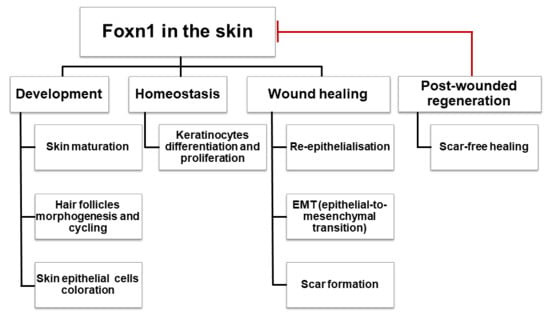
Foxn1 in Skin Development, Homeostasis and Wound Healing
Abstract
:1. Introduction
2. Foxn1 in Skin Development
2.1. Foxn1 over Evolution Time
2.2. Foxn1 in the Development of Skin Tissue and Skin Appendages
2.2.1. Foxn1 during Embryonic Development in Mice
2.2.2. Foxn1 during Postnatal Development
2.2.3. Foxn1 in Hair Follicle Morphogenesis
2.2.4. Foxn1 in Skin and Hair Pigmentation
2.2.5. Foxn1 Deficient (Nude) Mice
2.2.6. Foxn1 Deficient Human Nude/SCID Phenotype
2.2.7. Foxn1 Transgenic Animal Models
3. Foxn1 in Mature Skin Homeostasis
3.1. Foxn1 Localisation and Expression
3.2. The Role of Foxn1 in Epidermis: Proliferation, Differentiation and Apoptosis
3.3. Age Related Foxn1 Modulation
3.4. Foxn1 Regulation
3.5. Foxn1 and Dermal Compartment of the Skin
4. Foxn1 in Skin Wound Healing
4.1. Scar-Less Skin Wound Healing in Foxn1 Deficient (Nude) Mice
4.2. Foxn1 in Reparative (Scar-Forming) Skin Wound Healing
4.3. Foxn1 as a Transcriptional Switch between Scar-Free and Scar-Forming Skin Healing
4.4. Foxn1 among Transcription Factors and Signalling Pathways in the Skin Healing Process
5. Conclusions and Future Directions
Author Contributions:
Funding
Conflicts of Interest
References
- Slominski, A.T.; Manna, P.R.; Tuckey, R.C. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids 2015, 103, 72–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell. Biol. 2012, 212, 1–115. [Google Scholar]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Bellavia, G.; Fasanaro, P.; Melchionna, R.; Capogrossi, M.C.; Napolitano, M. Transcriptional control of skin reepithelialization. J. Dermatol. Sci. 2014, 73, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Teng, A.; Nair, M.; Wells, J.; Segre, J.A.; Dai, X. Strain-dependent perinatal lethality of Ovol1-deficient mice and identification of Ovol2 as a downstream target of Ovol1 in skin epidermis. Biochim. Biophys. Acta 2007, 1772, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.; Villarreal-Ponce, A.; Fallahi, M.; Ovadia, J.; Sun, P.; Yu, Q.C.; Ito, S.; Sinha, S.; Nie, Q.; Dai, X. Transcriptional mechanisms link epithelial plasticity to adhesion and differentiation of epidermal progenitor cells. Dev. Cell 2014, 29, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Mori, R.; Tanaka, K.; de Kerckhove, M.; Okamoto, M.; Kashiyama, K.; Kim, S.; Kawata, T.; Komatsu, T.; Park, S.; Ikematsu, K.; et al. Reduced FOXO1 expression accelerates skin wound healing and attenuates scarring. Am. J. Pathol. 2014, 184, 2465–2479. [Google Scholar] [CrossRef] [PubMed]
- Donati, G.; Proserpio, V.; Lichtenberger, B.M.; Natsuga, K.; Sinclair, R.; Fujiwara, H.; Watt, F.M. Epidermal Wnt/beta-catenin signaling regulates adipocyte differentiation via secretion of adipogenic factors. Proc. Natl. Acad. Sci. USA 2014, 111, E1501–E1509. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Werner, S. Transcriptional control of wound repair. Annu. Rev. Cell Dev. Biol. 2007, 23, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberger, B.M.; Mastrogiannaki, M.; Watt, F.M. Epidermal beta-catenin activation remodels the dermis via paracrine signalling to distinct fibroblast lineages. Nat. Commun. 2016, 7, 10537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbeaux, T.; Hess, I.; Swann, J.B.; Kanzler, B.; Haas-Assenbaum, A.; Boehm, T. Thymopoiesis in mice depends on a Foxn1-positive thymic epithelial cell lineage. Proc. Natl. Acad. Sci. USA 2010, 107, 16613–16618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehm, T.; Swann, J.B. Thymus involution and regeneration: Two sides of the same coin? Nat. Rev. Immunol. 2013, 13, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Brissette, J.L.; Li, J.; Kamimura, J.; Lee, D.; Dotto, G.P. The product of the mouse nude locus, WHN, regulates the balance between epithelial cell growth and differentiation. Genes Dev. 1996, 10, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Prowse, D.M.; Lee, D.; Weiner, L.; Jiang, N.; Magro, C.M.; Baden, H.P.; Brissette, J.L. Ectopic expression of the nude gene induces hyperproliferation and defects in differentiation: Implications for the self-renewal of cutaneous epithelia. Dev. Biol. 1999, 212, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Schlake, T. The nude gene and the skin. Exp. Dermatol. 2001, 10, 293–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawronska-Kozak, B.; Grabowska, A.; Kur-Piotrowska, A.; Kopcewicz, M. Foxn1 transcription factor regulates wound healing of skin through promoting epithelial-mesenchymal transition. PLoS ONE 2016, 11, e0150635. [Google Scholar] [CrossRef] [PubMed]
- Bredenkamp, N.; Nowell, C.S.; Blackburn, C.C. Regeneration of the aged thymus by a single transcription factor. Development 2014, 141, 1627–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terszowski, G.; Muller, S.M.; Bleul, C.C.; Blum, C.; Schirmbeck, R.; Reimann, J.; Pasquier, L.D.; Amagai, T.; Boehm, T.; Rodewald, H.R. Evidence for a functional second thymus in mice. Science 2006, 312, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Prowse, D.M.; Brissette, J.L. Association between mouse nude gene expression and the initiation of epithelial terminal differentiation. Dev. Biol. 1999, 208, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.M.; Brissette, J.L. Role of the nude gene in epithelial terminal differentiation. J. Investig. Dermatol. 2002, 118, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Kopcewicz, M.M.; Kur-Piotrowska, A.; Bukowska, J.; Gimble, J.M.; Gawronska-Kozak, B. Foxn1 and mmp-9 expression in intact skin and during excisional wound repair in young, adult, and old c57bl/6 mice. Wound Repair Regen. 2017, 25, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Nehls, M.; Pfeifer, D.; Schorpp, M.; Hedrich, H.; Boehm, T. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature 1994, 372, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Pignata, C.; Fiore, M.; Guzzetta, V.; Castaldo, A.; Sebastio, G.; Porta, F.; Guarino, A. Congenital alopecia and nail dystrophy associated with severe functional t-cell immunodeficiency in two sibs. Am. J. Med. Genet. 1996, 65, 167–170. [Google Scholar] [CrossRef]
- Flanagan, S.P. ‘Nude’, a new hairless gene with pleiotropic effects in the mouse. Genet. Res. 1966, 8, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Mecklenburg, L.; Tychsen, B.; Paus, R. Learning from nudity: Lessons from the nude phenotype. Exp. Dermatol. 2005, 14, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Barbul, A.; Shawe, T.; Rotter, S.M.; Efron, J.E.; Wasserkrug, H.L.; Badawy, S.B. Wound healing in nude mice: A study on the regulatory role of lymphocytes in fibroplasia. Surgery 1989, 105, 764–769. [Google Scholar] [PubMed]
- Schlake, T.; Schorpp, M.; Boehm, T. Formation of regulator/target gene relationships during evolution. Gene 2000, 256, 29–34. [Google Scholar] [CrossRef]
- Schorpp, M.; Hofmann, M.; Dear, T.N.; Boehm, T. Characterization of mouse and human nude genes. Immunogenetics 1997, 46, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Schlake, T.; Schorpp, M.; Nehls, M.; Boehm, T. The nude gene encodes a sequence-specific DNA binding protein with homologs in organisms that lack an anticipatory immune system. Proc. Natl. Acad. Sci. USA 1997, 94, 3842–3847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, K.L.; Halay, E.D.; Lai, E.; Burley, S.K. Co-crystal structure of the hnf-3/fork head DNA-recognition motif resembles histone h5. Nature 1993, 364, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.L.; Huang, C.Y.; Chang, C.H.; Sun, Y.J.; Chuang, W.J.; Hsiao, C.D. Crystal structure of the human foxk1a-DNA complex and its implications on the diverse binding specificity of winged helix/forkhead proteins. J. Biol. Chem. 2006, 281, 17400–17409. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Gisselbrecht, S.S.; Rogers, J.M.; Hartl, D.L.; Bulyk, M.L. DNA-binding specificity changes in the evolution of forkhead transcription factors. Proc. Natl. Acad. Sci. USA 2013, 110, 12349–12354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, N.; Dear, T.N.; Boehm, T. Whn and mha3 are components of the genetic hierarchy controlling hair follicle differentiation. Mech. Dev. 1999, 89, 215–221. [Google Scholar] [CrossRef]
- Schlake, T.; Schorpp, M.; Maul-Pavicic, A.; Malashenko, A.M.; Boehm, T. Forkhead/winged-helix transcription factor whn regulates hair keratin gene expression: Molecular analysis of the nude skin phenotype. Dev. Dyn. 2000, 217, 368–376. [Google Scholar] [CrossRef]
- Calautti, E.; Li, J.; Saoncella, S.; Brissette, J.L.; Goetinck, P.F. Phosphoinositide 3-kinase signaling to akt promotes keratinocyte differentiation versus death. J. Biol. Chem. 2005, 280, 32856–32865. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Baxter, R.M.; Weiner, L.; Goetinck, P.F.; Calautti, E.; Brissette, J.L. Foxn1 promotes keratinocyte differentiation by regulating the activity of protein kinase C. Differentiation 2007, 75, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Nehls, M.; Kyewski, B.; Messerle, M.; Waldschutz, R.; Schuddekopf, K.; Smith, A.J.; Boehm, T. Two genetically separable steps in the differentiation of thymic epithelium. Science 1996, 272, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Ihara, S.; Motobayashi, Y.; Nagao, E.; Kistler, A. Ontogenetic transition of wound healing pattern in rat skin occurring at the fetal stage. Development 1990, 110, 671–680. [Google Scholar] [PubMed]
- Colwell, A.S.; Longaker, M.T.; Lorenz, H.P. Mammalian fetal organ regeneration. Adv. Biochem. Eng. Biotechnol. 2005, 93, 83–100. [Google Scholar] [PubMed]
- Colwell, A.S.; Longaker, M.T.; Peter Lorenz, H. Identification of differentially regulated genes in fetal wounds during regenerative repair. Wound Repair Regen. 2008, 16, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Kur-Piotrowska, A.; Kopcewicz, M.; Kozak, L.P.; Sachadyn, P.; Grabowska, A.; Gawronska-Kozak, B. Neotenic phenomenon in gene expression in the skin of Foxn1- deficient (nude) mice—A projection for regenerative skin wound healing. BMC Genom. 2017, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Gawronska-Kozak, B. Scarless skin wound healing in Foxn1 deficient (nude) mice is associated with distinctive matrix metalloproteinase expression. Matrix Biol. 2011, 30, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Gawronska-Kozak, B.; Bogacki, M.; Rim, J.S.; Monroe, W.T.; Manuel, J.A. Scarless skin repair in immunodeficient mice. Wound Repair Regen. 2006, 14, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Kur-Piotrowska, A.; Bukowska, J.; Kopcewicz, M.M.; Dietrich, M.; Nynca, J.; Slowinska, M.; Gawronska-Kozak, B. Foxn1 expression in keratinocytes is stimulated by hypoxia: Further evidence of its role in skin wound healing. Sci. Rep. 2018, 8, 5425. [Google Scholar] [CrossRef] [PubMed]
- Janes, S.M.; Ofstad, T.A.; Campbell, D.H.; Watt, F.M.; Prowse, D.M. Transient activation of FOXN1 in keratinocytes induces a transcriptional programme that promotes terminal differentiation: Contrasting roles of FOXN1 and Akt. J. Cell Sci. 2004, 117, 4157–4168. [Google Scholar] [CrossRef] [PubMed]
- Janes, S.M.; Ofstad, T.A.; Campbell, D.H.; Eddaoudi, A.; Warnes, G.; Davies, D.; Watt, F.M. Pi3-kinase-dependent activation of apoptotic machinery occurs on commitment of epidermal keratinocytes to terminal differentiation. Cell Res. 2009, 19, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Gawronska-Kozak, B.; Kirk-Ballard, H. Cyclosporin a reduces matrix metalloproteinases and collagen expression in dermal fibroblasts from regenerative Foxn1 deficient (nude) mice. Fibrog. Tissue Repair 2013, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Brooks, Y.; Lefort, K.; Getsios, S.; Dotto, G.P. The retinoid-related orphan receptor roralpha promotes keratinocyte differentiation via Foxn1. PLoS ONE 2013, 8, e70392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, E. Scratching the surface of skin development. Nature 2007, 445, 834–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, L.; Fuchs, E. The hair cycle. J. Cell Sci. 2006, 119, 391–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt-Ullrich, R.; Paus, R. Molecular principles of hair follicle induction and morphogenesis. Bioessays 2005, 27, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Legue, E.; Nicolas, J.F. Hair follicle renewal: Organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development 2005, 132, 4143–4154. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Segre, J.A. Transcriptional control of epidermal specification and differentiation. Curr. Opin. Genet. Dev. 2004, 14, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.; Han, R.; Scicchitano, B.M.; Li, J.; Hasegawa, K.; Grossi, M.; Lee, D.; Brissette, J.L. Dedicated epithelial recipient cells determine pigmentation patterns. Cell 2007, 130, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Li, F.; Meng, Q.; Zhao, Y.; Chen, L.; Zhang, H.; Xue, L.; Zhang, X.; Lengner, C.; Yu, Z. Post-transcriptional regulation of keratinocyte progenitor cell expansion, differentiation and hair follicle regression by mir-22. PLoS Genet. 2015, 11, e1005253. [Google Scholar] [CrossRef] [PubMed]
- DasGupta, R.; Fuchs, E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 1999, 126, 4557–4568. [Google Scholar] [PubMed]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. Beta-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar] [CrossRef]
- Zhang, Y.; Andl, T.; Yang, S.H.; Teta, M.; Liu, F.; Seykora, J.T.; Tobias, J.W.; Piccolo, S.; Schmidt-Ullrich, R.; Nagy, A.; et al. Activation of beta-catenin signaling programs embryonic epidermis to hair follicle fate. Development 2008, 135, 2161–2172. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Lefort, K.; Qiu, W.; Nguyen, B.C.; Rajaram, R.D.; Castillo, E.; He, F.; Chen, Y.; Angel, P.; Brisken, C.; et al. Control of hair follicle cell fate by underlying mesenchyme through a CSL-Wnt5a-Foxn1 regulatory axis. Genes Dev. 2010, 24, 1519–1532. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ichinohe, M.; Hirata, M.; Matsuura, H.; Fujiwara, T.; Igarashi, T.; Nakahara, M.; Yamaguchi, H.; Yasugi, S.; Takenawa, T.; et al. Phospholipase c-delta1 is an essential molecule downstream of Foxn1, the gene responsible for the nude mutation, in normal hair development. FASEB J. 2008, 22, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Johns, S.A.; Soullier, S.; Rashbass, P.; Cunliffe, V.T. Foxn1 is required for tissue assembly and desmosomal cadherin expression in the hair shaft. Dev. Dyn. 2005, 232, 1062–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirobe, T. Keratinocytes regulate the function of melanocytes. Dermatol. Sin. 2014, 32, 200–204. [Google Scholar] [CrossRef]
- Pla, P.; Solov’eva, O.; Moore, R.; Alberti, C.; Kunisada, T.; Larue, L. Dct::Lacz es cells: A novel cellular model to study melanocyte determination and differentiation. Pigment Cell Res. 2004, 17, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bossche, K.; Naeyaert, J.M.; Lambert, J. The quest for the mechanism of melanin transfer. Traffic 2006, 7, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, G.; Ceccarelli, S.; Kovacs, D.; Aspite, N.; Lotti, L.V.; Torrisi, M.R.; Picardo, M. Keratinocyte growth factor promotes melanosome transfer to keratinocytes. J. Investig. Dermatol. 2005, 125, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hammer, J.A. Melanosome transfer: It is best to give and receive. Curr. Opin. Cell Biol. 2014, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Segre, J.A.; Nemhauser, J.L.; Taylor, B.A.; Nadeau, J.H.; Lander, E.S. Positional cloning of the nude locus: Genetic, physical, and transcription maps of the region and mutations in the mouse and rat. Genomics 1995, 28, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Balciunaite, G.; Keller, M.P.; Balciunaite, E.; Piali, L.; Zuklys, S.; Mathieu, Y.D.; Gill, J.; Boyd, R.; Sussman, D.J.; Hollander, G.A. Wnt glycoproteins regulate the expression of Foxn1, the gene defective in nude mice. Nat. Immunol. 2002, 3, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Eaton, G.J. Hair growth cycles and wave patterns in “nude” mice. Transplantation 1976, 22, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Pignata, C.; Panteleyev, A.A.; Prowse, D.M.; Baden, H.; Weiner, L.; Gaetaniello, L.; Ahmad, W.; Pozzi, N.; Cserhalmi-Friedman, P.B.; et al. Exposing the human nude phenotype. Nature 1999, 398, 473–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mecklenburg, L.; Paus, R.; Halata, Z.; Bechtold, L.S.; Fleckman, P.; Sundberg, J.P. Foxn1 is critical for onycholemmal terminal differentiation in nude (Foxn1) mice. J. Investig. Dermatol. 2004, 123, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Amorosi, S.; D’Armiento, M.; Calcagno, G.; Russo, I.; Adriani, M.; Christiano, A.M.; Weiner, L.; Brissette, J.L.; Pignata, C. Foxn1 homozygous mutation associated with anencephaly and severe neural tube defect in human athymic nude/scid fetus. Clin. Genet. 2008, 73, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Radha Rama Devi, A.; Panday, N.N.; Naushad, S.M. Foxn1 Italian founder mutation in Indian family: Implications in prenatal diagnosis. Gene 2017, 627, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Potter, C.S.; Pruett, N.D.; Kern, M.J.; Baybo, M.A.; Godwin, A.R.; Potter, K.A.; Peterson, R.L.; Sundberg, J.P.; Awgulewitsch, A. The nude mutant gene Foxn1 is a hoxc13 regulatory target during hair follicle and nail differentiation. J. Investig. Dermatol. 2011, 131, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Mangan, S.; Alon, U. Structure and function of the feed-forward loop network motif. Proc. Natl. Acad. Sci. USA 2003, 100, 11980–11985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohr, S.; Patel, S.J.; Vasko, R.; Shen, K.; Huang, G.; Yarmush, M.L.; Berthiaume, F. Highly upregulated lhx2 in the Foxn1−/− nude mouse phenotype reflects a dysregulated and expanded epidermal stem cell niche. PLoS ONE 2013, 8, e64223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, J.; Lee, J.; Kopan, R.; Ma, L. Genetic interplays between Msx2 and Foxn1 are required for notch1 expression and hair shaft differentiation. Dev. Biol. 2009, 326, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Vigliano, I.; Gorrese, M.; Fusco, A.; Vitiello, L.; Amorosi, S.; Panico, L.; Ursini, M.V.; Calcagno, G.; Racioppi, L.; Del Vecchio, L.; et al. Foxn1 mutation abrogates prenatal t-cell development in humans. J. Med. Genet. 2011, 48, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Coolen, N.A.; Schouten, K.C.; Middelkoop, E.; Ulrich, M.M. Comparison between human fetal and adult skin. Arch. Dermatol. Res. 2010, 302, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quigley, D.A.; Kandyba, E.; Huang, P.; Halliwill, K.D.; Sjolund, J.; Pelorosso, F.; Wong, C.E.; Hirst, G.L.; Wu, D.; Delrosario, R.; et al. Gene expression architecture of mouse dorsal and tail skin reveals functional differences in inflammation and cancer. Cell Rep. 2016, 16, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mirza, R.; Kwon, Y.; DiPietro, L.A.; Koh, T.J. The murine excisional wound model: Contraction revisited. Wound Repair Regen. 2015, 23, 874–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adriani, M.; Martinez-Mir, A.; Fusco, F.; Busiello, R.; Frank, J.; Telese, S.; Matrecano, E.; Ursini, M.V.; Christiano, A.M.; Pignata, C. Ancestral founder mutation of the nude (Foxn1) gene in congenital severe combined immunodeficiency associated with alopecia in southern Italy population. Ann. Hum. Genet. 2004, 68, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Zhang, Z.; Mu, L.; Burnley, P.; Wang, L.; Coder, B.; Zhuge, Q.; Su, D.M. Biological significance of Foxn1 gain-of-function mutations during t and b lymphopoiesis in juvenile mice. Cell Death Dis. 2014, 5, e1457. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Li, S.; Liu, C.; Bari, A.S.; Longaker, M.T.; Lorenz, H.P. Epithelial-mesenchymal transition occurs after epidermal development in mouse skin. Exp. Cell Res. 2006, 312, 3959–3968. [Google Scholar] [CrossRef] [PubMed]
- Wondimu, A.; Weir, L.; Robertson, D.; Mezentsev, A.; Kalachikov, S.; Panteleyev, A.A. Loss of ARNT (Hif1beta) in mouse epidermis triggers dermal angiogenesis, blood vessel dilation and clotting defects. Lab. Investig. 2012, 92, 110–124. [Google Scholar] [CrossRef] [PubMed]
- El Ghalbzouri, A.; Ponec, M. Diffusible factors released by fibroblasts support epidermal morphogenesis and deposition of basement membrane components. Wound Repair Regen. 2004, 12, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Maas-Szabowski, N.; Shimotoyodome, A.; Fusenig, N.E. Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J. Cell Sci. 1999, 112 Pt 12, 1843–1853. [Google Scholar] [PubMed]
- Bukowska, J.; Kopcewicz, M.; Kur-Piotrowska, A.; Szostek-Mioduchowska, A.Z.; Walendzik, K.; Gawronska-Kozak, B. Effect of TGFbeta1, TGFbeta3 and keratinocyte conditioned media on functional characteristics of dermal fibroblasts derived from reparative (Balb/c) and regenerative (Foxn1 deficient; nude) mouse models. Cell Tissue Res. 2018, 373, 1–15. [Google Scholar]
- Lanzini, J.; Dargere, D.; Regazzetti, A.; Tebani, A.; Laprevote, O.; Auzeil, N. Changing in lipid profile induced by the mutation of Foxn1 gene: A lipidomic analysis of nude mice skin. Biochimie 2015, 118, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Stemmer, K.; Kotzbeck, P.; Zani, F.; Bauer, M.; Neff, C.; Muller, T.D.; Pfluger, P.T.; Seeley, R.J.; Divanovic, S. Thermoneutral housing is a critical factor for immune function and diet-induced obesity in c57bl/6 nude mice. Int. J. Obes. 2015, 39, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Suzuki, M.; Ishii, R.; Satow, R.; Uchida, T.; Kitazumi, T.; Sasaki, T.; Kitamura, T.; Yamaguchi, H.; Nakamura, Y.; et al. Genetic defect in phospholipase cdelta1 protects mice from obesity by regulating thermogenesis and adipogenesis. Diabetes 2011, 60, 1926–1937. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.A.; Horsley, V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development 2013, 140, 1517–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera-Gonzalez, G.; Shook, B.; Horsley, V. Adipocytes in skin health and disease. Cold Spring Harb. Perspect. Med. 2014, 4, a015271. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.M.; Kasza, I.; Yen, C.L.; Reeder, S.B.; Hernando, D.; Gallo, R.L.; Jahoda, C.A.; Horsley, V.; MacDougald, O.A. Dermal white adipose tissue: A new component of the thermogenic response. J. Lipid Res. 2015, 56, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Kruglikov, I.L.; Scherer, P.E. Dermal adipocytes: From irrelevance to metabolic targets? Trends Endocrinol. Metab. 2016, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiannaki, M.; Lichtenberger, B.M.; Reimer, A.; Collins, C.A.; Driskell, R.R.; Watt, F.M. Beta-catenin stabilization in skin fibroblasts causes fibrotic lesions by preventing adipocyte differentiation of the reticular dermis. J. Investig. Dermatol. 2016, 136, 1130–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.E.; Parks, W.C. Metalloproteinases and their inhibitors: Regulators of wound healing. Int. J. Biochem. Cell Biol. 2008, 40, 1334–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, L.D.; Clark, R.K.; Heber-Katz, E. A new murine model for mammalian wound repair and regeneration. Clin. Immunol. Immunopathol. 1998, 88, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Goss, R.J.; Grimes, L.N. Epidermal downgrowths in regenerating rabbit ear holes. J. Morphol. 1975, 146, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Heydemann, A. The super super-healing MRL mouse strain. Front. Biol. 2012, 7, 522–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawronska-Kozak, B. Regeneration in the ears of immunodeficient mice: Identification and lineage analysis of mesenchymal stem cells. Tissue Eng. 2004, 10, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Manuel, J.A.; Gawronska-Kozak, B. Matrix metalloproteinase 9 (MMP-9) is upregulated during scarless wound healing in athymic nude mice. Matrix Biol. 2006, 25, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Gawronska-Kozak, B.; Grabowska, A.; Kopcewicz, M.; Kur, A. Animal models of skin regeneration. Reprod. Biol. 2014, 14, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.V.; Gardiner, D.M.; Carlson, M.R.; Nugas, C.A.; Bryant, S.V. Expression of MMP-9 and related matrix metalloproteinase genes during axolotl limb regeneration. Dev. Dyn. 1999, 216, 2–9. [Google Scholar] [CrossRef]
- Kato, T.; Miyazaki, K.; Shimizu-Nishikawa, K.; Koshiba, K.; Obara, M.; Mishima, H.K.; Yoshizato, K. Unique expression patterns of matrix metalloproteinases in regenerating newt limbs. Dev. Dyn. 2003, 226, 366–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wawersik, M.; Coulombe, P.A. Forced expression of keratin 16 alters the adhesion, differentiation, and migration of mouse skin keratinocytes. Mol. Biol. Cell 2000, 11, 3315–3327. [Google Scholar] [CrossRef] [PubMed]
- Iwano, M.; Plieth, D.; Danoff, T.M.; Xue, C.; Okada, H.; Neilson, E.G. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Investig. 2002, 110, 341–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longaker, M.T.; Whitby, D.J.; Adzick, N.S.; Crombleholme, T.M.; Langer, J.C.; Duncan, B.W.; Bradley, S.M.; Stern, R.; Ferguson, M.W.; Harrison, M.R. Studies in fetal wound healing, vi. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J. Pediatr. Surg. 1990, 25, 63–68. [Google Scholar] [CrossRef]
- Lorenz, H.P.; Adzick, N.S. Scarless skin wound repair in the fetus. West. J. Med. 1993, 159, 350–355. [Google Scholar] [PubMed]
- Sousounis, K.; Michel, C.S.; Bruckskotten, M.; Maki, N.; Borchardt, T.; Braun, T.; Looso, M.; Tsonis, P.A. A microarray analysis of gene expression patterns during early phases of newt lens regeneration. Mol. Vis. 2013, 19, 135–145. [Google Scholar] [PubMed]
- Gourevitch, D.; Kossenkov, A.V.; Zhang, Y.; Clark, L.; Chang, C.; Showe, L.C.; Heber-Katz, E. Inflammation and its correlates in regenerative wound healing: An alternate perspective. Adv. Wound Care 2014, 3, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Leferovich, J.; Zhang, X.M.; Bedelbaeva, K.; Gourevitch, D.; Hatcher, C.J.; Basson, C.T.; Heber-Katz, E.; Marx, K.A. Keratin gene expression profiles after digit amputation in c57bl/6 vs. Regenerative mrl mice imply an early regenerative keratinocyte activated-like state. Physiol. Genom. 2013, 45, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Stelnicki, E.J.; Arbeit, J.; Cass, D.L.; Saner, C.; Harrison, M.; Largman, C. Modulation of the human homeobox genes prx-2 and hoxb13 in scarless fetal wounds. J. Investig. Dermatol. 1998, 111, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Mack, J.A.; Abramson, S.R.; Ben, Y.; Coffin, J.C.; Rothrock, J.K.; Maytin, E.V.; Hascall, V.C.; Largman, C.; Stelnicki, E.J. Hoxb13 knockout adult skin exhibits high levels of hyaluronan and enhanced wound healing. FASEB J. 2003, 17, 1352–1354. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.; Brunet, A.; Grenier, J.M.; Datta, S.R.; Fornace, A.J., Jr.; DiStefano, P.S.; Chiang, L.W.; Greenberg, M.E. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the gadd45 protein. Science 2002, 296, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef]
- Brunet, A.; Park, J.; Tran, H.; Hu, L.S.; Hemmings, B.A.; Greenberg, M.E. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol. Cell Biol. 2001, 21, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Van der Horst, A.; Burgering, B.M. Stressing the role of FOXO proteins in lifespan and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Nakae, J.; Kitamura, T.; Kitamura, Y.; Biggs, W.H., III; Arden, K.C.; Accili, D. The forkhead transcription factor FOXO1 regulates adipocyte differentiation. Dev. Cell 2003, 4, 119–129. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Nakamura, N.; Sansal, I.; Bergeron, L.; Sellers, W.R. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell 2002, 2, 81–91. [Google Scholar] [CrossRef]
- Puigserver, P.; Rhee, J.; Donovan, J.; Walkey, C.J.; Yoon, J.C.; Oriente, F.; Kitamura, Y.; Altomonte, J.; Dong, H.; Accili, D.; et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-pgc-1alpha interaction. Nature 2003, 423, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Tia, N.; Singh, A.K.; Pandey, P.; Azad, C.S.; Chaudhary, P.; Gambhir, I.S. Role of forkhead box o (FOXO) transcription factor in aging and diseases. Gene 2018, 648, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Roupe, K.M.; Veerla, S.; Olson, J.; Stone, E.L.; Sorensen, O.E.; Hedrick, S.M.; Nizet, V. Transcription factor binding site analysis identifies FOXO transcription factors as regulators of the cutaneous wound healing process. PLoS ONE 2014, 9, e89274. [Google Scholar] [CrossRef] [PubMed]
- Ponugoti, B.; Xu, F.; Zhang, C.; Tian, C.; Pacios, S.; Graves, D.T. Foxo1 promotes wound healing through the up-regulation of tgf-beta1 and prevention of oxidative stress. J. Cell Biol. 2013, 203, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Reginelli, A.D.; Wang, Y.Q.; Sassoon, D.; Muneoka, K. Digit tip regeneration correlates with regions of msx1 (hox 7) expression in fetal and newborn mice. Development 1995, 121, 1065–1076. [Google Scholar] [PubMed]
- Stelnicki, E.J.; Komuves, L.G.; Holmes, D.; Clavin, W.; Harrison, M.R.; Adzick, N.S.; Largman, C. The human homeobox genes MSX-1, MSX-2, and MOX-1 are differentially expressed in the dermis and epidermis in fetal and adult skin. Differentiation 1997, 62, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.; Green, L.M.; Jiang, T.X.; Plikus, M.; Huang, E.; Chang, R.N.; Hughes, M.W.; Chuong, C.M.; Tuan, T.L. Accelerated closure of skin wounds in mice deficient in the homeobox gene MSX2. Wound Repair Regen. 2009, 17, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, J.; Wu, T.; Plikus, M.; Jiang, T.X.; Bi, Q.; Liu, Y.H.; Muller-Rover, S.; Peters, H.; Sundberg, J.P.; et al. ‘Cyclic alopecia’ in MSX2 mutants: Defects in hair cycling and hair shaft differentiation. Development 2003, 130, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Itasaki, N.; Hoppler, S. Crosstalk between Wnt and bone morphogenic protein signaling: A turbulent relationship. Dev. Dyn. 2010, 239, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Bastakoty, D.; Young, P.P. Wnt/beta-catenin pathway in tissue injury: Roles in pathology and therapeutic opportunities for regeneration. FASEB J. 2016, 30, 3271–3284. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.L.; Smith, A.A.; Helms, J.A. Wnt signaling and injury repair. Cold Spring Harb. Perspect. Biol. 2012, 4, a008078. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Rodriguez Esteban, C.; Raya, M.; Kawakami, H.; Marti, M.; Dubova, I.; Izpisua Belmonte, J.C. Wnt/beta-catenin signaling regulates vertebrate limb regeneration. Genes Dev. 2006, 20, 3232–3237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, X.; Tan, S.H.; Koh, W.L.; Chau, R.M.; Yan, K.S.; Kuo, C.J.; van Amerongen, R.; Klein, A.M.; Nusse, R. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science 2013, 342, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Yang, Z.; Andl, T.; Cui, C.; Kim, N.; Millar, S.E.; Cotsarelis, G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 2007, 447, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Cheon, S.S.; Cheah, A.Y.; Turley, S.; Nadesan, P.; Poon, R.; Clevers, H.; Alman, B.A. Beta-catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc. Natl. Acad. Sci. USA 2002, 99, 6973–6978. [Google Scholar] [CrossRef] [PubMed]
- Akhmetshina, A.; Palumbo, K.; Dees, C.; Bergmann, C.; Venalis, P.; Zerr, P.; Horn, A.; Kireva, T.; Beyer, C.; Zwerina, J.; et al. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat. Commun. 2012, 3, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathke, C.; Wilson, L.; Shah, K.; Kim, B.; Hocking, A.; Moon, R.; Isik, F. Wnt signaling induces epithelial differentiation during cutaneous wound healing. BMC Cell Boil. 2006, 7, 4. [Google Scholar]
- Mori, H.; Yao, Y.; Learman, B.S.; Kurozumi, K.; Ishida, J.; Ramakrishnan, S.K.; Overmyer, K.A.; Xue, X.; Cawthorn, W.P.; Reid, M.A.; et al. Induction of Wnt11 by hypoxia and hypoxia-inducible factor-1alpha regulates cell proliferation, migration and invasion. Sci. Rep. 2016, 6, 21520. [Google Scholar] [CrossRef] [PubMed]
- Scheid, A.; Wenger, R.H.; Christina, H.; Camenisch, I.; Ferenc, A.; Stauffer, U.G.; Gassmann, M.; Meuli, M. Hypoxia-regulated gene expression in fetal wound regeneration and adult wound repair. Pediatr. Surg. Int. 2000, 16, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Pasolli, H.A.; Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 2011, 144, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Blanpain, C.; Fuchs, E. Epidermal stem cells of the skin. Annu. Rev. Cell Dev. Biol. 2006, 22, 339–373. [Google Scholar] [CrossRef] [PubMed]
- Blanpain, C.; Lowry, W.E.; Geoghegan, A.; Polak, L.; Fuchs, E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 2004, 118, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.M.; Kubler, M.D.; Hotchin, N.A.; Nicholson, L.J.; Adams, J.C. Regulation of keratinocyte terminal differentiation by integrin-extracellular matrix interactions. J. Cell Sci. 1993, 106 Pt 1, 175–182. [Google Scholar] [PubMed]
- Rennert, R.C.; Sorkin, M.; Garg, R.K.; Gurtner, G.C. Stem cell recruitment after injury: Lessons for regenerative medicine. Regen. Med. 2012, 7, 833–850. [Google Scholar] [CrossRef] [PubMed]



| Characteristic of Foxn1 Deficiency | |
|---|---|
| Mouse (Nude) | Human (Nude/SCID) |
| Mutations in the Foxn1 gene identified in the mouse allele nu, located on chromosomes 11; a single base-pair deletion in the Foxn1 coding sequence leads to a frame shift and premature termination in the DBD [22,67]; | Mutation in human FOXN1 gene recognised as a homozygous C-to-T transition (CGA to TGA) at nucleotide position 792 resulted in a nonsense mutation at residue 255 (R255X) in exon 5 [70]; |
| The translated FOXN1, Foxn1, proteins are nonfunctional in human and mice, respectively and lead to similar defects [23,24,25]; Pleiotropic mutation categorised into two independent phenotypic responses: (i) impaired skin keratinisation and aberrant HFs development [13,19,23] and (ii) thymus dysgenesis that leads to T-cell deficiency [23,67]; | |
Mutants represent multiple skin defects [13,19]:
| Foetuses at 15th weeks show tight, shiny and smooth skin associated with a lack of thymus, anencephaly and spina bifida, indicating that beside its role in the thymus and skin epithelium, FOXN1 might also be involved in neurulation in humans [72]; Infant mutants demonstrate complete alopecia of the scalp, eyebrow, and eyelashes and nail dystrophy associated with a primary severe T-cell immunodeficiency [23,73]. |
| Attributes to Foxn1 Activity in the Mice Skin | Attributes to Foxn1 Deficiency in the Mice Skin | |
|---|---|---|
| Development | Prenatal stage: Foxn1 expression detected on E13 in the developing nasal region; and by E15.5–16.5 Foxn1 occupied entire epidermis including developing HF of the fur coat [19]; 2065 genes differentially regulated (up and down) between skin from B6 embryo at E14 (model of skin regeneration) and E18 (model of skin reparation) [41]; Early neonatal stage: Foxn1 expression (postpartum days P1–P2) reported in the hair shaft and throughout the developing IRS and in a matrix compartment of hair bulb suggesting that Foxn1 correlates with the onset of terminal differentiation [19]; | Prenatal stage: Lack of Foxn1 activity at E16.5 (Foxn1 priming) keeps skin of nude mice in the immature stage resembling the phenomena of neoteny [41]; |
| Homeostasis | Foxn1 expression in epithelial cells (epidermis) initiates terminal differentiation program and stimulates neighboring epithelial cell proliferation in paracrine manner [14,19,51]; Foxn1 participates in the development of skin epithelial cells coloration through activation of epithelial cells to emit signals (FGF-2) recognisable by melanocytes [54]; Epidermal Foxn1 expression impact the skin: (i) decreased levels of anti-fibrotic cytokine Tgfbeta3; (ii) cultured DF exhibited reduced adipogenic differentiation capacity [88]; | Impaired keratinisation of epidermis and hair shaft results in “hairless phenotype” [24,25]; Aberrant differentiation of epidermal keratinocytes manifests by inhibition of early differentiation markers (Krt1, -10), overproduction and accumulation of late differentiation markers (profilaggrin, loricrin, involucrin) and abnormal thickening of epidermis [13,14]; Increased amounts of cholesterol sulfate, phospholipids, sphingolipids and fatty acids in the skin when compare to the Foxn1 -active mouse models [89]; |
| Wound healing | The process of healing occurs with fibrosis and scar formation, a condition characterised by excessive deposition of ECM protein rich in collagens [97,98,99]; Foxn1 acts as a key molecular player in re-epithelialisation and EMT processes in post-wounded skin tissues [16]. | Perfect healing in the process of regeneration characterised by lack of a scar, high levels of hyaluronic acid, low levels of collagen and pro-scarring cytokines (PDGF-B and TGF β1), and unique bimodal pattern of Mmp-9 and Mmp-13 expression [42,43]. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukowska, J.; Kopcewicz, M.; Walendzik, K.; Gawronska-Kozak, B. Foxn1 in Skin Development, Homeostasis and Wound Healing. Int. J. Mol. Sci. 2018, 19, 1956. https://doi.org/10.3390/ijms19071956
Bukowska J, Kopcewicz M, Walendzik K, Gawronska-Kozak B. Foxn1 in Skin Development, Homeostasis and Wound Healing. International Journal of Molecular Sciences. 2018; 19(7):1956. https://doi.org/10.3390/ijms19071956
Chicago/Turabian StyleBukowska, Joanna, Marta Kopcewicz, Katarzyna Walendzik, and Barbara Gawronska-Kozak. 2018. "Foxn1 in Skin Development, Homeostasis and Wound Healing" International Journal of Molecular Sciences 19, no. 7: 1956. https://doi.org/10.3390/ijms19071956
APA StyleBukowska, J., Kopcewicz, M., Walendzik, K., & Gawronska-Kozak, B. (2018). Foxn1 in Skin Development, Homeostasis and Wound Healing. International Journal of Molecular Sciences, 19(7), 1956. https://doi.org/10.3390/ijms19071956


.png)