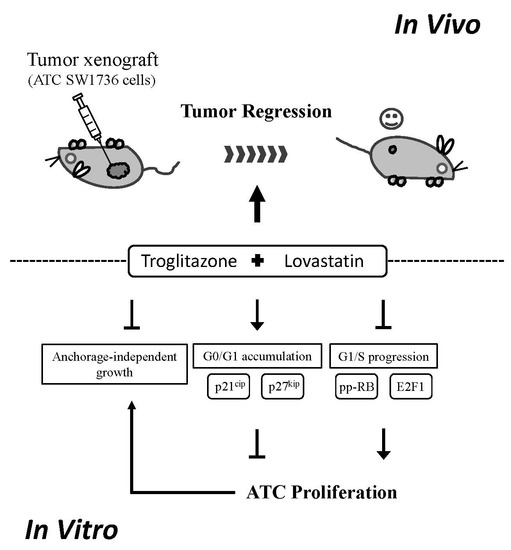
A Synergistic Anti-Cancer Effect of Troglitazone and Lovastatin in a Human Anaplastic Thyroid Cancer Cell Line and in a Mouse Xenograft Model
Abstract
1. Introduction
2. Results
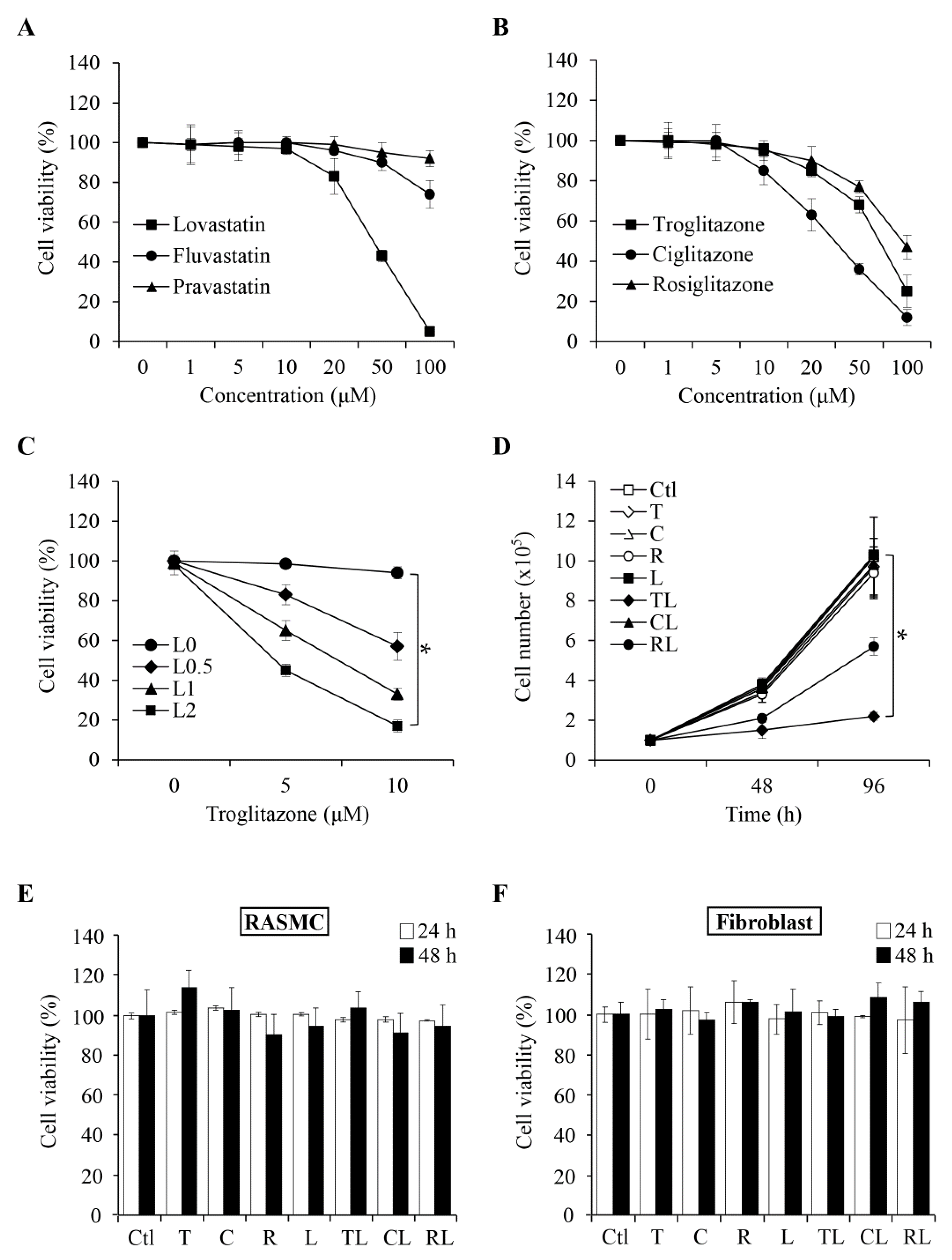
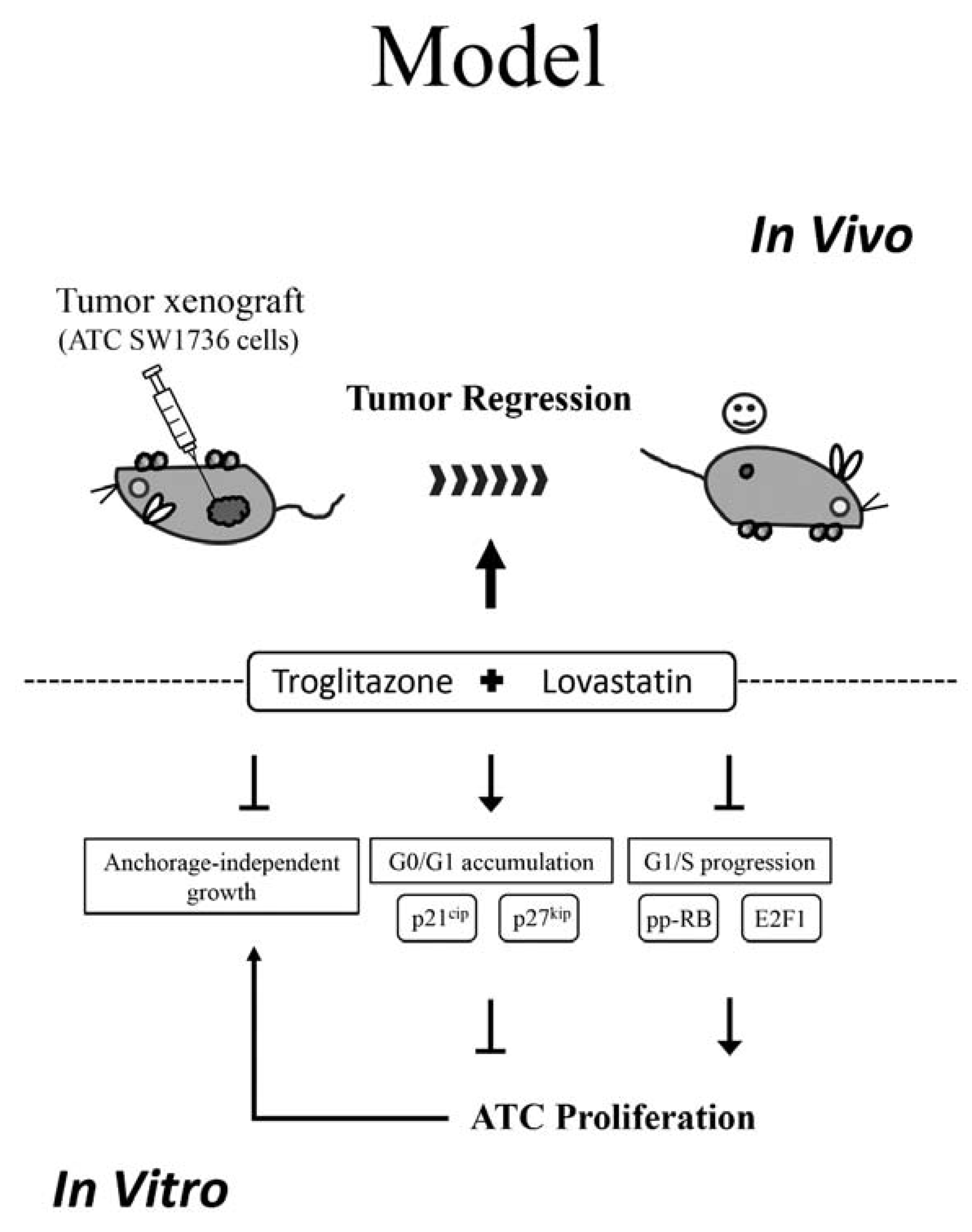
2.1. Combination of Troglitazone and Lovastatin Shows Tumor-Specific Anti-Proliferation Effect
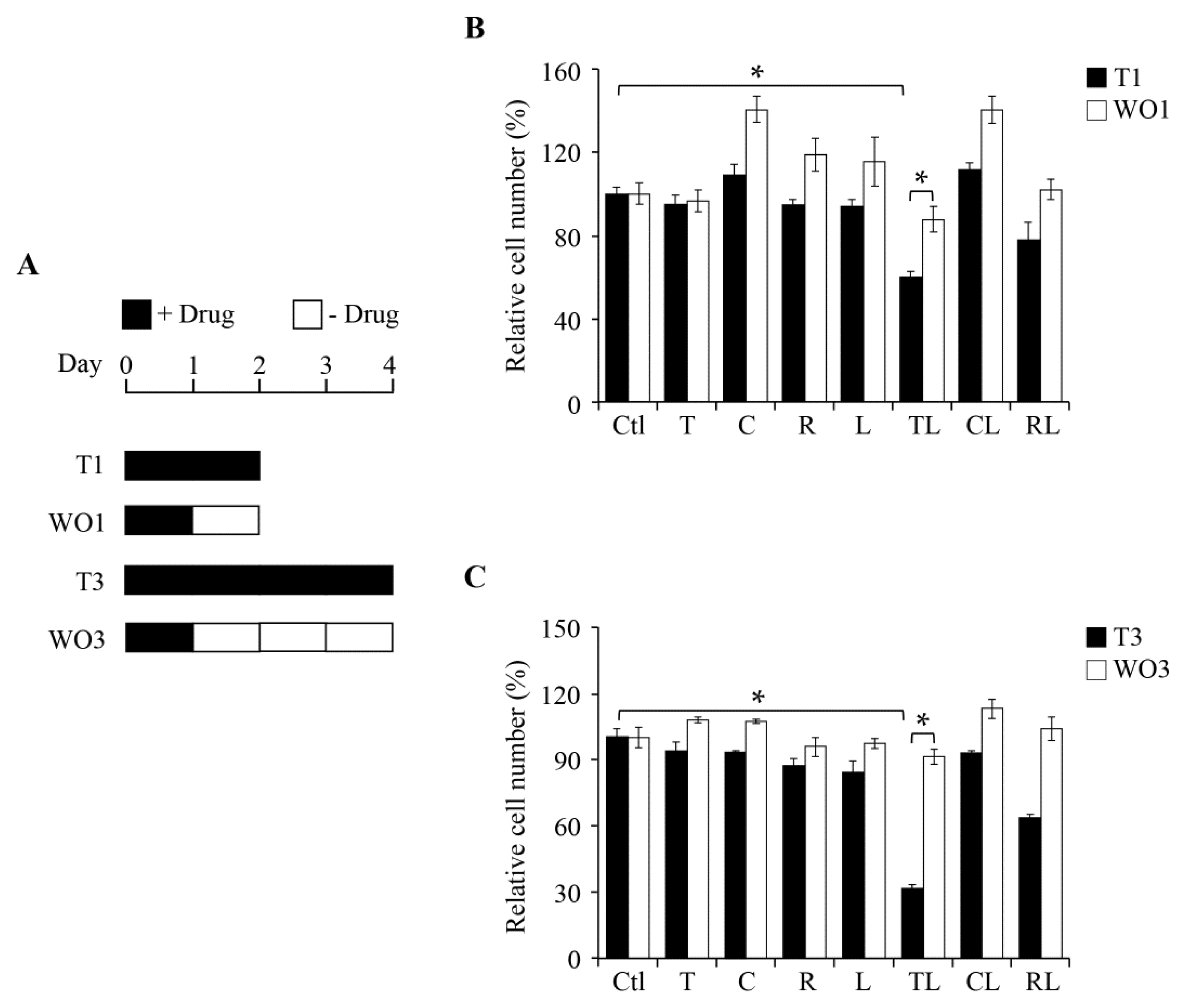
2.2. Inhibitory Effect of Combination Treatment Is Reversible
2.3. Combination of Sub-Lethal Concentrations of Lovastatin and Troglitazone Leads to Cell Cycle Arrest at the G0/G1 Phase
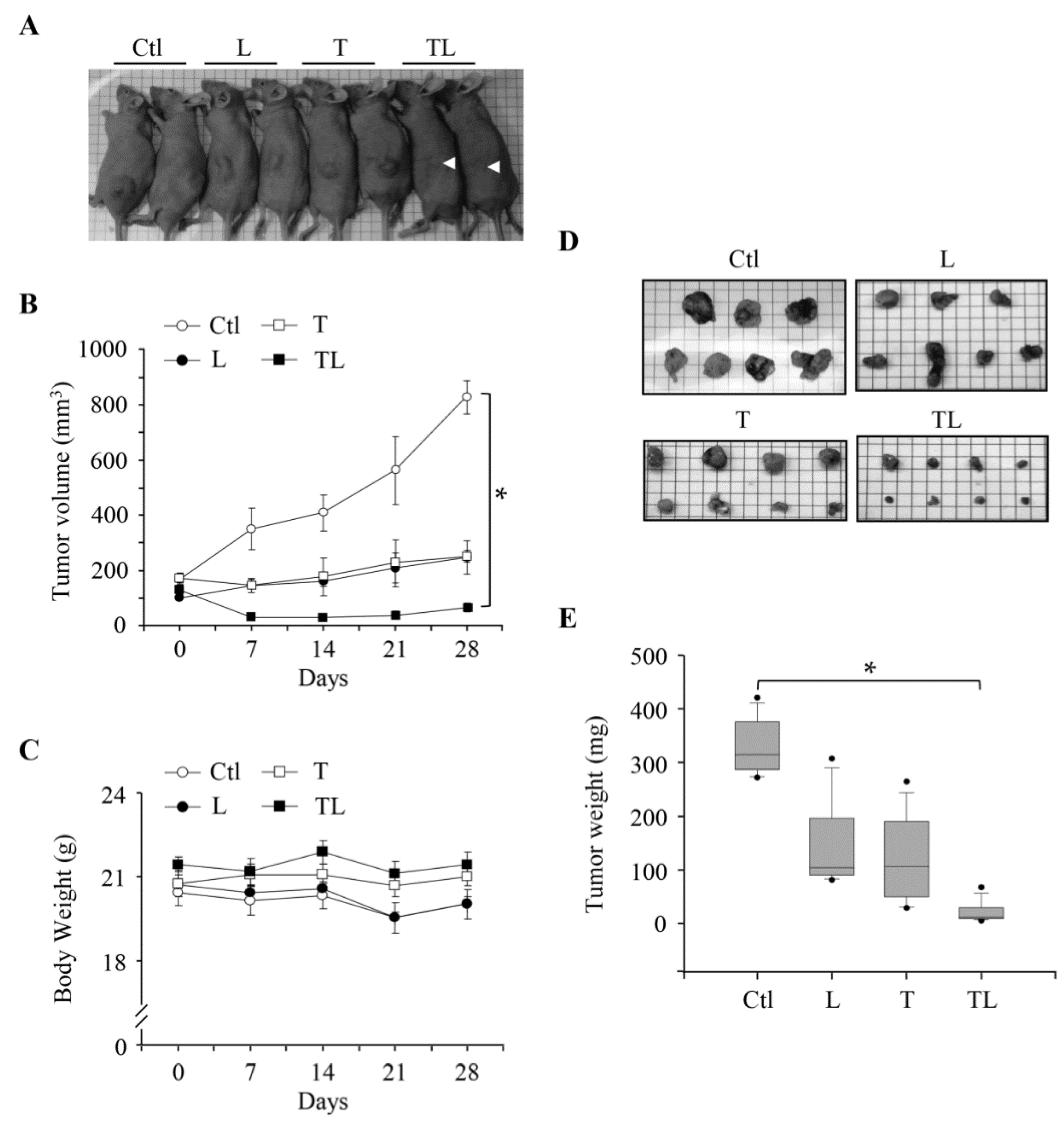
2.4. Combination of Troglitazone and Lovastatin Leads to Tumor Regression in a Mouse Xenograft Model
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents, and Antibodies
4.2. Cell Culture
4.3. MTT Assay
4.4. Soft Agar Colony Formation Assay
4.5. Flow Cytometry Analysis
4.6. Immunoblotting Analysis
4.7. Animal Experiments: Human Tumor Xenograft Models
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Untch, B.R.; Olson, J.A., Jr. Anaplastic thyroid carcinoma, thyroid lymphoma, and metastasis to thyroid. Surg. Oncol. Clin. N. Am. 2006, 15, 661–679. [Google Scholar] [CrossRef] [PubMed]
- McIver, B.; Hay, I.D.; Giuffrida, D.F.; Dvorak, C.E.; Grant, C.S.; Thompson, G.B.; van Heerden, J.A.; Goellner, J.R. Anaplastic thyroid carcinoma: A 50-year experience at a single institution. Surgery 2001, 130, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Mohebati, A.; Dilorenzo, M.; Palmer, F.; Patel, S.G.; Pfister, D.; Lee, N.; Tuttle, R.M.; Shaha, A.R.; Shah, J.P.; Ganly, I. Anaplastic thyroid carcinoma: A 25-year single-institution experience. Ann. Surg. Oncol. 2014, 21, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Sugitani, I.; Hasegawa, Y.; Sugasawa, M.; Tori, M.; Higashiyama, T.; Miyazaki, M.; Hosoi, H.; Orita, Y.; Kitano, H. Super-radical surgery for anaplastic thyroid carcinoma: A large cohort study using the anaplastic thyroid carcinoma research consortium of japan database. Head Neck 2014, 36, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Prasongsook, N.; Kumar, A.; Chintakuntlawar, A.V.; Foote, R.L.; Kasperbauer, J.; Molina, J.; Garces, Y.; Ma, D.; Neben Wittich, M.A.; Rubin, J.; et al. Survival in response to multimodal therapy in anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 2017, 102, 4506–4514. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Sashidhara, K.V. Lipid lowering agents of natural origin: An account of some promising chemotypes. Eur. J. Med. Chem. 2017, 140, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Cannon, C.P. The potential relevance of the multiple lipid-independent (pleiotropic) effects of statins in the management of acute coronary syndromes. J. Am. Coll. Cardiol. 2005, 46, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Buemi, M.; Senatore, M.; Corica, F.; Aloisi, C.; Romeo, A.; Cavallaro, E.; Floccari, F.; Tramontana, D.; Frisina, N. Statins and progressive renal disease. Med. Res. Rev. 2002, 22, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Yousaf, M.; Malecek, M.K.; Carneiro, B.; Chandra, S.; Kaplan, J.; Kalyan, A.; Sassano, A.; Platanias, L.C.; Giles, F. Statins as anti-cancer therapy; can we translate preclinical and epidemiologic data into clinical benefit? Discov. Med. 2015, 20, 413–427. [Google Scholar] [PubMed]
- Hindler, K.; Cleeland, C.S.; Rivera, E.; Collard, C.D. The role of statins in cancer therapy. Oncologist 2006, 11, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Mullen, P.J.; Yu, R.; Longo, J.; Archer, M.C.; Penn, L.Z. The interplay between cell signalling and the mevalonate pathway in cancer. Nat. Rev. Cancer 2016, 16, 718–731. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wakelee, H.A.; Aragaki, A.K.; Tang, J.Y.; Kurian, A.W.; Manson, J.E.; Stefanick, M.L. Protective effects of statins in cancer: Should they be prescribed for high-risk patients? Curr. Atheroscler. Rep. 2016, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Gorniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Janani, C.; Ranjitha Kumari, B.D. PPAR gamma gene—A review. Diabetes Metab. Syndr. 2015, 9, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Mughal, A.; Kumar, D.; Vikram, A. Effects of thiazolidinediones on metabolism and cancer: Relative influence of PPARγ and IGF-1 signaling. Eur. J. Pharmacol. 2015, 768, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, E.; Wahl, R. Chemotherapy and chemoprevention by thiazolidinediones. BioMed Res. Int. 2015, 2015, 845340. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Zhong, W.B.; Chang, T.C.; Lai, S.M.; Tsai, Y.F. Lovastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, induces apoptosis and differentiation in human anaplastic thyroid carcinoma cells. J. Clin. Endocrinol. Metab. 2003, 88, 3021–3026. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.B.; Hsu, S.P.; Ho, P.Y.; Liang, Y.C.; Chang, T.C.; Lee, W.S. Lovastatin inhibits proliferation of anaplastic thyroid cancer cells through up-regulation of p27 by interfering with the Rho/ROCK-mediated pathway. Biochem. Pharmacol. 2011, 82, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.B.; Liang, Y.C.; Wang, C.Y.; Chang, T.C.; Lee, W.S. Lovastatin suppresses invasiveness of anaplastic thyroid cancer cells by inhibiting Rho geranylgeranylation and RhoA/ROCK signaling. Endocr. Relat. Cancer 2005, 12, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.B.; Wang, C.Y.; Chang, T.C.; Lee, W.S. Lovastatin induces apoptosis of anaplastic thyroid cancer cells via inhibition of protein geranylgeranylation and de novo protein synthesis. Endocrinology 2003, 144, 3852–3859. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.J.; Crist, H.S.; Durvesh, S.; Bruggeman, R.D.; Goldenberg, D. A comparative study of cell cycle mediator protein expression patterns in anaplastic and papillary thyroid carcinoma. Cancer Biol. Ther. 2012, 13, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kobayashi, T.; Takeda, T.; Komoike, Y.; Wakasugi, E.; Tamaki, Y.; Tsujimoto, M.; Matsuura, N.; Monden, M. Expression of p21 (WAF1/CIP1) protein in clinical thyroid tissues. Br. J. Cancer 1996, 74, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Erickson, L.A.; Jin, L.; Wollan, P.C.; Thompson, G.B.; van Heerden, J.; Lloyd, R.V. Expression of p27kip1 and Ki-67 in benign and malignant thyroid tumors. Mod. Pathol. 1998, 11, 169–174. [Google Scholar] [PubMed]
- Onda, M.; Nagai, H.; Yoshida, A.; Miyamoto, S.; Asaka, S.; Akaishi, J.; Takatsu, K.; Nagahama, M.; Ito, K.; Shimizu, K.; et al. Up-regulation of transcriptional factor E2F1 in papillary and anaplastic thyroid cancers. J. Hum. Genet. 2004, 49, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Tiedje, V.; Stuschke, M.; Weber, F.; Dralle, H.; Moss, L.; Fuhrer, D. Anaplastic thyroid carcinoma: Review of treatment protocols. Endocr. Relat. Cancer 2018, 25, R153–R161. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, M.G.; Ozyer, T.; Polat, F.; Alhajj, R. Realizing drug repositioning by adapting a recommendation system to handle the process. BMC Bioinform. 2018, 19, 136. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J.; Esserman, L.J.; Zhou, Y.; Shoemaker, M.; Lobo, M.; Borman, E.; Baehner, F.; Kumar, A.S.; Adduci, K.; Marx, C.; et al. Breast cancer growth prevention by statins. Cancer Res. 2006, 66, 8707–8714. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.W.L.; Tan, M.M.; Xia, Z.L.; Dimitroulakos, J.; Minden, M.D.; Penn, L.Z. Cerivastatin triggers tumor-specific apoptosis with higher efficacy than lovastatin. Clin. Cancer Res. 2001, 7, 2067–2075. [Google Scholar] [PubMed]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S.; Rawat, R. Peroxisome proliferator-activated receptors (PPARs) and PPAR agonists: The ‘future’ in dermatology therapeutics? Arch. Dermatol. Res. 2015, 307, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Robbins, G.T.; Nie, D. PPARγ, bioactive lipids, and cancer progression. Front. Biosci. 2012, 17, 1816–1834. [Google Scholar] [CrossRef]
- Palakurthi, S.S.; Aktas, H.; Grubissich, L.M.; Mortensen, R.M.; Halperin, J.A. Anticancer effects of thiazolidinediones are independent of peroxisome proliferator-activated receptor γ and medicated by inhibition of translation initiation. Cancer Res. 2001, 61, 6213–6218. [Google Scholar] [PubMed]
- Panigrahy, D.; Singer, S.; Shen, L.Q.; Butterfield, C.E.; Freedman, D.A.; Chen, E.J.; Moses, M.A.; Kilroy, S.; Duensing, S.; Fletcher, C.; et al. PPARγ ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesis. J. Clin. Investig. 2002, 110, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Wakino, S.; Hayashi, K.; Kanda, T.; Tatematsu, S.; Homma, K.; Yoshioka, K.; Takamatsu, I.; Saruta, T. Peroxisome proliferator-activated receptor γ ligands inhibit Rho/Rho kinase pathway by inducing protein tyrosine phosphatase SHP-2. Circ. Res. 2004, 95, e45–e55. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.J.; Lai, G.M.; Chan, C.F.; Cheng, A.L.; Yang, Y.Y.; Chuang, S.E. Dramatic synergistic anticancer effect of clinically achievable doses of lovastatin and troglitazone. Int. J. Cancer 2006, 118, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.H.; Hsu, S.P.; Zhong, W.B.; Liang, Y.C. Involvement of cysteine-rich protein 61 in the epidermal growth factor-induced migration of human anaplastic thyroid cancer cells. Mol. Carcinog. 2016, 55, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.H.; Hsu, S.P.; Zhong, W.B.; Liang, Y.C. Correction: Combined treatment with troglitazone and lovastatin inhibited epidermal growth factor-induced migration through the downregulation of cysteine-rich protein 61 in human anaplastic thyroid cancer cells. PLoS ONE 2017, 12, e0177545. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, M.; Sandberg, C.; Nygren, P.; Larsson, R. Effects of lovastatin on a human myeloma cell line: Increased sensitivity of a multidrug-resistant subline that expresses the 170 kDa P-glycoprotein. Anticancer Drugs 1994, 5, 598–600. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.; Tomlinson, B. Targeting the abcg2-overexpressing multidrug resistant (MDR) cancer cells by PPARγ agonists. Br. J. Pharmacol. 2013, 170, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Shui, H.A.; Chang, T.C. In vivo evidence of duality effects for lovastatin in a nude mouse cancer model. Int. J. Cancer 2010, 126, 578–582. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, W.-B.; Tsai, Y.-C.; Chin, L.-H.; Tseng, J.-H.; Tang, L.-W.; Horng, S.; Fan, Y.-C.; Hsu, S.-P. A Synergistic Anti-Cancer Effect of Troglitazone and Lovastatin in a Human Anaplastic Thyroid Cancer Cell Line and in a Mouse Xenograft Model. Int. J. Mol. Sci. 2018, 19, 1834. https://doi.org/10.3390/ijms19071834
Zhong W-B, Tsai Y-C, Chin L-H, Tseng J-H, Tang L-W, Horng S, Fan Y-C, Hsu S-P. A Synergistic Anti-Cancer Effect of Troglitazone and Lovastatin in a Human Anaplastic Thyroid Cancer Cell Line and in a Mouse Xenograft Model. International Journal of Molecular Sciences. 2018; 19(7):1834. https://doi.org/10.3390/ijms19071834
Chicago/Turabian StyleZhong, Wen-Bin, Yuan-Chin Tsai, Li-Han Chin, Jen-Ho Tseng, Li-Wen Tang, Steve Horng, Yu-Ching Fan, and Sung-Po Hsu. 2018. "A Synergistic Anti-Cancer Effect of Troglitazone and Lovastatin in a Human Anaplastic Thyroid Cancer Cell Line and in a Mouse Xenograft Model" International Journal of Molecular Sciences 19, no. 7: 1834. https://doi.org/10.3390/ijms19071834
APA StyleZhong, W.-B., Tsai, Y.-C., Chin, L.-H., Tseng, J.-H., Tang, L.-W., Horng, S., Fan, Y.-C., & Hsu, S.-P. (2018). A Synergistic Anti-Cancer Effect of Troglitazone and Lovastatin in a Human Anaplastic Thyroid Cancer Cell Line and in a Mouse Xenograft Model. International Journal of Molecular Sciences, 19(7), 1834. https://doi.org/10.3390/ijms19071834