production using enzymatic transesterification—Current state and perspectives. Renew. Energy 2012, 39, 10–16. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Ling, F.W.; Jun, L.S. Proposed kinetic mechanism of the production of biodiesel from palm oil using lipase. Process Biochem. 2007, 42, 951–960. [Google Scholar] [CrossRef]
- Karimpil, J.J.; Melo, J.S.; D’Souza, S.F. Hen egg white as a feeder protein for lipase immobilization. J. Mol. Catal. B Enzym. 2011, 71, 113–118. [Google Scholar] [CrossRef]
- Zou, B.; Hu, Y.; Yu, D.; Xia, J.; Tang, S.; Liu, W.; Huang, H. Immobilization of porcine pancreatic lipase onto ionic liquid modified mesoporous silica SBA-15. Biochem. Eng. J. 2010, 53, 150–153. [Google Scholar] [CrossRef]
- Zou, B.; Hu, Y.; Yu, D.; Jiang, L.; Liu, W.; Song, P. Functionalized ionic liquid modified mesoporous silica SBA-15: A novel, designable and efficient carrier for porcine pancreas lipase. Colloids Surf. B Biointerfaces 2011, 88, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yu, N.; Ding, Y.; Tan, R.; Liu, C.; Yin, D.; Qiu, H.; Yin, D. Task-specific basic ionic liquid immobilized on mesoporous silicas: Efficient and reusable catalysts for Knoevenagel condensation in aqueous media. Microporous Mesoporous Mater. 2010, 136, 10–17. [Google Scholar] [CrossRef]
- Abdullah, A.Z.; Sulaiman, N.S.; Kamaruddin, A.H. Biocatalytic esterification of citronellol with lauric acid by immobilized lipase on aminopropyl-grafted mesoporous SBA-15. Biochem. Eng. J. 2009, 44, 263–270. [Google Scholar] [CrossRef]
- Lü, Y.; Guo, Y.; Wang, Y.; Liu, X.; Wang, Y.; Guo, Y.; Zhang, Z.; Lu, G. Immobilized penicillin G acylase on mesoporous silica: The influence of pore size, pore volume and mesophases. Microporous Mesoporous Mater. 2008, 114, 507–510. [Google Scholar] [CrossRef]
- Poppe, J.K.; Costa, A.P.O.; Brasil, M.C.; Rodrigues, R.C.; Ayub, M.A.Z. Multipoint covalent immobilization of lipases on aldehyde-activated support: Characterization and application in transesterification reaction. J. Mol. Catal. B Enzym. 2013, 94, 57–62. [Google Scholar] [CrossRef]
- Portaccio, M.; Della Ventura, B.; Mita, D.G.; Manolova, N.; Stoilova, O.; Rashkov, I.; Lepore, M. FT-IR microscopy characterization of sol-gel layers prior and after glucose oxidase immobilization for biosensing applications. J. Sol-Gel Sci. Technol. 2011, 57, 204–211. [Google Scholar] [CrossRef]
- Anuar, S.T.; Zhao, Y.-Y.; Mugo, S.M.; Curtis, J.M. The development of a capillary microreactor for transesterification reactions using lipase immobilized onto a silica monolith. J. Mol. Catal. B Enzym. 2013, 92, 62–70. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Guldhe, A.; Singh, B.; Mutanda, T.; Permaul, K.; Bux, F. Advances in synthesis of biodiesel via enzyme catalysis: Novel and sustainable approaches. Renew. Sustain. Energy Rev. 2015, 41, 1447–1464. [Google Scholar] [CrossRef]
- Murugesan, A.; Umarani, C.; Chinnusamy, T.R.; Krishnan, M.; Subramanian, R.; Neduzchezhain, N. Production and analysis of bio-diesel from non-edible oils—A review. Renew. Sustain. Energy Rev. 2009, 13, 825–834. [Google Scholar] [CrossRef]
- Shahid, E.M.; Jamal, Y. Production of biodiesel: A technical review. Renew. Sustain. Energy Rev. 2011, 15, 4732–4745. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- ANVISA Resolução RDC no 482, de 23 de setembro de 1999. Agência Nac. Vigilância Sanitária 1999.
- Polaina, J.; MacCabe, A.P. Industrial Enzymes: Structure, Function and Applications; Polaina, J., MacCabe, A.P., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2007; ISBN 978-1-4020-5376-4. [Google Scholar]
- Soares, C.M.F.; De Castro, H.F.; De Moraes, F.F.; Zanin, G.M. Characterization and utilization of Candida rugosa lipase immobilized on controlled pore silica. Appl. Biochem. Biotechnol. 1999, 79, 745–757. [Google Scholar] [CrossRef]
- Santos, J.C.; Paula, A. V; Nunes, G.F.M.; de Castro, H.F. Pseudomonas fluorescens lipase immobilization on polysiloxane–polyvinyl alcohol composite chemically modified with epichlorohydrin. J. Mol. Catal. B Enzym. 2008, 52–53, 49–57. [Google Scholar] [CrossRef]







| Biocatalysts | Vmax (U·g−1) | Km (mM) |
|---|---|---|
| LBC | 1836 ± 141 | 249 ± 31 |
| CBE-SC | 2718 ± 237 | 99 ± 14 |
| CBE-SIL | 3801 ± 296 | 149 ± 25 |
| Sample | Superficial Area (m2·g−1) | Pore Volume (cm3·g−1) | Pore Diameter (Å) |
|---|---|---|---|
| SC | 799.5 | 0.57 | 30.25 |
| SIL | 853.5 | 1.02 | 41.76 |
| CBE-SC | 349.4 | 0.67 | 42.55 |
| CBE-SIL | 371.8 | 0.35 | 46.03 |
| Samples | Temperature (°C) | Partial Mass Loss (%) |
|---|---|---|
| LBC | 25–200 | 8.0 |
| 200–600 | 83.5 | |
| 600–1000 | 8.4 | |
| SC | 25–200 | 21.2 |
| 200–600 | 4.9 | |
| 600–1000 | 1.4 | |
| SIL | 25–200 | 20.0 |
| 200–600 | 9.8 | |
| 600–1000 | 1.5 | |
| CBE-SC | 25–200 | 23.4 |
| 200–600 | 17.7 | |
| 600–1000 | 2.2 | |
| CBE-SIL | 25–200 | 10.2 |
| 200–600 | 15.1 | |
| 600–1000 | 1.7 |
| Fatty Acids | Nomenclature | Soybean Oil | Sunflower Oil | Colza Oil |
|---|---|---|---|---|
| C 14:0 | Myristic | <0.5 | <0.5 | <0.2 |
| C 16:0 | Palmitic | 7.0–14.0 | 3.0–10.0 | 2.5–6.5 |
| C 16:1 | Palmitoleic | <0.5 | <1.0 | <0.6 |
| C 18:0 | Stearic | 1.4–5.5 | 1.0–10.0 | 0.8–3.0 |
| C 18:1 | Oleic | 19.0–30.0 | 14.0–35.0 | 53.0–70.0 |
| C 18:2 | Linoleic | 44.0–62.0 | 55.0–75.0 | 15.0–30.0 |
| C 18:3 | Linolenic | 4.0–11.0 | <0.3 | 5.0–13.0 |
| C 20:0 | Arachidic | <1.0 | <1.5 | 0.1–1.2 |
| C 20:1 | Eicosenoic | <1.0 | <0.5 | 0.1–4.3 |
| C 22:0 | Behenic | <0.5 | <1.0 | <0.6 |
| C 22:1 | Erucic | <0.5 | <2.0 | |
| C 24:0 | Lignoceric | <0.5 | <0.2 | |
| C 24:1 | Tetracosenóico | <0.5 | <0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, N.B.; Vidal, B.T.; Barbosa, A.S.; Pereira, M.M.; Mattedi, S.; Freitas, L.D.S.; Lima, Á.S.; Soares, C.M.F. Lipase Immobilization on Silica Xerogel Treated with Protic Ionic Liquid and its Application in Biodiesel Production from Different Oils. Int. J. Mol. Sci. 2018, 19, 1829. https://doi.org/10.3390/ijms19071829
Carvalho NB, Vidal BT, Barbosa AS, Pereira MM, Mattedi S, Freitas LDS, Lima ÁS, Soares CMF. Lipase Immobilization on Silica Xerogel Treated with Protic Ionic Liquid and its Application in Biodiesel Production from Different Oils. International Journal of Molecular Sciences. 2018; 19(7):1829. https://doi.org/10.3390/ijms19071829
Chicago/Turabian StyleCarvalho, Nayára B., Bruna T. Vidal, Anderson S. Barbosa, Matheus M. Pereira, Silvana Mattedi, Lisiane Dos S. Freitas, Álvaro S. Lima, and Cleide M. F. Soares. 2018. "Lipase Immobilization on Silica Xerogel Treated with Protic Ionic Liquid and its Application in Biodiesel Production from Different Oils" International Journal of Molecular Sciences 19, no. 7: 1829. https://doi.org/10.3390/ijms19071829





