Biotransformation of α-Acetylbutyrolactone in Rhodotorula Strains
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Analysis
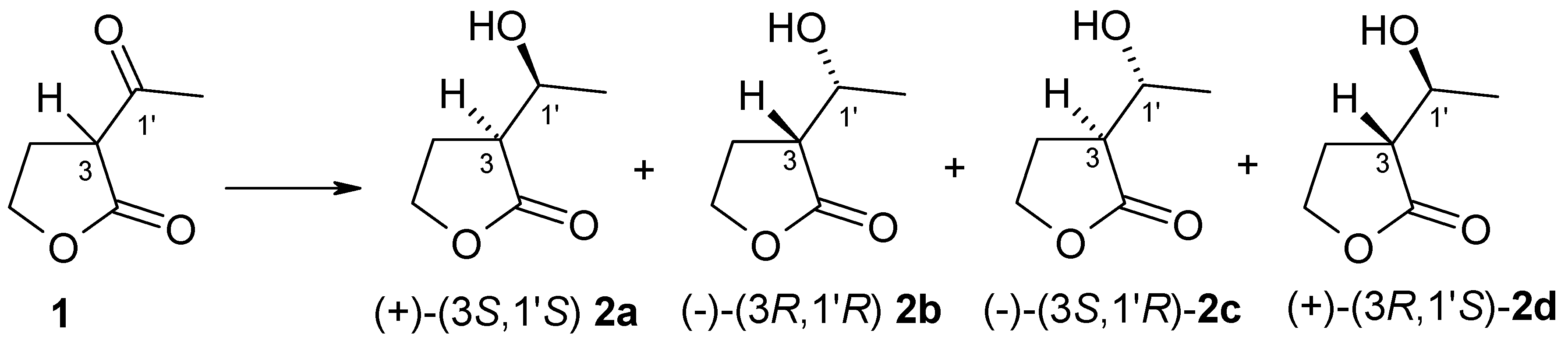
3.2. Synthesis of Standards
3.3. Microorganisms
3.4. Cultivation Media
3.5. Screening Procedure
3.6. Preparative Biotransformation
3.7. Preparation of Resting Cell Suspension
3.8. Biotransformation in the Presence of Organic Solvents
3.9. Biotransformation in the Presence of Deep Eutectic Solvents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kot, A.M.; Błażejak, S.; Kurcz, A.; Gientka, I.; Kieliszek, M. Rhodotorula glutinis—Potential source of lipids, carotenoids, and enzymes for use in industries. Appl. Microbiol. Biotechnol. 2016, 100, 6103–6117. [Google Scholar] [CrossRef] [PubMed]
- Wirth, F.; Goldani, L.Z. Epidemiology of Rhodotorula: An emerging pathogen, interdisciplinary perspectives on infectious diseases. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Banzatto, D.; Aline de Freita, L.; Rossini Mutton, M.J. Carotenoid production by Rhodotorula rubra cultivated in sugarcane juice, molasses, and syrup. Ciênc. Tecnol. Aliment. 2013, 33, 14–18. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kurcz, A.; Bryś, J.; Gientka, I.; Bzducha-Wróbel, A.; Maliszewska, M.; Reczek, L. Effect of initial pH of medium with potato wastewater and glycerol on protein, lipid and carotenoid biosynthesis by Rhodotorula glutinis. Electron. J. Biotechnol. 2017, 27, 25–31. [Google Scholar] [CrossRef]
- Aguirre-Pranzoni, C.; Bisogno, F.R.; Orden, A.A.; Kurina-Sanz, M. Lyophilized Rhodotorula yeast as all-in-one redox biocatalyst: Access to enantiopure building blocks by simple chemoenzymatic one-pot procedures. J. Mol. Catal. B Enzym. 2015, 114, 19–24. [Google Scholar] [CrossRef]
- Janeczko, T.; Dymarska, M.; Kostrzewa-Susłow, E. Highly enantioselective production of (R)-halohydrins with whole cells of Rhodotorula rubra KCh 82 culture. Int. J. Mol. Sci. 2014, 15, 22392–22404. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, T.; Kostrzewa-Susłow, E. Enantioselective reduction of propiophenone formed from 3-chloropropiophenone and stereoinversion of resulting alcohols in selected yeast cultures. Tetrahedron Asymmetry 2014, 25, 1264–1269. [Google Scholar] [CrossRef]
- De Oliveira Lopes, R.; Benzaquem Ribeiro, J.; de Souza Ramos, A.; Miranda, L.S.M.; Ramos Leal, I.C.; Gomes Ferreira Leite, S.; Mendoça Alves de Souza, R.O. Highly enantioselective bioreduction of bromoacetophenone. Tetrahedron Asymmetry 2011, 22, 1763–1766. [Google Scholar] [CrossRef]
- Fardelone, L.C.; Rodrigues, J.A.R.; Moran, P.J.S. Bioreduction of 2-azido-1-arylethanones mediated by Geotrichum candidum and Rhodotorula glutinis. J. Mol. Catal. B Enzym. 2006, 39, 9–12. [Google Scholar] [CrossRef]
- De Souza Ramos, A.; Benzaquem Ribeiro, J.; de Oliveira Lopes, R.; Mendonc, R.O.; de Souza, A. Whole cells in enantioselective reduction of tert-butyl acetoacetate. Synth. Commun. 2013, 43, 1611–1618. [Google Scholar] [CrossRef]
- Benzaquem Ribeiro, J.; Andrade de Sousa, L.M.; da Volta Soares, M.; da Conceição, M.; Ramos, K.V.; Aquino Neto, F.R.; Mansour Fraga, C.A.; Ferreira Leite, S.G.; Cordeiro, Y.; Antunes, O.A.C. Microbial reduction of α-acetyl-γ-butyrolactone. Tetrahedron Asymmetry 2006, 17, 984–988. [Google Scholar] [CrossRef]
- Teixeira, L.H.P.; de Souza, M.C.B.V.; da Conceição, K.V.; Ramos, M.; de Aquino Neto, F.R.; Barreiro, E.J.; Fraga, C.A.M. Studies on the diastereoselective reduction of 2-acetyl-2-alkyl-γ-butyrolactones with boron hydrides. Synth. Commun. 2002, 32, 505–526. [Google Scholar] [CrossRef]
- Bräutigam, S.; Dennewald, D.; Schürmann, M.; Lutje-Spelberg, J.; Pitner, W.-R.; Weuster-Botz, D. Whole-cell biocatalysis: Evaluation of new hydrophobic ionic liquids for efficient asymmetric reduction of prochiral ketones. Enzym. Microb. Technol. 2009, 45, 310–316. [Google Scholar] [CrossRef]
- Dennewald, D.; Pitner, W.-R.; Weuster-Botz, D. Recycling of the ionic liquid phase in process integrated biphasic whole-cell biocatalysis. Proc. Biochem. 2011, 46, 1132–1137. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef]
- Juneidi, I.; Hayyana, M.; Hashim, M.A. Intensification of biotransformations using deep eutectic solvents: Overview and outlook. Process Biochem. 2018, 66, 33–60. [Google Scholar] [CrossRef]
- Abo-Hamad, A.; Hayyan, M.; AlSaadi, M.A.; Hashim, M.A. Potential application of deep eutectic solvents in nanotechnology. Chem. Eng. J. 2015, 273, 551–567. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Hayyan, M.; Alsaadi, M.A.; Akib, S.; Hayyan, A.; Hashim, M.A. Glycerol-based deep eutectic solvents: Physical properties. J. Mol. Liq. 2016, 215, 98–103. [Google Scholar] [CrossRef]
- Fantin, G.; Fogagnolo, M.; Giovannini, P.P.; Medici, A.; Pedrini, P.; Gardini, F.; Lanciotti, R. Anti-Prelog microbial reduction of prochiral carbonyl compounds. Tetrahedron 1996, 52, 3547–3552. [Google Scholar] [CrossRef]
- Fantin, G.; Fogagnolo, M.; Giovannini, P.; Medici, A.; Pagnotta, E.; Pedrini, P.; Trincone, A. Synthesis of homochiral syn- and anti-α-(hydroxyethyl)-γ-buttyrolactones via microbial reduction. Tetrahedron Asymmetry 1994, 5, 1631–1634. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, X.; Huang, H.; Li, S. Efficient synthesis of (R)-2-chloro-1-phenylethol using a yeast carbonyl reductase with broad substrate spectrum and 2-propanol as cosubstrate. Biochem. Eng. J. 2015, 103, 277–285. [Google Scholar] [CrossRef]
- Johanson, T.; Katz, M.; Gorwa-Grauslund, M.F. Strain engineering for stereoselective bioreduction of dicarbonyl compounds by yeast reductases. FEMS Yeast Res. 2005, 5, 513–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Strain | Medium | Time (h) | Substrate | Stereoisomers of Product | enol (%) | ||
|---|---|---|---|---|---|---|---|
| 1 (%) | 2a + 2b (syn) (%) | 2c + 2d (anti) (%) | de (%) | ||||
| Rhodotorula glutinis AM242 | A (YPG) | 1 | 83.3 | 4.6 | 2.7 | 26.0 | 9.4 |
| 2 | 75.3 | 8.9 | 8.0 | 5.3 | 7.8 | ||
| 3 | 66.3 | 13.5 | 14.3 | 2.9 | 5.8 | ||
| 4 | 52.1 | 17.8 | 27.5 | 21.4 | 2.6 | ||
| 5 | 34.9 | 22.6 | 40.1 | 27.9 | 2.4 | ||
| B (S) | 1 | 85.6 | 7.8 | 1.4 | 69.5 | 5.2 | |
| 2 | 82.5 | 10.7 | 2.5 | 62.1 | 3.7 | ||
| 3 | 78.5 | 13.7 | 4.5 | 50.5 | 3.3 | ||
| 4 | 70.3 | 17.3 | 9.0 | 31.5 | 3.4 | ||
| 5 | 67.0 | 15.6 | 10.0 | 21.9 | 7.4 | ||
| C (MCM) | 1 | 83.5 | 5.7 | 0.5 | 83.9 | 10.3 | |
| 2 | 82.3 | 6.6 | 1.2 | 69.2 | 9.9 | ||
| 3 | 78.6 | 10.4 | 2.8 | 57.6 | 8.2 | ||
| 4 | 72.9 | 11.6 | 5.1 | 38.9 | 10.4 | ||
| 5 | 70.2 | 12.2 | 6.0 | 34.1 | 11.5 | ||
| Strain | Medium | Time (h) | Substrate | Stereoisomers of Product | enol (%) | ||
|---|---|---|---|---|---|---|---|
| 1 (%) | 2a + 2b (syn) (%) | 2c + 2d (anti) (%) | de (%) | ||||
| Rhodotorula marina AM77 | A (YPG) | 1 | 77.8 | 6.8 | 8.1 | 8.7 | 7.2 |
| 2 | 36.0 | 15.8 | 37.8 | 41.1 | 10.4 | ||
| 3 | 0 | 21.3 | 67.6 | 52.1 | 11.1 | ||
| B (S) | 1 | 86.3 | 4.0 | 8.7 | 37.0 | 0.9 | |
| 2 | 50.1 | 11.8 | 33.1 | 47.4 | 5.0 | ||
| 3 | 0 | 19.4 | 77.2 | 59.8 | 3.3 | ||
| C (MCM) | 1 | 86.4 | 4.0 | 2.8 | 17.6 | 6.7 | |
| 2 | 79.6 | 5.5 | 7.0 | 12.0 | 7.7 | ||
| 3 | 48.1 | 11.2 | 34.0 | 50.4 | 6.7 | ||
| 4 | 8.2 | 14.8 | 68.3 | 64.4 | 8.7 | ||
| 5 | 0 | 12.8 | 59.8 | 64.7 | 27.3 | ||
| Strain | Medium | Time (h) | Substrate | Stereoisomers of Product | enol (%) | ||
|---|---|---|---|---|---|---|---|
| 1 (%) | 2a + 2b (syn) (%) | 2c + 2d (anti) (%) | de (%) | ||||
| Rhodotorula rubra C9 | A (YPG) | 1 | 20.9 | 12.8 | 62.4 | 66.0 | 3.9 |
| 2 | 0.6 | 14.2 | 83.1 | 70.8 | 1.8 | ||
| 3 | 0 | 10.1 | 89.9 | 79.8 | 0 | ||
| B (S) | 1 | 84.6 | 7.4 | 4.0 | 29.8 | 4.0 | |
| 2 | 72.6 | 9.4 | 12.6 | 14.5 | 5.4 | ||
| 3 | 43.9 | 17.5 | 34.5 | 32.7 | 4.1 | ||
| 4 | 0 | 24.8 | 73.4 | 49.5 | 1.8 | ||
| C (MCM) | 1 | 85.2 | 6.6 | 2.1 | 51.7 | 6.0 | |
| 2 | 76.9 | 10.8 | 8.3 | 13.1 | 4.0 | ||
| 3 | 33.4 | 26.6 | 32.1 | 9.4 | 7.9 | ||
| 4 | 0 | 34.4 | 60.2 | 27.3 | 5.3 | ||
| Strain | Time (h) | 1 (%) | syn | ee (%) | anti | ee (%) | ||
|---|---|---|---|---|---|---|---|---|
| 2a (3S, 1′S) (%) | 2b (3R, 1′R) (%) | 2c (3S, 1′R) (%) | 2d (3R, 1′S) (%) | |||||
| Rhodotorula glutinis AM242 | 5 | 39.3 | 3.7 | 17.9 | 65.7 | 1.2 | 38.0 | 93.9 |
| Rhodotorula marina AM77 | 3 | 0 | 2.7 | 16.4 | 71.7 | 0 | 80.9 | 100 |
| Rhodotorula rubra C9 | 3 | 0 | 1.7 | 6.8 | 60.0 | 0 | 91.5 | 100 |
| Strain | Time (h) | 1 (%) | syn | ee (%) | anti | ee (%) | ||
|---|---|---|---|---|---|---|---|---|
| 2a (3S, 1′S) (%) | 2b (3R, 1′R) (%) | 2c (3S, 1′R) (%) | 2d (3R, 1′S) (%) | |||||
| Rhodotorula glutinis AM242 | 3 | 90.2 | 0.3 | 5.8 | 90.2 | 0 | 3.7 | 100 |
| 5 | 87.2 | 0.3 | 7.3 | 92.1 | 0.2 | 5.1 | 92.4 | |
| 8 | 81.4 | 0.4 | 9.2 | 87.3 | 0.3 | 8.7 | 93.5 | |
| 24 | 2.4 | 3.6 | 35.7 | 81.7 | 0.7 | 58.6 | 97.6 | |
| 48 | 0 | 3.9 | 36 | 80.0 | 0.9 | 59.2 | 96.0 | |
| Rhodotorula marina AM77 | 3 | 40.6 | 0 | 5.7 | 100 | 0 | 53.7 | 100 |
| 5 | 6.2 | 0 | 5.5 | 100 | 0 | 88.3 | 100 | |
| 8 | 0 | 0 | 1.6 | 100 | 0 | 98.3 | 100 | |
| Rhodotorula rubra C9 | 3 | 7.8 | 4.6 | 16.8 | 57.0 | 0 | 70.8 | 100 |
| 5 | 0 | 4.6 | 17.4 | 58.2 | 0 | 78.0 | 100 | |
| Strain | Time (h) | 1 (%) | syn | ee (%) | anti | ee (%) | ||
|---|---|---|---|---|---|---|---|---|
| 2a (3S, 1′S) (%) | 2b (3R, 1′R) (%) | 2c (3S, 1′R) (%) | 2d (3R, 1′S) (%) | |||||
| Rhodotorula glutinis AM242 | 3 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 89.6 | 0.4 | 5.1 | 85.5 | 0 | 4.9 | 100 | |
| 8 | 78.5 | 0.6 | 7.3 | 84.4 | 0 | 13.6 | 100 | |
| 24 | 17.6 | 3.6 | 32.7 | 80.2 | 0.6 | 45.4 | 97.4 | |
| 48 | 0 | 3.4 | 37.2 | 83.0 | 1.1 | 58.3 | 96.0 | |
| Rhodotorula marina AM77 | 3 | 57 | 0.9 | 6.2 | 74.6 | 0 | 35.9 | 100 |
| 5 | 1.3 | 1.8 | 9.6 | 68.0 | 0 | 87.3 | 100 | |
| 8 | 0 | 0.9 | 6.7 | 76.3 | 0 | 92.4 | 100 | |
| Rhodotorula rubra C9 | 3 | 2.9 | 4.6 | 10.6 | 39.0 | 0 | 81.9 | 100 |
| 5 | 0 | 3.8 | 5.4 | 17.4 | 0 | 90.8 | 100 | |
| Solvent | % of Solvent | 1 (%) | syn | ee (%) | anti | ee (%) | ||
|---|---|---|---|---|---|---|---|---|
| 2a (3S, 1′S) (%) | 2b (3R, 1′R) (%) | 2c (3S, 1′R) (%) | 2d (3R, 1′S) (%) | |||||
| ethanol | 5 | 52.2 | 0 | 0 | 0 | 0 | 47.8 | 100 |
| 10 | 87.2 | 0 | 0 | 0 | 0 | 12.8 | 100 | |
| 20 | 99.5 | 0 | 0 | 0 | 0 | 0.5 | 100 | |
| glycerol | 5 | 0 | 0 | 0 | 0 | 0 | 100 | 100 |
| 10 | 7.8 | 0 | 0 | 0 | 0 | 92.2 | 100 | |
| 20 | 80.6 | 2.8 | 1.6 | 27.0 | 0 | 15 | 100 | |
| hexane | 5 | 73.9 | 0 | 0 | 0 | 0 | 26.1 | 100 |
| 10 | 90.8 | 0 | 0 | 0 | 0 | 9.2 | 100 | |
| 20 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | |
| isopropanol | 5 | 76.7 | 0 | 0 | 0 | 0 | 23.3 | 100 |
| 10 | 98.8 | 0 | 0 | 0 | 0 | 1.2 | 100 | |
| 20 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | |
| DES | Time | 1 (%) | syn | ee (%) | anti | ee (%) | ||
|---|---|---|---|---|---|---|---|---|
| 2a (3S, 1′S) (%) | 2b (3R, 1′R) (%) | 2c (3S, 1′R) (%) | 2d (3R, 1′S) (%) | |||||
| ChCl:Gly 10% | 5 h | 1.5 | 0 | 4.4 | 100 | 0 | 94.1 | 100 |
| 1 d | 0 | 0 | 4.7 | 100 | 0 | 95.3 | 100 | |
| ChCl:Gly 25% | 5 h | 100 | 0 | 0 | - | 0 | 0 | - |
| 1 d | 2.4 | 2.0 | 12.8 | 73.0 | 0 | 82.8 | 100 | |
| 2 d | 0 | 2.1 | 13.3 | 72.7 | 0 | 84.6 | 100 | |
| ChCl:Gly 50% | 5 h | 100 | 0 | 0 | - | 0 | 0 | - |
| 1 d | 88.8 | 1.3 | 6.2 | 65.3 | 0 | 3.7 | 100 | |
| 4 d | 31.4 | 4.8 | 35.4 | 76.0 | 0 | 27.9 | 100 | |
| 7 d | 1.6 | 5.2 | 39.8 | 76.9 | 0 | 53.4 | 100 | |
| ChCl:Gly 66% | 5 h | 100 | 0 | 0 | - | 0 | 0 | - |
| 1 d | 93.7 | 0.7 | 4.0 | 70.2 | 0 | 1.6 | - | |
| 4 d | 76.3 | 3.2 | 14.7 | 64.0 | 0 | 5.8 | 100 | |
| 7 d | 55.2 | 8.8 | 24.6 | 63.2 | 0 | 11.4 | 100 | |
| ChCl:Gly:Glc 10% | 5 h | 4.9 | 1.6 | 14.3 | 80.0 | 0 | 79.2 | 100 |
| 1 d | 0 | 1.4 | 5.8 | 61.0 | 0 | 92.8 | 100 | |
| ChCl:Gly:Glc 25% | 5 h | 81.2 | 0 | 2.5 | 100 | 0 | 16.3 | 100 |
| 1 d | 0 | 0 | 13.6 | 100 | 0 | 86.4 | 100 | |
| ChCl:Gly:Glc 50% | 1 d | 83.7 | 1.7 | 8.2 | 66.0 | 0 | 6.4 | 100 |
| 4 d | 32.4 | 6.2 | 33.8 | 69.0 | 0 | 27.3 | 100 | |
| 7 d | 29.5 | 6.5 | 31.5 | 66.0 | 0 | 31.8 | 100 | |
| ChCl:Gly:Glc 66% | 5h | 100 | 0 | 0 | - | 0 | 0 | - |
| 1 d | 100 | 0 | 0 | - | 0 | 0 | - | |
| 4 d | 92.5 | 0.6 | 3.0 | 67.0 | 0 | 3.2 | 100 | |
| 7 d | 87.5 | 2.1 | 6.0 | 65.0 | 0 | 4.4 | 100 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mączka, W.; Wińska, K.; Grabarczyk, M.; Żarowska, B. Biotransformation of α-Acetylbutyrolactone in Rhodotorula Strains. Int. J. Mol. Sci. 2018, 19, 2106. https://doi.org/10.3390/ijms19072106
Mączka W, Wińska K, Grabarczyk M, Żarowska B. Biotransformation of α-Acetylbutyrolactone in Rhodotorula Strains. International Journal of Molecular Sciences. 2018; 19(7):2106. https://doi.org/10.3390/ijms19072106
Chicago/Turabian StyleMączka, Wanda, Katarzyna Wińska, Małgorzata Grabarczyk, and Barbara Żarowska. 2018. "Biotransformation of α-Acetylbutyrolactone in Rhodotorula Strains" International Journal of Molecular Sciences 19, no. 7: 2106. https://doi.org/10.3390/ijms19072106







